Emergency Department Visits in Children Associated with Exposure to Ambient PM1 within Several Hours
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Statistical Analyses
2.2.1. Main Analysis
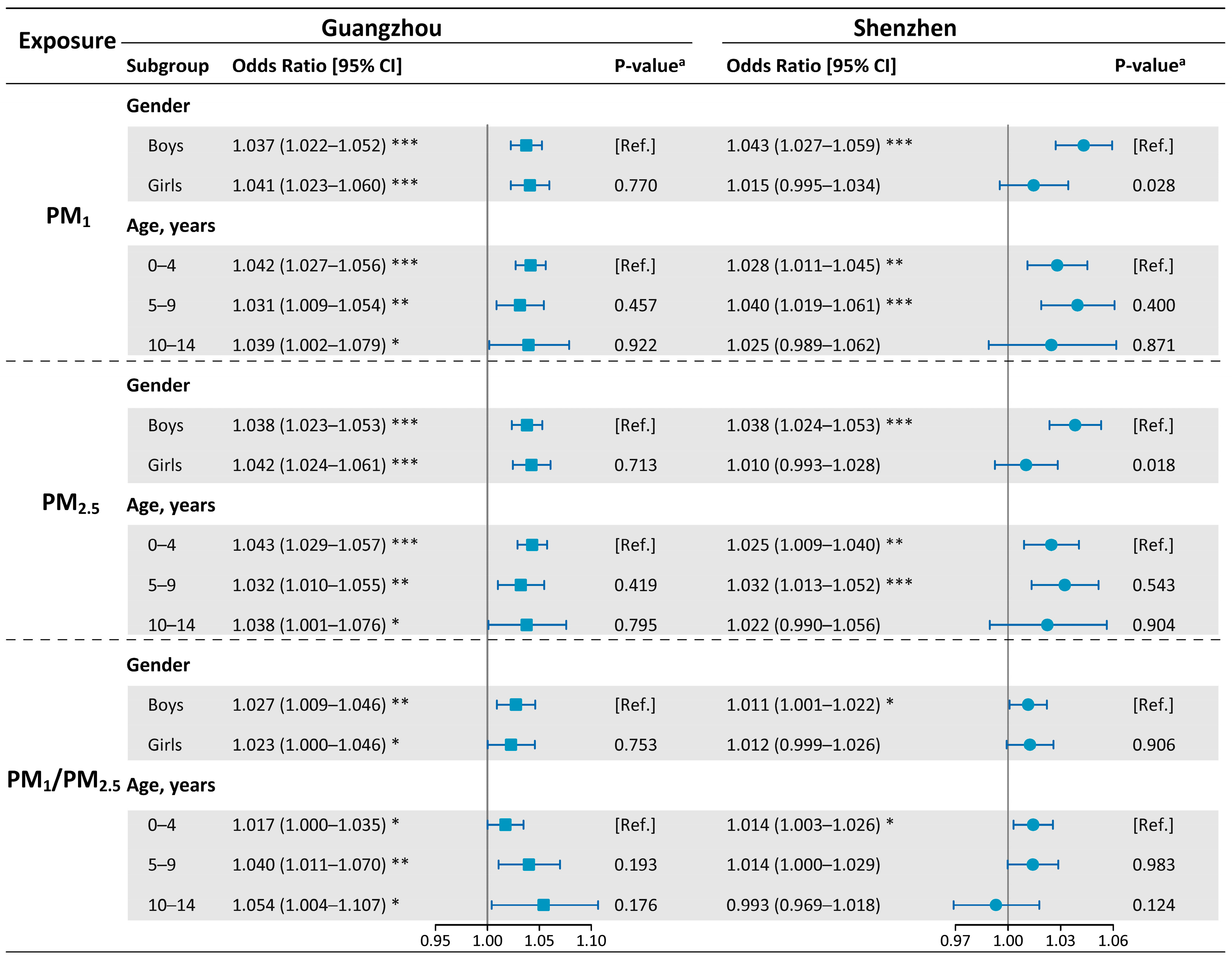
2.2.2. Subgroup Analyses
2.2.3. Sensitivity Analyses
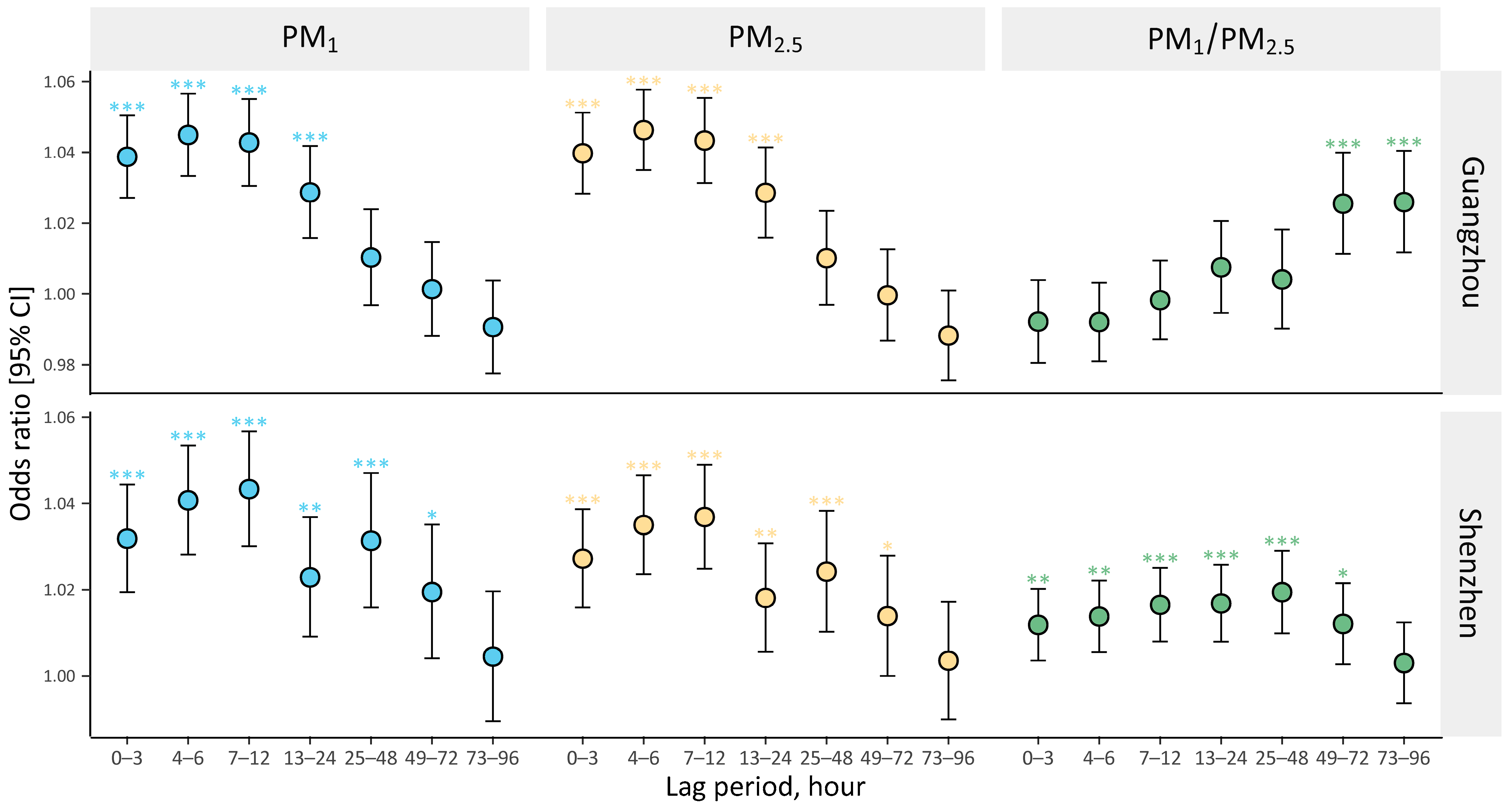
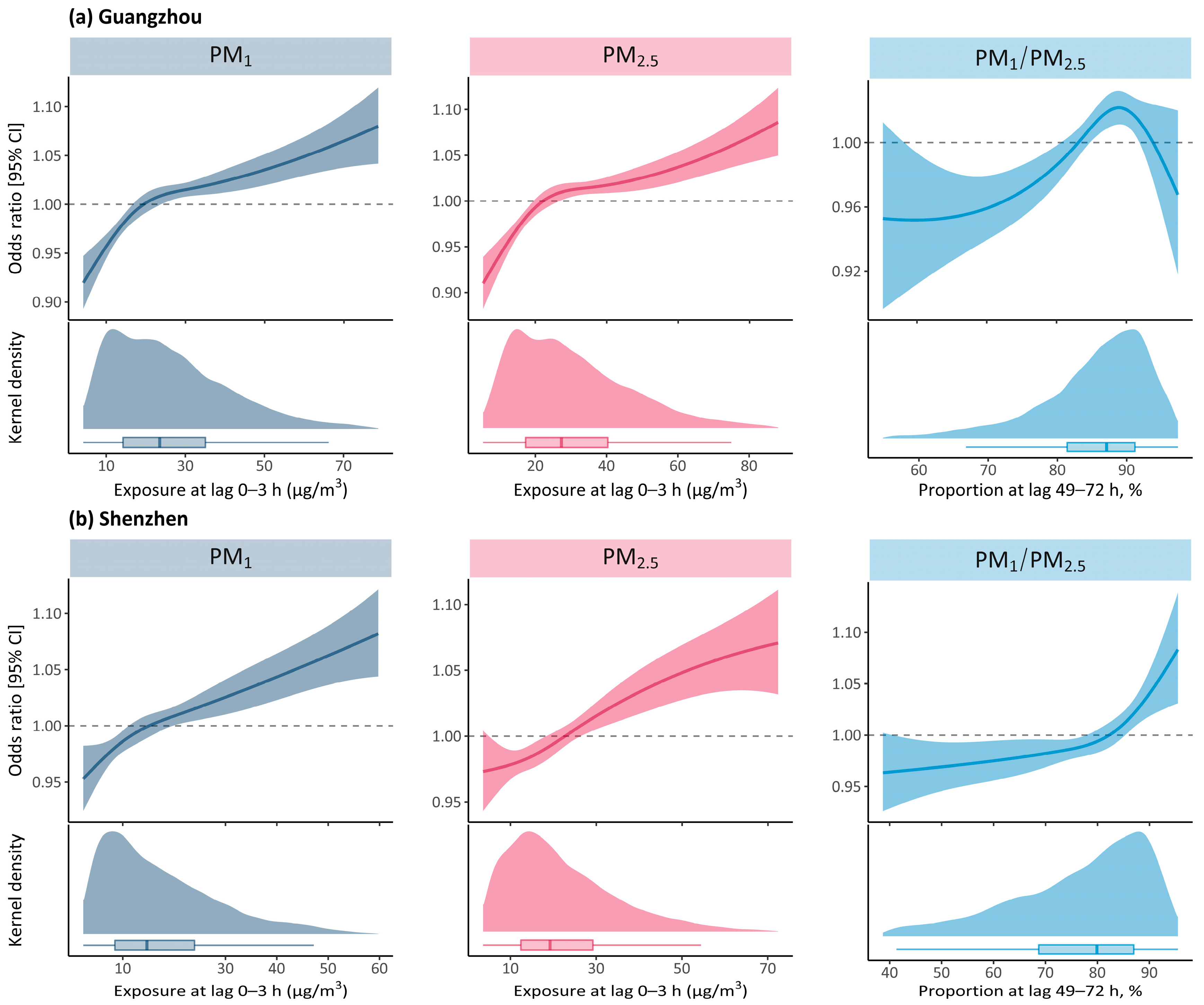
3. Results
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PM | particulate matter |
| PM0.1 | particulate matter with aerodynamic diameter ≤ 0.1 μm |
| PM1 | particulate matter with aerodynamic diameter ≤ 1 μm |
| PM2.5 | particulate matter with aerodynamic diameter ≤ 2.5 μm |
| NO2 | nitrogen dioxide |
| SO2 | sulfur dioxide |
| CO | carbon monoxide |
| O3 | ozone |
| EDVs | emergency department visits |
| PEDVs | pediatric emergency department visits |
| CI | confidence interval |
| OR | odds ratio |
| TSCC | time-stratified case-crossover design |
| CLR | conditional logistic regression |
| NCS | cubic spline function |
| df | degree of freedom |
| IQR | interquartile range |
| MR | meta regression |
| SD | standard deviation |
References
- Sorensen, C.; Lehmann, E.; Holder, C.; Hu, J.; Krishnan, A.; Munzel, T.; Mb, R.; Rn, S. Reducing the health impacts of ambient air pollution. BMJ 2022, 379, e069487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ma, R.; Wang, Y.; Jiang, N.; Zhang, Y.; Li, T. The relationship between particulate matter and lung function of children: A systematic review and meta-analysis. Environ. Pollut. 2022, 309, 119735. [Google Scholar] [CrossRef] [PubMed]
- Nhung, N.T.T.; Amini, H.; Schindler, C.; Kutlar Joss, M.; Dien, T.M.; Probst-Hensch, N.; Perez, L.; Kunzli, N. Short-term association between ambient air pollution and pneumonia in children: A systematic review and meta-analysis of time-series and case-crossover studies. Environ. Pollut. 2017, 230, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Castagna, A.; Mascheroni, E.; Fustinoni, S.; Montirosso, R. Air pollution and neurodevelopmental skills in preschool- and school-aged children: A systematic review. Neurosci. Biobehav. Rev. 2022, 136, 104623. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Ho, H.C.; Su, H.; Huang, C.; Zheng, H.; Zhang, W.; Tao, J.; Hossain, M.Z.; Zhang, Y.; Hu, K.; et al. A systematic review and meta-analysis of intraday effects of ambient air pollution and temperature on cardiorespiratory morbidities: First few hours of exposure matters to life. EBioMedicine 2022, 86, 104327. [Google Scholar] [CrossRef] [PubMed]
- Zong, Z.; Zhao, M.; Zhang, M.; Xu, K.; Zhang, Y.; Zhang, X.; Hu, C. Association between PM1 Exposure and Lung Function in Children and Adolescents: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 15888. [Google Scholar] [CrossRef]
- Yan, M.; Xu, J.; Li, C.; Guo, P.; Yang, X.; Tang, N.J. Associations between ambient air pollutants and blood pressure among children and adolescents: A systemic review and meta-analysis. Sci. Total Environ. 2021, 785, 147279. [Google Scholar] [CrossRef]
- Zhang, Q.; Meng, X.; Shi, S.; Kan, L.; Chen, R.; Kan, H. Overview of particulate air pollution and human health in China: Evidence, challenges, and opportunities. Innovation 2022, 3, 100312. [Google Scholar] [CrossRef]
- Wang, W.; Mao, F.; Zou, B.; Guo, J.; Wu, L.; Pan, Z.; Zang, L. Two-stage model for estimating the spatiotemporal distribution of hourly PM1.0 concentrations over central and east China. Sci. Total Environ. 2019, 675, 658–666. [Google Scholar] [CrossRef]
- Zang, L.; Mao, F.; Guo, J.; Gong, W.; Wang, W.; Pan, Z. Estimating hourly PM1 concentrations from Himawari-8 aerosol optical depth in China. Environ. Pollut. 2018, 241, 654–663. [Google Scholar] [CrossRef]
- Chen, G.; Li, S.; Zhang, Y.; Zhang, W.; Li, D.; Wei, X.; He, Y.; Bell, M.L.; Williams, G.; Marks, G.B.; et al. Effects of ambient PM1 air pollution on daily emergency hospital visits in China: An epidemiological study. Lancet. Planet. Health 2017, 1, e221–e229. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Tao, J.; Du, Y.; Liu, T.; Qian, Z.; Tian, L.; Di, Q.; Rutherford, S.; Guo, L.; Zeng, W.; et al. Particle size and chemical constituents of ambient particulate pollution associated with cardiovascular mortality in Guangzhou, China. Environ. Pollut. 2016, 208, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.S.; Mason, A.; Quan, T.P.; Fawcett, N.J.; Watkinson, P.; Llewelyn, M.; Stoesser, N.; Finney, J.; Davies, J.; Wyllie, D.H. Mortality risks associated with emergency admissions during weekends and public holidays: An analysis of electronic health records. Lancet 2017, 390, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Y.; Zhang, W.; Chen, G.; Lu, P.; Guo, Y.; Li, S. Short-term effect of PM1 on hospital admission for ischemic stroke: A multi-city case-crossover study in China. Environ. Pollut. 2020, 260, 113776. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Z.; Xiang, Q.; Wang, W.; Huang, L.; Mao, F. Short-term effects of ambient PM1 and PM2.5 air pollution on hospital admission for respiratory diseases: Case-crossover evidence from Shenzhen, China. Int. J. Hyg. Environ. Health 2020, 224, 113418. [Google Scholar] [CrossRef]
- Shim, S.R.; Kim, S.J.; Lee, J.; Rucker, G. Network meta-analysis: Application and practice using R software. Epidemiol. Health 2019, 41, e2019013. [Google Scholar] [CrossRef]
- Song, P.; Zhang, Y.; Yu, J.; Zha, M.; Zhu, Y.; Rahimi, K.; Rudan, I. Global Prevalence of Hypertension in Children: A Systematic Review and Meta-analysis. JAMA Pediatr. 2019, 173, 1154–1163. [Google Scholar] [CrossRef]
- Shanahan, K.H.; Subramanian, S.V.; Burdick, K.J.; Monuteaux, M.C.; Lee, L.K.; Fleegler, E.W. Association of Neighborhood Conditions and Resources for Children With Life Expectancy at Birth in the US. JAMA Netw. Open 2022, 5, e2235912. [Google Scholar] [CrossRef]
- Chen, T.T.; Zhan, Z.Y.; Yu, Y.M.; Xu, L.J.; Guan, Y.; Ou, C.Q. Effects of hourly levels of ambient air pollution on ambulance emergency call-outs in Shenzhen, China. Environ. Sci. Pollut. Res. Int. 2020, 27, 24880–24888. [Google Scholar] [CrossRef]
- Phung, V.L.H.; Ueda, K.; Seposo, X.; Takami, A.; Sugata, S.; Yoshino, A.; Michikawa, T.; Yamazaki, S.; Honda, A.; Takano, H. Hourly association between ambient PM2.5 and emergency ambulance dispatches in 11 cities in Japan. Environ. Res. 2020, 185, 109448. [Google Scholar] [CrossRef]
- Yamazaki, S.; Shima, M.; Ando, M.; Nitta, H.; Watanabe, H.; Nishimuta, T. Effect of hourly concentration of particulate matter on peak expiratory flow in hospitalized children: A panel study. Environ. Health 2011, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Tong, S.; Su, H.; Xu, Z. Association between sub-daily exposure to ambient air pollution and risk of asthma exacerbations in Australian children. Environ. Res. 2022, 212, 113556. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhang, F.; Yu, C.; Jiao, A.; Xiang, Q.; Yu, Y.; Mayvaneh, F.; Hu, K.; Ding, Z.; Zhang, Y. Hourly associations between exposure to ambient particulate matter and emergency department visits in an urban population of Shenzhen, China. Atmos. Environ. 2019, 209, 78–85. [Google Scholar] [CrossRef]
- Wu, Y.; Jin, T.; He, W.; Liu, L.; Li, H.; Liu, C.; Zhou, Y.; Hong, J.; Cao, L.; Lu, Y.; et al. Associations of fine particulate matter and constituents with pediatric emergency room visits for respiratory diseases in Shanghai, China. Int. J. Hyg. Environ. Health 2021, 236, 113805. [Google Scholar] [CrossRef]
- He, M.; Zhong, Y.; Chen, Y.; Zhong, N.; Lai, K. Association of short-term exposure to air pollution with emergency visits for respiratory diseases in children. iScience 2022, 25, 104879. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, Y.; Wei, J.; Zhao, Z.; Norback, D.; Zhang, X.; Lu, C.; Yu, W.; Wang, T.; Zheng, X. Associations of Early-Life Exposure to Submicron Particulate Matter With Childhood Asthma and Wheeze in China. JAMA Netw. Open 2022, 5, e2236003. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, Y.; Wei, J.; Bovet, P.; Zhao, M.; Liu, W.; Xi, B. Association between short-term exposure to ambient PM1 and PM2.5 and forced vital capacity in Chinese children and adolescents. Environ. Sci. Pollut. Res. Int. 2022, 29, 71665–71675. [Google Scholar] [CrossRef]
- Johnson, N.M.; Hoffmann, A.R.; Behlen, J.C.; Lau, C.; Pendleton, D.; Harvey, N.; Shore, R.; Li, Y.; Chen, J.; Tian, Y.; et al. Air pollution and children’s health-a review of adverse effects associated with prenatal exposure from fine to ultrafine particulate matter. Environ. Health Prev. Med. 2021, 26, 72. [Google Scholar] [CrossRef]
- Samoli, E.; Rodopoulou, S.; Schneider, A.; Morawska, L.; Stafoggia, M.; Renzi, M.; Breitner, S.; Lanki, T.; Pickford, R.; Schikowski, T.; et al. Meta-analysis on short-term exposure to ambient ultrafine particles and respiratory morbidity. Eur. Respir. Rev. 2020, 29, 200116. [Google Scholar] [CrossRef]
- Huang, C.; Tang, M.; Li, H.; Wen, J.; Wang, C.; Gao, Y.; Hu, J.; Lin, J.; Chen, R. Particulate matter air pollution and reduced heart rate variability: How the associations vary by particle size in Shanghai, China. Ecotoxicol. Environ. Saf. 2021, 208, 111726. [Google Scholar] [CrossRef]
- Feng, D.; Cao, K.; He, Z.Z.; Knibbs, L.D.; Jalaludin, B.; Leskinen, A.; Roponen, M.; Komppula, M.; Jalava, P.; Guo, P.Y.; et al. Short-Term Effects of Particle Sizes and Constituents on Blood Biomarkers among Healthy Young Adults in Guangzhou, China. Environ. Sci. Technol. 2021, 55, 5636–5647. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Fiotakis, K.; Vlachogianni, T. Airborne particulate matter and human health: Toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2008, 26, 339–362. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Vanbilloen, H.; Hoylaerts, M.F.; Hoet, P.H.; Verbruggen, A.; Nemery, B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am. J. Respir. Crit. Care Med. 2001, 164, 1665–1668. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, C.; Cheng, Y.; Guo, S.; Sun, Q.; Kan, L.; Chen, R.; Kan, H.; Bai, H.; Cao, J. Association between ambient particulate matter air pollution and ST-elevation myocardial infarction: A case-crossover study in a Chinese city. Chemosphere 2019, 219, 724–729. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Hajat, S.; Armstrong, B.; Haines, A.; Herrett, E.; Wilkinson, P.; Smeeth, L. The effects of hourly differences in air pollution on the risk of myocardial infarction: Case crossover analysis of the MINAP database. BMJ 2011, 343, d5531. [Google Scholar] [CrossRef]
- Bai, L.; Su, X.; Zhao, D.; Zhang, Y.; Cheng, Q.; Zhang, H.; Wang, S.; Xie, M.; Su, H. Exposure to traffic-related air pollution and acute bronchitis in children: Season and age as modifiers. J. Epidemiol. Community Health 2018, 72, 426–433. [Google Scholar] [CrossRef]
- Bell, M.L.; Ebisu, K.; Peng, R.D.; Walker, J.; Samet, J.M.; Zeger, S.L.; Dominici, F. Seasonal and regional short-term effects of fine particles on hospital admissions in 202 US counties, 1999-2005. Am. J. Epidemiol. 2008, 168, 1301–1310. [Google Scholar] [CrossRef]
| Characteristics | Guangzhou | Shenzhen |
|---|---|---|
| Total included visits , No. | 97,508 | 101,639 |
| Hourly visits, No. | ||
| Mean (SD) | 5.6 (5.5) | 6.3 (4.3) |
| Median (IQR) | 4.0 (8.0) | 6.0 (6.0) |
| Sex, No. (%) | ||
| Boy | 58,941 (60.4) | 61,889 (60.9) |
| Girl | 38,567 (39.6) | 39,750 (39.1) |
| Age, No. (%) | ||
| 0–4 year | 64,930 (66.6) | 55,680 (54.8) |
| 5–9 year | 24,046 (24.7) | 34,556 (34.0) |
| 10–14 year | 8532 (8.8) | 11,403 (11.2) |
| Variables | Guangzhou | Shenzhen | ||||||
|---|---|---|---|---|---|---|---|---|
| Min | Mean (SD) | Median (IQR) | Max | Min | Mean (SD) | Median (IQR) | Max | |
| On case hours | n = 97,508 | n = 101,639 | ||||||
| Particulate pollutants | ||||||||
| PM1, μg/m3 | 0.6 | 26.9 (16.6) | 23.8 (21.6) | 121.5 | 1.0 | 17.9 (13.1) | 14.3 (16.0) | 118.7 |
| PM2.5, μg/m3 | 1.0 | 31.3 (18.5) | 27.7 (23.7) | 153.3 | 1.5 | 22.7 (14.8) | 19.0 (17.2) | 141.0 |
| PM1/PM2.5, % | 11.4 | 84.5 (8.8) | 86.2 (10.7) | 99.6 | 25.7 | 75.9 (14.3) | 79.6 (20.3) | 98.2 |
| Gaseous pollutants | ||||||||
| NO2, μg/m3 | 4.0 | 52.9 (27.3) | 47.0 (30.0) | 226.0 | 6.0 | 33.4 (14.9) | 30.0 (17.0) | 125.0 |
| SO2, μg/m3 | 2.0 | 11.6 (5.7) | 10.0 (6.0) | 100.0 | 3.0 | 8.0 (2.9) | 7.0 (3.0) | 37.0 |
| CO, mg/m3 | 0.4 | 1.0 (0.3) | 0.9 (0.3) | 3.1 | 0.4 | 0.8 (0.2) | 0.8 (0.2) | 1.7 |
| O3, μg/m3 | 1.0 | 41.4 (43.7) | 25.0 (45.0) | 342.0 | 10.0 | 58.3 (33.3) | 49.0 (41.0) | 245.0 |
| Weather conditions | ||||||||
| Temperature, °C | 2.5 | 22.8 (6.2) | 24.5 (8.0) | 37.5 | 3.0 | 24.7 (5.4) | 26.0 (8.0) | 37.0 |
| Relative humidity, % | 16.5 | 80.1 (14.0) | 83.2 (19.3) | 100.0 | 16.5 | 79.4 (12.1) | 83.0 (15.2) | 100.0 |
| On control hours | n = 332,289 | n = 347,231 | ||||||
| Particulate pollutants | ||||||||
| PM1, μg/m3 | 0.6 | 26.4 (16.4) | 23.3 (21.5) | 121.5 | 1.0 | 17.7 (13.0) | 14.1 (16.0) | 118.7 |
| PM2.5, μg/m3 | 1.0 | 30.7 (18.2) | 27.3 (23.7) | 164.1 | 1.5 | 22.6 (14.7) | 18.9 (16.9) | 141.0 |
| PM1/PM2.5, % | 11.4 | 84.5 (8.9) | 86.1 (10.8) | 99.6 | 25.7 | 75.7 (14.4) | 79.4 (20.7) | 98.2 |
| Gaseous pollutants | ||||||||
| NO2, μg/m3 | 4.0 | 51.9 (26.8) | 47.0 (28.0) | 226.0 | 6.0 | 33.2 (14.8) | 30.0 (17.0) | 125.0 |
| SO2, μg/m3 | 2.0 | 11.4 (5.7) | 10.0 (6.0) | 100.0 | 3.0 | 7.9 (2.9) | 7.0 (3.0) | 37.0 |
| CO, mg/m3 | 0.4 | 1.0 (0.3) | 0.9 (0.3) | 3.1 | 0.4 | 0.8 (0.2) | 0.8 (0.2) | 1.7 |
| O3, μg/m3 | 1.0 | 40.7 (42.9) | 25.0 (45.0) | 342.0 | 10.0 | 57.9 (33.2) | 49.0 (41.0) | 245.0 |
| Weather conditions | ||||||||
| Temperature, °C | 2.5 | 22.7 (6.3) | 24.5 (9.0) | 37.5 | 3.0 | 24.5 (5.6) | 26.0 (8.0) | 37.0 |
| Relative humidity, % | 16.5 | 80.2 (14.1) | 83.4 (19.5) | 100.0 | 16.5 | 79.5 (12.3) | 83.1 (15.2) | 100.0 |
| Exposure | Guangzhou | Shenzhen | ||
|---|---|---|---|---|
| OR [95% CI] | P for Heterogeneity | OR [95% CI] | P for Heterogeneity | |
| PM1, lag 0–3 h | 0.004 | 0.011 | ||
| Cold | 1.068 [1.053 to 1.084] | 1.037 [1.021 to 1.054] | ||
| Warm | 1.037 [1.021 to 1.052] | 1.010 [0.998 to 1.023] | ||
| PM2.5, lag 0–3 h | 0.005 | 0.025 | ||
| Cold | 1.072 [1.057 to 1.088] | 1.034 [1.019 to 1.051] | ||
| Warm | 1.040 [1.025 to 1.056] | 1.011 [0.998 to 1.024] | ||
| PM1/PM2.5, lag 49–72 h | 0.025 | 0.576 | ||
| Cold | 1.044 [1.009 to 1.080] | 1.015 [0.991 to 1.040] | ||
| Warm | 1.000 [0.984 to 1.017] | 1.008 [0.997 to 1.018] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhu, L.; Wang, Y.; Tang, Z.; Huang, Y.; Wang, Y.; Zhang, J.; Zhang, Y. Emergency Department Visits in Children Associated with Exposure to Ambient PM1 within Several Hours. Int. J. Environ. Res. Public Health 2023, 20, 4910. https://doi.org/10.3390/ijerph20064910
Li Y, Zhu L, Wang Y, Tang Z, Huang Y, Wang Y, Zhang J, Zhang Y. Emergency Department Visits in Children Associated with Exposure to Ambient PM1 within Several Hours. International Journal of Environmental Research and Public Health. 2023; 20(6):4910. https://doi.org/10.3390/ijerph20064910
Chicago/Turabian StyleLi, Yachen, Lifeng Zhu, Yaqi Wang, Ziqing Tang, Yuqian Huang, Yixiang Wang, Jingjing Zhang, and Yunquan Zhang. 2023. "Emergency Department Visits in Children Associated with Exposure to Ambient PM1 within Several Hours" International Journal of Environmental Research and Public Health 20, no. 6: 4910. https://doi.org/10.3390/ijerph20064910
APA StyleLi, Y., Zhu, L., Wang, Y., Tang, Z., Huang, Y., Wang, Y., Zhang, J., & Zhang, Y. (2023). Emergency Department Visits in Children Associated with Exposure to Ambient PM1 within Several Hours. International Journal of Environmental Research and Public Health, 20(6), 4910. https://doi.org/10.3390/ijerph20064910







