Therapeutic Approach of Whole-Body Vibration Exercise on Wound Healing in Animal Models: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Appraisal of Risk of Bias (RoB)
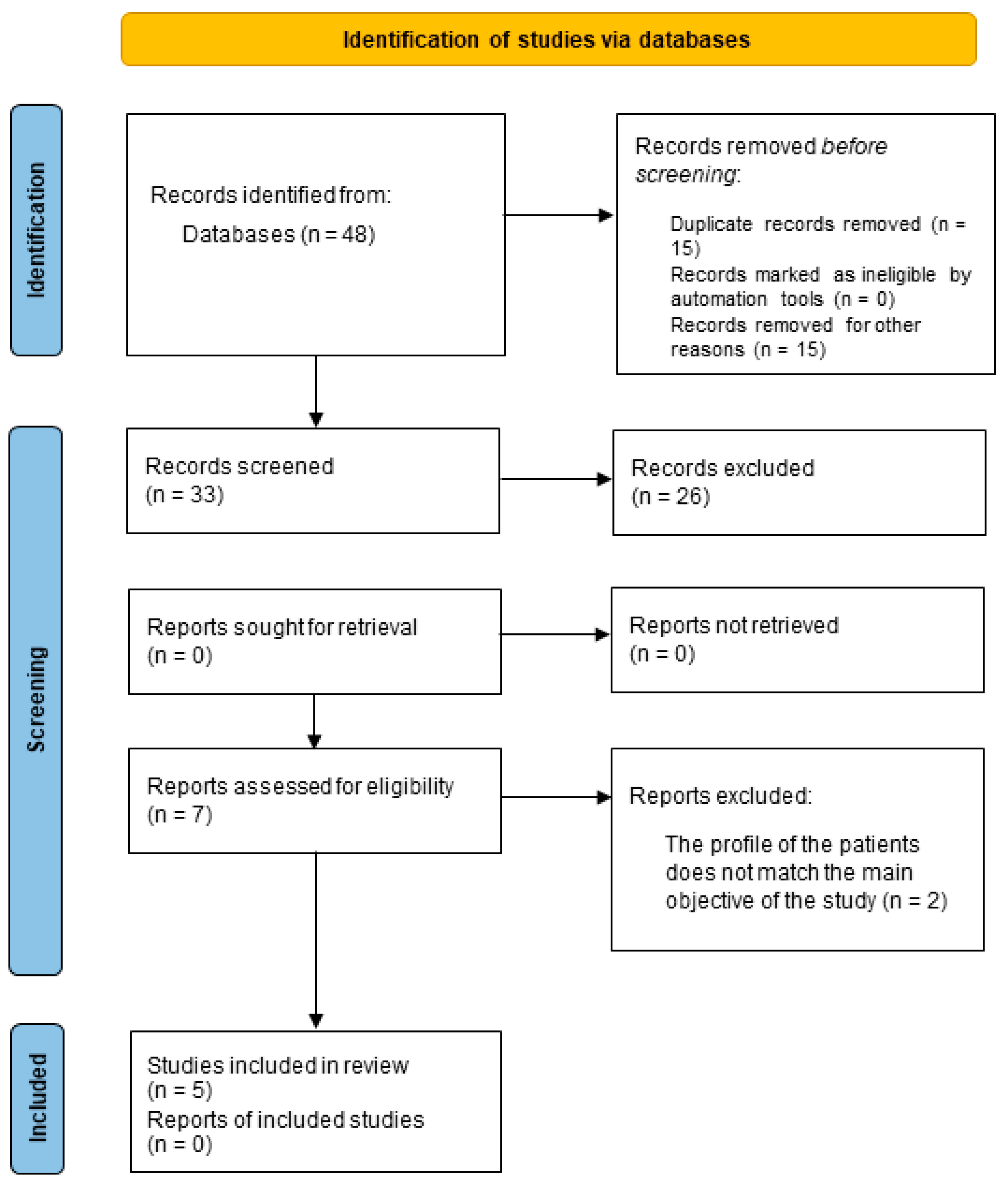
3. Results
3.1. Selected Studies
3.2. Main Findings
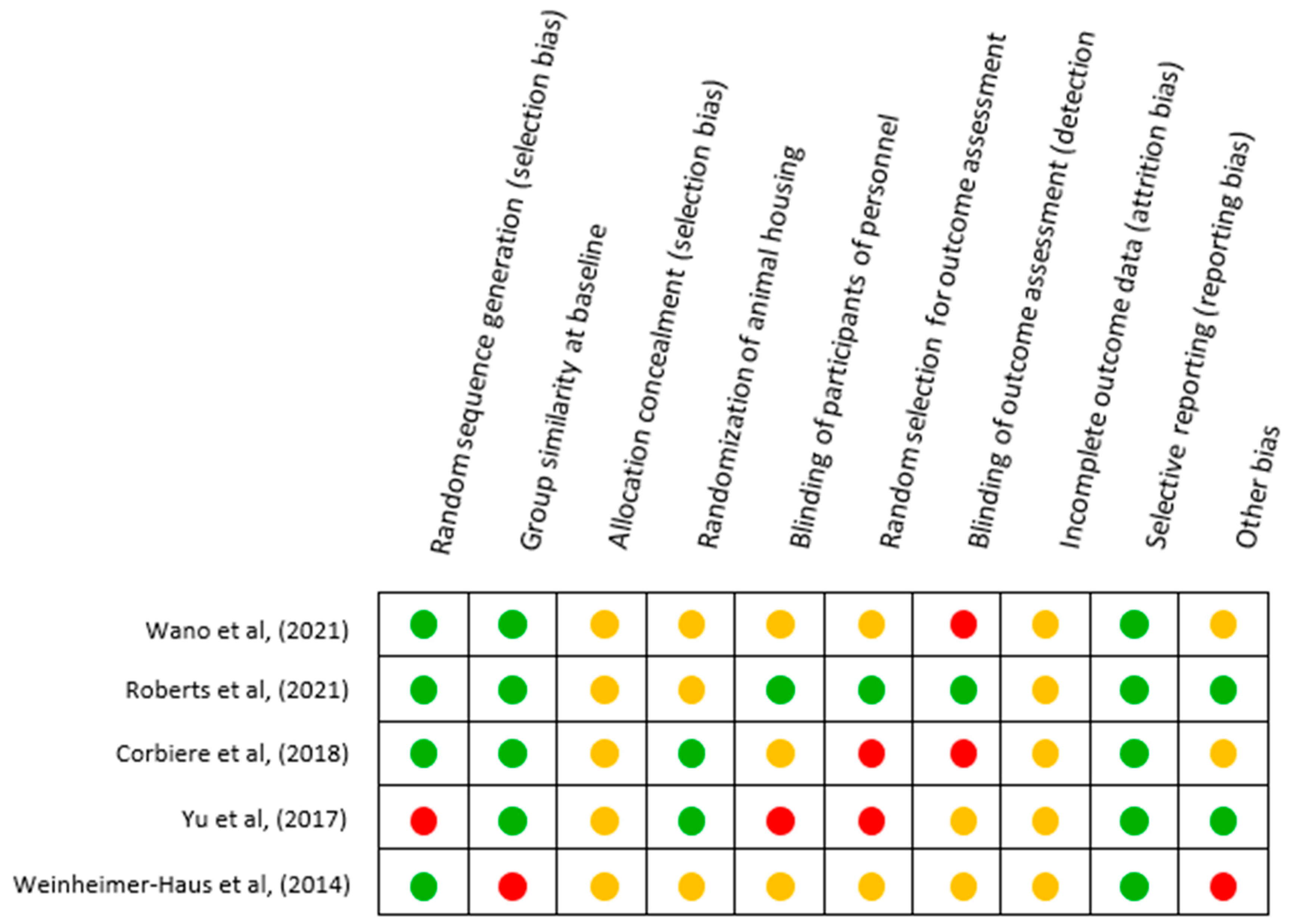
3.3. Risk of Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fayne, R.A.; Borda, L.G.; Egger, A.N.; Canic, M.T. The Potential Impact of Social Genomics on Wound Healing. Adv. Wound Care 2020, 9, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Shou, K.; Li, Z.; Jian, C.; Qi, B.; Yu, A. Negative pressure wound therapy promotes vessel destabilization and maturation at various stages of wound healing and thus influences wound prognosis. Exp. Ther. Med. 2016, 11, 1307–1317. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Li, S.; Yu, Z.; Liu, B.; Song, B.; Su, Y. The clinical dynamic changes of macrophage phenotype and function in different stages of human wound healing and hypertrophic scar formation. Int. Wound J. 2019, 16, 360–369. [Google Scholar] [CrossRef]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J.; Deptuła, M.; Karpowicz, P.; Wardowska, A.; Sass, P.; Sosnowski, P.; Mieczkowska, A.; Filipowicz, N.; Dzierżyńska, M.; et al. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Monteiro-Oliveira, B.B.; Coelho-Oliveira, A.C.; Paineiras-Domingos, L.L.; Sonza, A.; Sá-Caputo, D.D.C.D.; Bernardo-Filho, M. Use of surface electromyography to evaluate effects of whole-body vibration exercises on neuromuscular activation and muscle strength in the elderly: A systematic review. Disabil. Rehabilitation 2022, 44, 7368–7377. [Google Scholar] [CrossRef]
- Lindley, L.E.; Stojadinovic, O.; Pastar, I.; Tomic-Canic, M. Biology and Biomarkers for Wound Healing. Plast. Reconstr. Surg. 2016, 138, 18S–28S. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T.J. Wound healing and treating wounds Chronic wound care and management. J. Am. Acad. Dermatol. 2016, 74, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Sharma, A.K.; Gupta, V.; Yashavarddhan, M. Pharmacological control of inflammation in wound healing. J. Tissue Viability 2019, 28, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, E.; Liu, P.Y.; Schultz, G.S.; Martins-Green, M.M.; Tanaka, R.; Weir, D.; Gould, L.J.; Armstrong, D.G.; Gibbons, G.W.; Wolcott, R.; et al. Chronic wounds: Treatment consensus. Wound Repair Regen. 2022, 30, 156–171. [Google Scholar] [CrossRef]
- Boyko, T.V.; Longaker, M.T.; Yang, G.P. Review of the Current Management of Pressure Ulcers. Adv. Wound Care 2018, 7, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, L.T. Wound Healing and Infection in Surgery. Arch. Surg. 2012, 147, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Citty, S.; Cowan, L.J.; Wingfield, Z.; Stechmiller, J. Optimizing Nutrition Care for Pressure Injuries in Hospitalized Patients. Adv. Wound Care 2019, 8, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Sá-Caputo, D.C.; Seixas, A.; Taiar, R.; Bernardo-Filho, M. Vibration Therapy for Health Promotion. Complement. Ther. 2022. [Google Scholar] [CrossRef]
- Bonanni, R.; Cariati, I.; Romagnoli, C.; D’Arcangelo, G.; Annino, G.; Tancredi, V. Whole Body Vibration: A Valid Alternative Strategy to Exercise? J. Funct. Morphol. Kinesiol. 2022, 7, 99. [Google Scholar] [CrossRef]
- van Heuvelen, M.J.G.; Rittweger, J.; Judex, S.; Sañudo, B.; Seixas, A.; Fuermaier, A.B.M.; Tucha, O.; Nyakas, C.; Marín, P.J.; Taiar, R.; et al. Reporting Guidelines for Whole-Body Vibration Studies in Humans, Animals and Cell Cultures: A Consensus Statement from an International Group of Experts. Biology 2021, 10, 965. [Google Scholar] [CrossRef]
- Rittweger, J. Vibration as an exercise modality: How it may work, and what its potential might be. Eur. J. Appl. Physiol. 2010, 108, 877–904. [Google Scholar] [CrossRef]
- Weinheimer-Haus, E.M.; Judex, S.; Ennis, W.J.; Koh, T.J. Low-Intensity Vibration Improves Angiogenesis and Wound Healing in Diabetic Mice. PLoS ONE 2014, 9, e91355. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Lan, Q.; Chen, Y.; Chan, Y.W.J.; Mahady, G.; Lee, S.M.-Y. Low-Magnitude High-Frequency Vibration Decreases Body Weight Gain and Increases Muscle Strength by Enhancing the p38 and AMPK Pathways in db/db Mice. Diabetes Metab. Syndr. Obes. 2020, 13, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.K.H.; Ho, C.Y.; Wong, H.W.; Chim, Y.N.; Wong, R.M.-Y.; Cheung, W.H. Efficacy of low-magnitude high-frequency vibration (LMHFV) on musculoskeletal health of participants on wheelchair: A study protocol for a single-blinded randomised controlled study. BMJ Open 2020, 10, e038578. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.-Y.; Leung, K.-S.; Qin, J.-H.; Chow, S.K.-H.; Cheung, W.H. Effect of Low-Magnitude, High-Frequency Vibration Treatment on Retardation of Sarcopenia: Senescence-Accelerated Mouse-P8 Model. Rejuvenation Res. 2016, 19, 293–302. [Google Scholar] [CrossRef]
- Steppe, L.; Liedert, A.; Ignatius, A.; Haffner-Luntzer, M. Influence of Low-Magnitude High-Frequency Vibration on Bone Cells and Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 595139. [Google Scholar] [CrossRef]
- Kaeding, T.S.; Karch, A.; Schwarz, R.; Flor, T.; Wittke, T.-C.; Kück, M.; Böselt, G.; Tegtbur, U.; Stein, L. Whole-body vibration training as a workplace-based sports activity for employees with chronic low-back pain. Scand. J. Med. Sci. Sport. 2017, 27, 2027–2039. [Google Scholar] [CrossRef]
- Choi, W.; Han, D.; Kim, J.; Lee, S. Whole-Body Vibration Combined with Treadmill Training Improves Walking Performance in Post-Stroke Patients: A Randomized Controlled Trial. Med. Sci. Monit. 2017, 23, 4918–4925. [Google Scholar] [CrossRef]
- Reis-Silva, A.; Coelho-Oliveira, A.C.; Martins-Anjos, E.; Moura-Fernandes, M.C.; Mulder, A.; Xavier, V.L.; Mendonça, V.A.; Lacerda, A.C.R.; Paineiras-Domingos, L.L.; Taiar, R.; et al. Impact of Two Whole-Body Vibration Exercise Protocols on Body Composition of Patients with Metabolic Syndrome: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2022, 20, 436. [Google Scholar] [CrossRef]
- Abbasi, M.; Yoosefinejad, A.K.; Poursadeghfard, M.; Jahromi, F.P.; Motealleh, A.; Sobhani, S. Whole body vibration improves core muscle strength and endurance in ambulant individuals with multiple sclerosis: A randomized clinical trial. Mult. Scler. Relat. Disord. 2019, 32, 88–93. [Google Scholar] [CrossRef]
- Borges, D.T.; Macedo, L.B.; Lins, C.A.A.; Sousa, C.O.; Brasileiro, J.S. Effects of Whole Body Vibration on the Neuromuscular Amplitude of Vastus Lateralis Muscle. J. Sport. Sci. Med. 2017, 16, 414–420. [Google Scholar]
- Han, Y.-G.; Lee, S.-W.; Yun, C.-K. The immediate influence of various whole-body vibration frequency on balance and walking ability in children with cerebral palsy: A pilot study. J. Exerc. Rehabil. 2019, 15, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Arauz, Y.L.A.; van der Zee, E.A.; Kamsma, Y.P.T.; van Heuvelen, M.J.G. Short-term effects of side-alternating Whole-Body Vibration on cognitive function of young adults. PLoS ONE 2023, 18, e0280063. [Google Scholar] [CrossRef]
- Arauz, Y.L.A.; Ahuja, G.; Kamsma, Y.P.T.; Kortholt, A.; van der Zee, E.A.; van Heuvelen, M.J.G. Potential of Whole-Body Vibration in Parkinson’s Disease: A Systematic Review and Meta-Analysis of Human and Animal Studies. Biology 2022, 11, 1238. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Guzmán, R.; Jiménez-Diaz, F.; Ramírez, C.; Esteban, P.; Abián-Vicén, J. Whole-Body–Vibration Training and Balance in Recreational Athletes With Chronic Ankle Instability. J. Athl. Train. 2018, 53, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Alghadir, A.; Anwer, S.; Zafar, H.; Iqbal, Z. Effect of localised vibration on muscle strength in healthy adults: A systematic review. Physiotherapy 2018, 104, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.; Sá-Caputo, D.C.; Asad, N.R.; van Heuvelen, M.J.; van der Zee, E.A.; Ribeiro-Carvalho, A.; Bernardo-Filho, M. Beneficial effects of whole-body vibration exercise for brain disorders in experimental studies with animal models: A systematic review. Behav. Brain Res. 2022, 431, 113933. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, D.J. Vibration Exercise: The Potential Benefits. Int. J. Sport. Med. 2010, 32, 75–99. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Lin, W.; Li, X.; Andersen, L.L.; Wang, Y. Efficacy of whole body vibration therapy on pain and functional ability in people with non-specific low back pain: A systematic review. BMC Complement. Med. Ther. 2020, 20, 158. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Roberts, R.E.; Bilgen, O.; Kineman, R.D.; Koh, T.J. Parameter-Dependency of Low-Intensity Vibration for Wound Healing in Diabetic Mice. Front. Bioeng. Biotechnol. 2021, 9, 654920. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.O.-L.; Leung, K.-S.; Jiang, J.L.; Wang, T.B.-Y.; Chow, S.K.-H.; Cheung, W.-H. Low-Magnitude High-Frequency Vibration Accelerated the Foot Wound Healing of n5-streptozotocin-induced Diabetic Rats by Enhancing Glucose Transporter 4 and Blood Microcirculation. Sci. Rep. 2017, 7, 11631. [Google Scholar] [CrossRef] [PubMed]
- Wano, N.; Sanguanrungsirikul, S.; Keelawat, S.; Somboonwong, J. The effects of whole-body vibration on wound healing in a mouse pressure ulcer model. Heliyon 2021, 7, e06893. [Google Scholar] [CrossRef] [PubMed]
- Corbiere, T.F.; Weinheimer-Haus, E.M.; Judex, S.; Koh, T.J. Low-Intensity Vibration Improves Muscle Healing in a Mouse Model of Laceration Injury. J. Funct. Morphol. Kinesiol. 2018, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Arashi, M.; Sugama, J.; Sanada, H.; Konya, C.; Okuwa, M.; Nakagami, G.; Inoue, A.; Tabata, K. Vibration Therapy Accelerates Healing of Stage I Pressure Ulcers in Older Adult Patients. Adv. Ski. Wound Care 2010, 23, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Judex, S.; Lei, X.; Han, D.; Rubin, C. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J. Biomech. 2007, 40, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Stuermer, E.K.; Komrakova, M.; Werner, C.; Wicke, M.; Kolios, L.; Sehmisch, S.; Tezval, M.; Utesch, C.; Mangal, O.; Zimmer, S.; et al. Musculoskeletal Response to Whole-Body Vibration During Fracture Healing in Intact and Ovariectomized Rats. Calcif. Tissue Int. 2010, 87, 168–180. [Google Scholar] [CrossRef]
- Gnyubkin, V.; Guignandon, A.; Laroche, N.; Vanden-Bossche, A.; Malaval, L.; Vico, L. High-acceleration whole body vibration stimulates cortical bone accrual and increases bone mineral content in growing mice. J. Biomech. 2016, 49, 1899–1908. [Google Scholar] [CrossRef]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef]
- Norton, K.-A.; Popel, A.S. Effects of endothelial cell proliferation and migration rates in a computational model of sprouting angiogenesis. Sci. Rep. 2016, 6, 36992. [Google Scholar] [CrossRef]
- Bach, L.A. Endothelial cells and the IGF system. J. Mol. Endocrinol. 2015, 54, R1–R13. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.A.; Mangino, M.J.; Bassuk, J.; Kurlansky, P.; Sackner, M.A. Regional blood flow during periodic acceleration. Crit. Care Med. 2001, 29, 1983–1988. [Google Scholar] [CrossRef] [PubMed]
- Delavary, B.M.; van der Veer, W.M.; van Egmond, M.; Niessen, F.B.; Beelen, R.H. Macrophages in skin injury and repair. Immunobiology 2011, 216, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.L.; Bryer, S.C.; Cheng, M.; Nguyen, M.-H.; Conley, K.L.; Cunningham, A.K.; Xue, B.; Sisson, T.H.; You, J.-S.; Hornberger, T.A.; et al. Macrophage-Specific Expression of Urokinase-Type Plasminogen Activator Promotes Skeletal Muscle Regeneration. J. Immunol. 2011, 187, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.Y.; Chow, S.K.; Leung, K.S.; Qin, J.; Guo, A.; Yu, O.L.; Li, G.; Cheung, W.H. Low-magnitude high-frequency vibration enhanced mesenchymal stem cell recruitment in osteoporotic fracture healing through the SDF-1/CXCR4 pathway. Eur. Cells Mater. 2016, 31, 341–354. [Google Scholar] [CrossRef]
- Sim, S.L.; Kumari, S.; Kaur, S.; Khosrotehrani, K. Macrophages in Skin Wounds: Functions and Therapeutic Potential. Biomolecules 2022, 12, 1659. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Games, K.E.; Sefton, J.M.; Wilson, A.E. Whole-Body Vibration and Blood Flow and Muscle Oxygenation: A Meta-Analysis. J. Athl. Train. 2015, 50, 542–549. [Google Scholar] [CrossRef]
- Murray, A.K.; Gorodkin, R.E.; Moore, T.; Gush, R.; Herrick, A.; King, T.A. Comparison of red and green laser doppler imaging of blood flow. Lasers Surg. Med. 2004, 35, 191–200. [Google Scholar] [CrossRef]

| Authors | Species | Aim | WBV Intervention | Period (Days a Week) Duration of Bouts | Study Design | Results |
|---|---|---|---|---|---|---|
| Weinheimer-Haus et al., (2014) [21] | Male Mice db/db (N = Undefined) | The objective was to assess effects of whole-body LIV on wound healing in diabetic db/db mice. | LIV: Vertically F: 45 Hz apeak of: 0.4 g | 2-Weeks (5 days) 1 bout: 30 min |
| LIV may exert beneficial effects accelerating wound closure by enhancing angiogenesis and granulation tissue formation, and these changes are associated with increases in pro-angiogenic growth factors. Wound closure was significantly higher in the WBV group compared to the CON group (p = 0.066). |
| Yu et al., (2017) [42] | Female Wistar Rats (N = 96) | The objective was to investigate effects of LIV on the DM at Days 1, 4, 8 and 13 post-wounding. | LIV: F: 35 Hz /apeak: 0.3 g | 2-Weeks (5 days) 1 bout: 20 min |
| LIV accelerates the foot wound healing; in DM_V and DM, there was reduction in the wound size; in DM-V, there was reduction in the blood glucose level and the glucose transporter 4 expression and enhanced blood microcirculation. Wound size decreased significantly from day 8, there was a significant difference between the DM_V group compared to the control group (p = 0.036). |
| Corbiere et al., (2018) [44] | Male C57BL/6J Mice (N = 78) | The objective was sought to determine whether mechanical stimulation via LIV could improve muscle healing following traumatic injury | LIV: F: 90 Hz apeak: 0.2 g or F: 45 Hz apeak: 0.4 g | 2-Weeks (7 days) 1 bout 30 min |
| LIV can improve muscle healing by increasing myofiber growth and reducing fibrosis. Statistical differences were observed in LIV groups compared to the control group (p ≤ 0.05). |
| Wano et al., (2021) [43] | Male ICR Mice (N = 32) | The objective was to examine effects of WBV on the healing of stage II pressure ulcers in a mouse model. | LIV: Vertically F: 45 Hz apeak: 0.4 g | 2-Weeks (5 days) 1 bout: 30 min |
| TNF-α levels and neutrophil infiltration were significantly decreased in wounds on days 7 and 14 of WBV treatment; wound closure rate and collagen deposition were remarkably accelerated on day 14. On day 14, the WBV group showed an improvement in healing compared to the control group (p < 0.01). |
| Roberts et al., (2021) [41] | Male db/db Mice (N = Undefined) | The objective was to identify LIV amplitudes and frequencies that promote healing in diabetic mice. | LIV: F: 45 and 90 Hz apeak: 0.3 g and 0.6 g | 2-Weeks (7 days) 1 bout: 30 min |
| The 45 Hz/0.3 g group was the only one that improved wound healing and increased angiogenesis and granulation tissue formation, rapid re-epithelialization and wound closure. The LL group had a mean value significantly different from the control group (p ≤ 0.05). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brites-Ferreira, A.; Taiar, R.; Cardoso, A.L.B.D.; De Souza-Santos, D.; da Costa-Borges, P.P.; Torres-Nunes, L.; Jaques-Albuquerque, L.T.; Monteiro-Oliveira, B.B.; Boyer, F.C.; da Cunha Sá-Caputo, D.; et al. Therapeutic Approach of Whole-Body Vibration Exercise on Wound Healing in Animal Models: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 4925. https://doi.org/10.3390/ijerph20064925
Brites-Ferreira A, Taiar R, Cardoso ALBD, De Souza-Santos D, da Costa-Borges PP, Torres-Nunes L, Jaques-Albuquerque LT, Monteiro-Oliveira BB, Boyer FC, da Cunha Sá-Caputo D, et al. Therapeutic Approach of Whole-Body Vibration Exercise on Wound Healing in Animal Models: A Systematic Review. International Journal of Environmental Research and Public Health. 2023; 20(6):4925. https://doi.org/10.3390/ijerph20064925
Chicago/Turabian StyleBrites-Ferreira, Adrielli, Redha Taiar, André Luiz Bandeira Dionizio Cardoso, Daysa De Souza-Santos, Patricia Prado da Costa-Borges, Luiza Torres-Nunes, Luelia Teles Jaques-Albuquerque, Bruno Bessa Monteiro-Oliveira, Francois Constant Boyer, Danúbia da Cunha Sá-Caputo, and et al. 2023. "Therapeutic Approach of Whole-Body Vibration Exercise on Wound Healing in Animal Models: A Systematic Review" International Journal of Environmental Research and Public Health 20, no. 6: 4925. https://doi.org/10.3390/ijerph20064925
APA StyleBrites-Ferreira, A., Taiar, R., Cardoso, A. L. B. D., De Souza-Santos, D., da Costa-Borges, P. P., Torres-Nunes, L., Jaques-Albuquerque, L. T., Monteiro-Oliveira, B. B., Boyer, F. C., da Cunha Sá-Caputo, D., Rapin, A., & Bernardo-Filho, M. (2023). Therapeutic Approach of Whole-Body Vibration Exercise on Wound Healing in Animal Models: A Systematic Review. International Journal of Environmental Research and Public Health, 20(6), 4925. https://doi.org/10.3390/ijerph20064925









