Bibliometric Analysis of Global Scientific Production on COVID-19 and Vaccines
Abstract
1. Introduction
2. Materials and Methods
3. Results
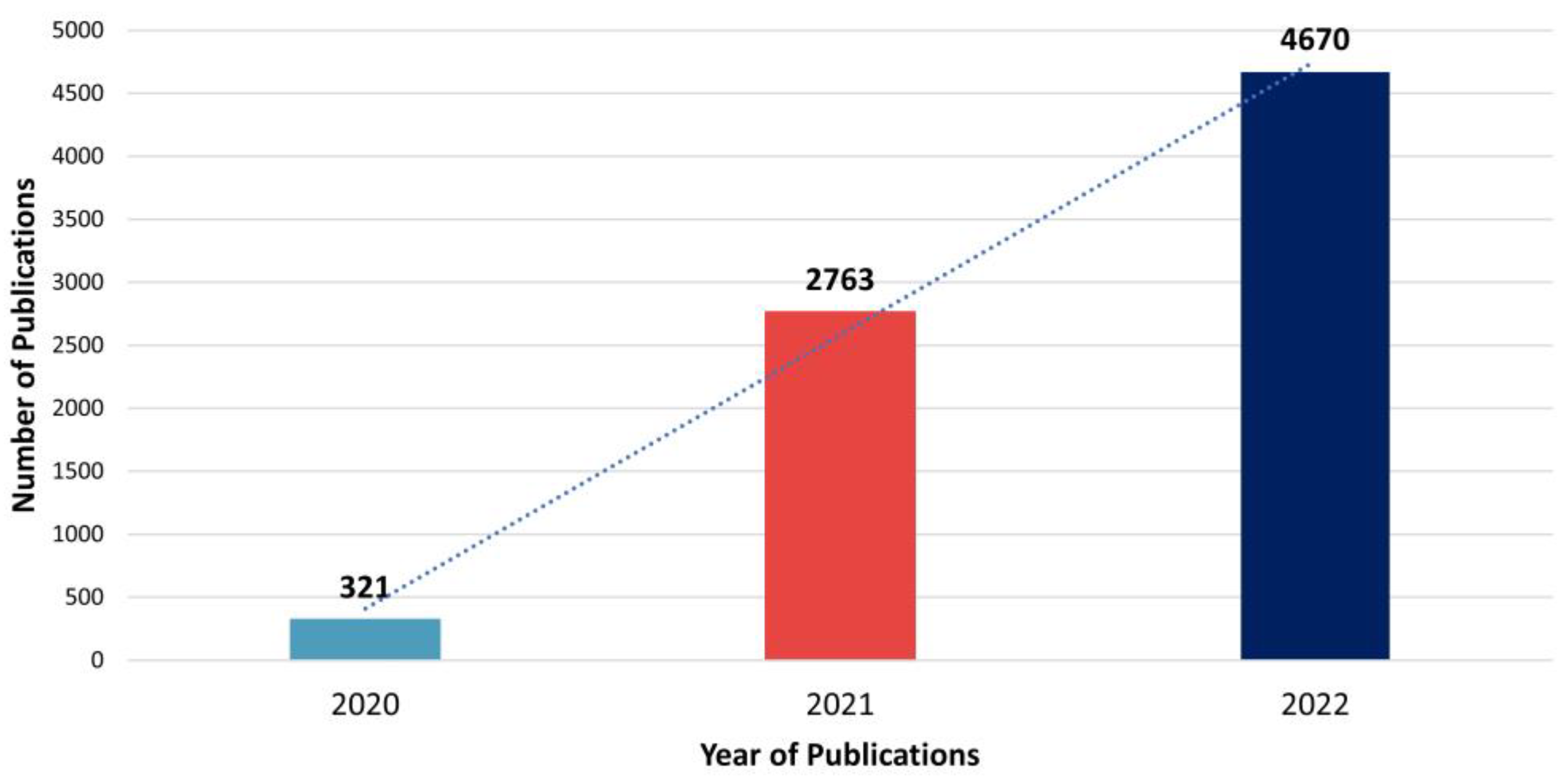
3.1. Publication Year
3.2. Scientific Journals
3.3. Affiliate Institutions
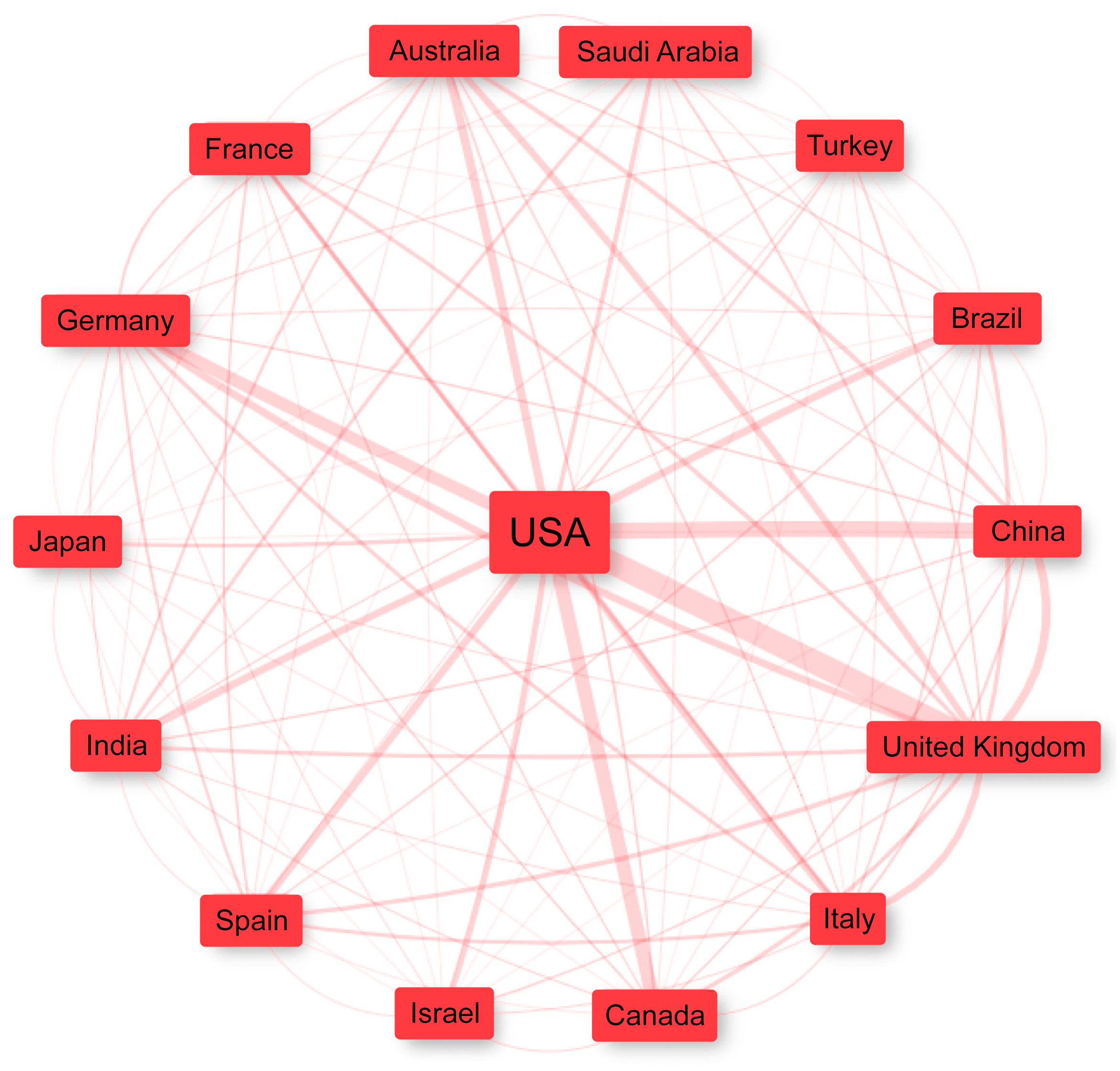
3.4. Countries and Collaborations
3.5. Most Cited Articles
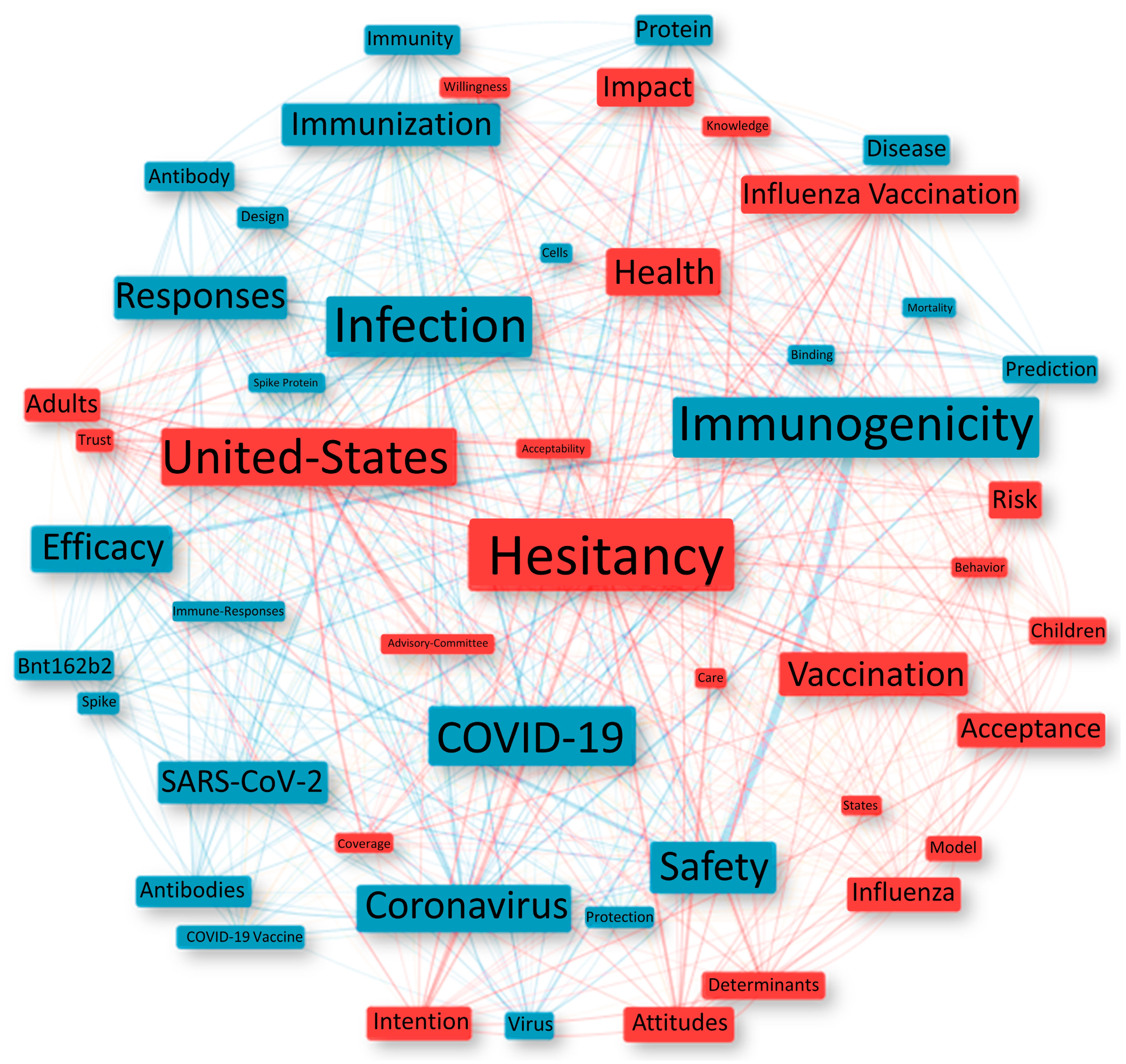
3.6. Conceptual Structure
3.7. Financing Agencies
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tabur, A.; Arslanoğlu, A. A 50-Year Overview of the Coronavirus Family with Science Mapping Techniques: A Review. Iran. J. Public Health 2021, 50, 649. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, S.; Teng, T.; Abdalla, A.E.; Zhu, W.; Xie, L.; Wang, Y.; Guo, X. Systematic Comparison of Two Animal-to-Human Transmitted Human Coronaviruses: SARS–CoV-2 and SARS-CoV. Viruses 2020, 12, 244. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Adil, S.F.; Alkhathlan, H.Z.; Tahir, M.N.; Saif, S.; Khan, M.; Khan, S.T. COVID-19: A Global Challenge with Old History, Epidemiology and Progress So Far. Molecules 2020, 26, 39. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Advice for the Public: Coronavirus Disease (COVID-19). Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public (accessed on 1 January 2023).
- Meyer, H.; Ehmann, R.; Smith, G.L. Smallpox in the post-eradication era. Viruses 2020, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Ghasemiyeh, P.; Mohammadi-Samani, S.; Firouzabadi, N.; Dehshahri, A.; Vazin, A. A Focused Review on Technologies, Mechanisms, Safety, and Efficacy of Available COVID-19 Vaccines. Int. Immunopharmacol. 2021, 100, 108162. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Timeline: WHO’s COVID-19 Response. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline (accessed on 5 January 2023).
- Oliveira, E.M.N.; Carvalho, A.R.B.; Silva, J.S.E.; Neto, A.R.; Moura, M.E.B.; Freitas, D.R.J. Analysis of scientific production on the new coronavirus (COVID-19): A bibliometric analysis. Sao Paulo Med. J. 2021, 139, 3–9. [Google Scholar] [CrossRef]
- Zupic, I.; Cater, T. Bibliometric methods in management and organization. Organ. Res. Methods 2015, 18, 429–472. [Google Scholar] [CrossRef]
- Deng, Z.; Chen, J.; Wang, T. Bibliometric and visualization analysis of human coronaviruses: Prospects and implications for COVID-19 research. Front. Cell. Infect. Microbiol. 2020, 10, 581404. [Google Scholar] [CrossRef]
- Dada, O. A model of entrepreneurial autonomy in franchised outlets: A systematic review of the empirical evidence. Int. J. Manag. Rev. 2018, 20, 206–226. [Google Scholar] [CrossRef]
- Rey-Martí, A.; Ribeiro-Soriano, D.; Palacios-Marqués, D. A bibliometric analysis of social entrepreneurship. J. Bus. Res. 2016, 69, 1651–1655. [Google Scholar] [CrossRef]
- Clarivate. Web of Science. Confident Research Begins Here. Available online: https://clarivate.com/webofsciencegroup/solutions/web-of-science/ (accessed on 5 January 2023).
- Ekundayo, T.C.; Okoh, A.I. A global bibliometric analysis of Plesiomonas-related research (1990–2017). PLoS ONE 2018, 13, e0207655. [Google Scholar] [CrossRef]
- Okaiyeto, K.; Ekundayo, T.C.; Okoh, A.I. Global research trends on bioflocculant potentials in wastewater remediation from 1990 to 2019 using a bibliometric approach. Lett. Appl. Microbiol. 2020, 71, 567–579. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. Bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Traag, V.A.; Waltman, L.; van Eck, N.J. From Louvain to Leiden: Guaranteeing well-connected communities. Sci. Rep. 2019, 9, 5233. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2021, 383, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J.L.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Ratzan, S.C.; Palayew, A.; Gostin, L.O.; Larson, H.J.; Rabin, K.; Kimball, S.; El-Mohandes, A. A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med. 2021, 27, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Dror, A.A.; Eisenbach, N.; Taiber, S.; Morozov, N.G.; Mizrachi, M.; Zigron, A.; Srouji, S.; Sela, E. Vaccine hesitancy: The next challenge in the fight against COVID-19. Eur. J. Epidemiol. 2020, 35, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Hubert, J.J. Linguistic indicators. Soc. Indic. Res. 1980, 8, 223–255. [Google Scholar] [CrossRef]
- Garfeld, E.; Sher, I.H. Keywords plusTM—Algorithmic derivative indexing. J. Am. Soc. Inf. 1993, 44, 298–299. [Google Scholar] [CrossRef]
- Forliano, C.; Bernardi, P.; Yahiaoui, D. Entrepreneurial universities: A bibliometric analysis within the business and management domains. Technol. Forecast. Soc. Chang. 2021, 165, 120522. [Google Scholar] [CrossRef]
- Aria, M.; Alterisio, A.; Scandurra, A.; Pinelli, C.; D’Aniello, B. The scholar’s best friend: Research trends in dog cognitive and behavioral studies. Anim. Cogn. 2021, 24, 541–553. [Google Scholar] [CrossRef]
- VIPER Group COVID19 Vaccine Tracker Team. World Health Organization. 11 Vaccines Granted Emergency Use Listing (EUL) by WHO. Available online: https://covid19.trackvaccines.org/agency/who/ (accessed on 20 January 2023).
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Imunologia Celular E Molecular, 9th ed.; Guanabara Koogan: São Paulo, Brazil, 2019. [Google Scholar]
- Canouï, E.; Launay, O. Histoire et principes de la vaccination [History and principles of vaccination]. Rev. Mal. Respir. 2019, 36, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jing, R.; Lai, X.; Zhang, H.; Lyu, Y.; Knoll, M.D.; Fang, H. Acceptance of COVID-19 Vaccination during the COVID-19 Pandemic in China. Vaccines 2020, 8, 482. [Google Scholar] [CrossRef]
- Reiter, P.L.; Pennell, M.L.; Katz, M.L. Acceptability of a COVID-19 vaccine among adults in the United States: How many people would get vaccinated? Vaccine 2020, 38, 6500–6507. [Google Scholar] [CrossRef]
- Wong, L.P.; Alias, H.; Wong, P.F.; Lee, H.Y.; AbuBakar, S. The use of the health belief model to assess predictors of intent to receive the COVID-19 vaccine and willingness to pay. Hum. Vaccin. Immunother. 2020, 16, 2204–2214. [Google Scholar] [CrossRef]
- Bok, K.; Sitar, S.; Graham, B.S.; Mascola, J.R. Accelerated COVID-19 vaccine development: Milestones, lessons, and prospects. Immunity 2021, 54, 1636–1651. [Google Scholar] [CrossRef]
- Kim, Y.C.; Dema, B.; Reyes-Sandoval, A. COVID-19 vaccines: Breaking record times to first-in-human trials. NPJ Vaccines 2020, 5, 34. [Google Scholar] [CrossRef]
- Lambert, P.H.; Ambrosino, D.M.; Andersen, S.R.; Baric, R.S.; Black, S.B.; Chen, R.T.; Dekker, C.L.; Didierlaurent, A.M.; Graham, B.S.; Martin, S.D.; et al. Consensus summary report for CEPI/BC March 12-13, 2020 meeting: Assessment of risk of disease enhancement with COVID-19 vaccines. Vaccine 2020, 38, 4783–4791. [Google Scholar] [CrossRef]
- Graham, S.P.; McLean, R.K.; Spencer, A.J.; Belij-Rammerstorfer, S.; Wright, D.; Ulaszewska, M.; Edwards, J.C.; Hayes, J.W.P.; Martini, V.; Thakur, N.; et al. Evaluation of the immunogenicity of prime-boost vaccination with the replication-deficient viral vectored COVID-19 vaccine candidate ChAdOx1 nCoV-19. NPJ Vaccines 2020, 5, 69. [Google Scholar] [CrossRef]
- VIPER Group COVID19 Vaccine Tracker Team. Oxford/AstraZeneca: Vaxzevria. Available online: https://covid19.trackvaccines.org/vaccines/4/ (accessed on 20 January 2023).
- Madhi, S.A.; Baillie, V.; Cutland, C.L.; Voysey, M.; Koen, A.L.; Fairlie, L.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. Efficacy of the ChAdOx1 nCoV-19 COVID-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1885–1898. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Member States. Available online: https://www.un.org/en/about-us/member-states (accessed on 18 February 2023).
- Li, Y.; Tenchov, R.; Smoot, J.; Liu, C.; Watkins, S.; Zhou, Q.A. Comprehensive Review of the Global Efforts on COVID-19 Vaccine Development. ACS Cent. Sci. 2021, 7, 512–533. [Google Scholar] [CrossRef] [PubMed]
- Human Development Report. Human Development Report 2021–22. Uncertain Times, Unsettled Lives: Shaping Our Future in a Transforming World. Available online: https://hdr.undp.org/content/human-development-report-2021-22 (accessed on 20 January 2023).
- Cohen, J. Shots of hope. Science 2020, 370, 1392–1394. [Google Scholar] [CrossRef]
- VIPER Group COVID19 Vaccine Tracker Team. Pfizer/BioNTech: Comirnaty. Available online: https://covid19.trackvaccines.org/vaccines/6 (accessed on 20 January 2023).
- VIPER Group COVID19 Vaccine Tracker Team. Moderna: Spikevax. Available online: https://covid19.trackvaccines.org/vaccines/22 (accessed on 20 January 2023).
- VIPER Group COVID19 Vaccine Tracker Team. Janssen (Johnson & Johnson): Jcovden. Available online: https://covid19.trackvaccines.org/vaccines/1 (accessed on 20 January 2023).
- VIPER Group COVID19 Vaccine Tracker Team. Gamaleya: Sputnik, V. Available online: https://covid19.trackvaccines.org/vaccines/12 (accessed on 20 January 2023).
- MacDonald, N.E.; SAGE Working Group on Vaccine Hesitancy. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef]
- Karafillakis, E.; Larson, H.J.; ADVANCE Consortium. The benefit of the doubt or doubts over benefits? A systematic literature review of perceived risks of vaccines in European populations. Vaccine 2017, 35, 4840–4850. [Google Scholar] [CrossRef]
- Cobos Muñoz, D.; Monzón Llamas, L.; Bosch-Capblanch, X. Exposing concerns about vaccination in low- and middle-income countries: A systematic review. Int. J. Public Health 2015, 60, 767–780. [Google Scholar] [CrossRef]
- Fisher, K.A.; Bloomstone, S.J.; Walder, J.; Crawford, S.; Fouayzi, H.; Mazor, K.M. Attitudes Toward a Potential SARS-CoV-2 Vaccine: A Survey of U.S. Adults. Ann. Intern. Med. 2020, 173, 964–973. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, Z.; Zhao, Q.; Alias, H.; Danaee, M.; Wong, L.P. Understanding COVID-19 vaccine demand and hesitancy: A nationwide online survey in China. PLoS Negl. Trop. Dis. 2020, 14, e0008961. [Google Scholar] [CrossRef]
- Carrion-Alvarez, D.; Tijerina-Salina, P.X. Fake news in COVID-19: A perspective. Health Promot. Perspect. 2020, 7, 290–291. [Google Scholar] [CrossRef]
- Lee, S.K.; Sun, J.; Jang, S. Misinformation of COVID-19 vaccines and vaccine hesitancy. Sci. Rep. 2022, 12, 13681. [Google Scholar] [CrossRef] [PubMed]
- Hildreth, J.E.K.; Alcendor, D.J. Targeting COVID-19 Vaccine Hesitancy in Minority Populations in the US: Implications for Herd Immunity. Vaccines 2021, 9, 489. [Google Scholar] [CrossRef] [PubMed]
- Solís Arce, J.S.; Warren, S.S.; Meriggi, N.F.; Scacco, A.; McMurry, N.; Voors, M.; Syunyaev, G.; Malik, A.A.; Aboutajdine, S.; Adeojo, O.; et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat. Med. 2021, 27, 1385–1394. [Google Scholar] [CrossRef]
- Shekhar, R.; Sheikh, A.B.; Upadhyay, S.; Singh, M.; Kottewar, S.; Mir, H.; Barrett, E.; Pal, S. COVID-19 Vaccine Acceptance among Health Care Workers in the United States. Vaccines 2021, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Skjefte, M.; Ngirbabul, M.; Akeju, O.; Escudero, D.; Hernandez-Diaz, S.; Wyszynski, D.F.; Wu, J.W. COVID-19 vaccine acceptance among pregnant women and mothers of young children: Results of a survey in 16 countries. Eur. J. Epidemiol. 2021, 36, 197–211. [Google Scholar] [CrossRef]
- Latkin, C.; Dayton, L.A.; Yi, G.; Konstantopoulos, A.; Park, J.; Maulsby, C.; Kong, X. COVID-19 vaccine intentions in the United States, a social-ecological framework. Vaccine 2021, 39, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; St Denis, K.J.; Hoelzemer, A.; Lam, E.C.; Nitido, A.D.; Sheehan, M.L.; Berrios, C.; Ofoman, O.; Chang, C.C.; Hauser, B.M.; et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2022, 185, 457–466. [Google Scholar] [CrossRef]
- Freeman, D.; Loe, B.S.; Chadwick, A.; Vaccari, C.; Waite, F.; Rosebrock, L.; Jenner, L.; Petit, A.; Lewandowsky, S.; Vanderslott, S.; et al. COVID-19 vaccine hesitancy in the UK: The Oxford coronavirus explanations, attitudes, and narratives survey (Oceans) II. Psychol. Med. 2022, 52, 3127–3141. [Google Scholar] [CrossRef]
- Thanh Le, T.; Andreadakis, Z.; Kumar, A.; Gómez Román, R.; Tollefsen, S.; Saville, M.; Mayhew, S. The COVID-19 vaccine development landscape. Nat. Rev. Drug. Discov. 2020, 19, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Lee, Y.-F.; Koumi, K. COVID-19 Vaccination: Sociopolitical and Economic Impact in the United States. Epidemiologia 2022, 3, 502–517. [Google Scholar] [CrossRef] [PubMed]
- Forman, R.; Shah, S.; Jeurissen, P.; Jit, M.; Mossialos, E. COVID-19 vaccine challenges: What have we learned so far and what remains to be done? Health Policy 2021, 125, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Ntoumi, F.; Hui, D.S.; Abubakar, A.; Kramer, L.D.; Obiero, C.; Tambyah, P.A.; Blumberg, L.; Yapi, R.; Al-Abri, S.; et al. Emergence of new SARS-CoV-2 Variant of Concern Omicron (B.1.1.529)—Highlights Africa’s research capabilities, but exposes major knowledge gaps, inequities of vaccine distribution, inadequacies in global COVID-19 response and control efforts. Int. J. Infect. Dis. 2022, 114, 268–272. [Google Scholar] [CrossRef]
- World Health Organization. African Region. WHO Welcomes Canadian Commitment of CAD$ 30 Million to Reinforce COVID-19 Vaccination Efforts and Strengthen Health Systems in Africa. Available online: https://www.afro.who.int/news/who-welcomes-canadian-commitment-cad-30-million-reinforce-covid-19-vaccination-efforts-and (accessed on 18 February 2023).


| Rank | Journal | WoS Categories | Articles |
|---|---|---|---|
| 1 | Vaccines | Medicine, Research and Experimental/Immunology | 788 |
| 2 | Vaccines | Medicine, Research and Experimental/Immunology | 288 |
| 3 | Hum. Vaccin. Immunother. | Biotechnology and Applied Microbiology/Immunology | 232 |
| 4 | Front. Immunol. | Immunology | 168 |
| 5 | PloS One | Multidisciplinary Sciences | 144 |
| 6 | Int. J. Environ. Res. Public Health | Public, Environmental and Occupational Health | 127 |
| 7 | Front. Public Health | Public, Environmental and Occupational Health | 121 |
| 8 | Cureus J. Med. Sci. | Medicine, General and Internal | 94 |
| 9 | Clin. Infect. Dis. | Microbiology/Infectious Diseases/Immunology | 82 |
| 10 | Sci. Rep. | Multidisciplinary Sciences | 77 |
| 11 | Nat. Commun. | Multidisciplinary Sciences | 68 |
| 12 | J. Med. Virol. | Virology | 57 |
| 13 | Morb. Mortal. Wkly. Rep. | Public, Environmental and Occupational Health | 57 |
| 14 | Int. J. Infect. Dis. | Infectious Diseases | 56 |
| 15 | NPJ Vaccines | Medicine, Research and Experimental/Immunology | 55 |
| Rank | Affiliate Institutions | Country | Frequency |
|---|---|---|---|
| 1 | University of Oxford | United Kingdom | 368 |
| 2 | The University of Hong Kong | China | 204 |
| 3 | Harvard Medical School | United States | 199 |
| 4 | Tel Aviv University | Israel | 191 |
| 5 | University of Pennsylvania | United States | 190 |
| 6 | University of Washington | United States | 190 |
| 7 | University of Toronto | Canada | 189 |
| 8 | Stanford University | United States | 166 |
| 9 | Emory University | United States | 157 |
| 10 | University of Michigan | United States | 155 |
| 11 | Johns Hopkins University | United States | 136 |
| 12 | Fudan University | China | 135 |
| 13 | Washington University | United States | 135 |
| 14 | University of California San Francisco | United States | 134 |
| 15 | Imperial College London | United Kingdom | 129 |
| Rank | Country | Articles | SCP | MCP | MCP_Rate |
|---|---|---|---|---|---|
| 1 | United States | 1997 | 1631 | 366 | 18% |
| 2 | China | 812 | 626 | 186 | 23% |
| 3 | Italy | 465 | 388 | 77 | 17% |
| 4 | United Kingdom | 400 | 239 | 161 | 40% |
| 5 | India | 276 | 213 | 63 | 23% |
| 6 | Israel | 225 | 180 | 45 | 20% |
| 7 | Japan | 223 | 202 | 21 | 9% |
| 8 | Canada | 204 | 133 | 71 | 35% |
| 9 | Turkey | 187 | 180 | 7 | 4% |
| 10 | Spain | 186 | 151 | 35 | 19% |
| 11 | Saudi Arabia | 173 | 108 | 65 | 38% |
| 12 | Germany | 163 | 94 | 69 | 42% |
| 13 | France | 162 | 114 | 48 | 30% |
| 14 | Australia | 155 | 106 | 49 | 32% |
| 15 | Korea | 126 | 104 | 22 | 18% |
| Rank | Author (Year), Journal | Title | Total Citations (TC) |
|---|---|---|---|
| 1 [18] | Polack, F.P. et al. (2020), N. Engl. J. Med. | Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine | 6729 |
| 2 [19] | Baden, L.R. et al. (2021), N. Engl. J. Med. | Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine | 4486 |
| 3 [20] | Voysey, M. et al. (2021), Lancet | Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa and the UK | 2259 |
| 4 [21] | Jackson, L.A. et al. (2020), N. Engl. J. Med. | An mRNA Vaccine against SARS-CoV-2—Preliminary Report | 1715 |
| 5 [22] | Bernal, J.L. et al. (2021), N. Engl. J. Med. | Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant | 1527 |
| 6 [23] | Dagan, N. et al. (2021), N. Engl. J. Med. | BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Mass Vaccination Setting | 1312 |
| 7 [24] | Folegatti, P.M. et al. (2020), Lancet | Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial | 1304 |
| 8 [25] | Walsh, E.E. et al. (2020), N. Engl. J. Med. | Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates | 1299 |
| 9 [26] | Lazarus, J.V. et al. (2021), Nat. Med. | A global survey of potential acceptance of a COVID-19 vaccine | 1248 |
| 10 [27] | Sadoff, J. et al. (2021), N. Engl. J. Med. | Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19 | 1188 |
| 11 [28] | Lugunov, D.Y. et al. (2021), Lancet | Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia | 838 |
| 12 [29] | Mulligan, M.J. et al. (2020), Nature | Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults | 827 |
| 13 [30] | Dror, A.A. et al. (2020), Eur. J. Epidemiol. | Vaccine hesitancy: the next challenge in the fight against COVID-19 | 814 |
| 14 [31] | Anderson, E.J. et al. (2020), N. Engl. J. Med. | Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults | 790 |
| 15 [32] | Haas, E.J. et al. (2021), Lancet | Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalizations and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data | 779 |
| Rank | Funding Source | Country | Type | Frequency |
|---|---|---|---|---|
| 1 | U.S. Department of Health Human Services (HHS) | United States | Government Department | 670 |
| 2 | National Institutes of Health (NIH) | United States | Government Agency | 604 |
| 3 | National Natural Science Foundation of China (NSFC) | China | Government Agency | 238 |
| 4 | European Commission (EC) | Belgium | Parliament | 174 |
| 5 | National Institute of Allergy and Infectious Diseases (NIAID) | United States | Government Agency | 164 |
| 6 | UK Research and Innovation (UKRI) | United Kingdom | Government Agency | 147 |
| 7 | Medical Research Council (MRC) | United Kingdom | Government Agency | 99 |
| 8 | Centers For Disease Control Prevention (CDC) | United States | Information Service | 83 |
| 9 | Wellcome Trust | United Kingdom | Philanthropic Institution | 83 |
| 10 | Bill and Melinda Gates Foundation (BMGF) | United States | Philanthropic Institution | 78 |
| 11 | National Science Foundation (NSF) | United States | Government Agency | 77 |
| 12 | National Institute for Health and Care Research (NIHR) | United Kingdom | Government Agency | 74 |
| 13 | Canadian Institutes of Health Research (CIHR) | Canada | Government Agency | 64 |
| 14 | National Cancer Institute (NCI) | United States | Government Agency | 59 |
| 15 | Pfizer | United States | Company | 48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa Neto, A.R.d.; Carvalho, A.R.B.d.; Ferreira da Silva, M.D.; Rêgo Neta, M.M.; Sena, I.V.d.O.; Almeida, R.N.; Filha, F.S.S.C.; Lima e Silva, L.L.; Costa, G.R.d.; Lira, I.M.d.S.; et al. Bibliometric Analysis of Global Scientific Production on COVID-19 and Vaccines. Int. J. Environ. Res. Public Health 2023, 20, 4796. https://doi.org/10.3390/ijerph20064796
Sousa Neto ARd, Carvalho ARBd, Ferreira da Silva MD, Rêgo Neta MM, Sena IVdO, Almeida RN, Filha FSSC, Lima e Silva LL, Costa GRd, Lira IMdS, et al. Bibliometric Analysis of Global Scientific Production on COVID-19 and Vaccines. International Journal of Environmental Research and Public Health. 2023; 20(6):4796. https://doi.org/10.3390/ijerph20064796
Chicago/Turabian StyleSousa Neto, Antonio Rosa de, Ana Raquel Batista de Carvalho, Márcia Daiane Ferreira da Silva, Marly Marques Rêgo Neta, Inara Viviane de Oliveira Sena, Rosângela Nunes Almeida, Francidalma Soares Sousa Carvalho Filha, Laianny Luize Lima e Silva, Girlene Ribeiro da Costa, Ivana Mayra da Silva Lira, and et al. 2023. "Bibliometric Analysis of Global Scientific Production on COVID-19 and Vaccines" International Journal of Environmental Research and Public Health 20, no. 6: 4796. https://doi.org/10.3390/ijerph20064796
APA StyleSousa Neto, A. R. d., Carvalho, A. R. B. d., Ferreira da Silva, M. D., Rêgo Neta, M. M., Sena, I. V. d. O., Almeida, R. N., Filha, F. S. S. C., Lima e Silva, L. L., Costa, G. R. d., Lira, I. M. d. S., Portela, D. M. M. C., Oliveira e Silva, A. T., Rabêlo, C. B. d. M., Valle, A. R. M. d. C., Moura, M. E. B., & Freitas, D. R. J. d. (2023). Bibliometric Analysis of Global Scientific Production on COVID-19 and Vaccines. International Journal of Environmental Research and Public Health, 20(6), 4796. https://doi.org/10.3390/ijerph20064796







