Preliminary Support for the Use of Motivational Interviewing to Improve Parent/Adult Caregiver Behavior for Obesity and Cancer Prevention
Abstract
1. Introduction
2. Materials and Methods
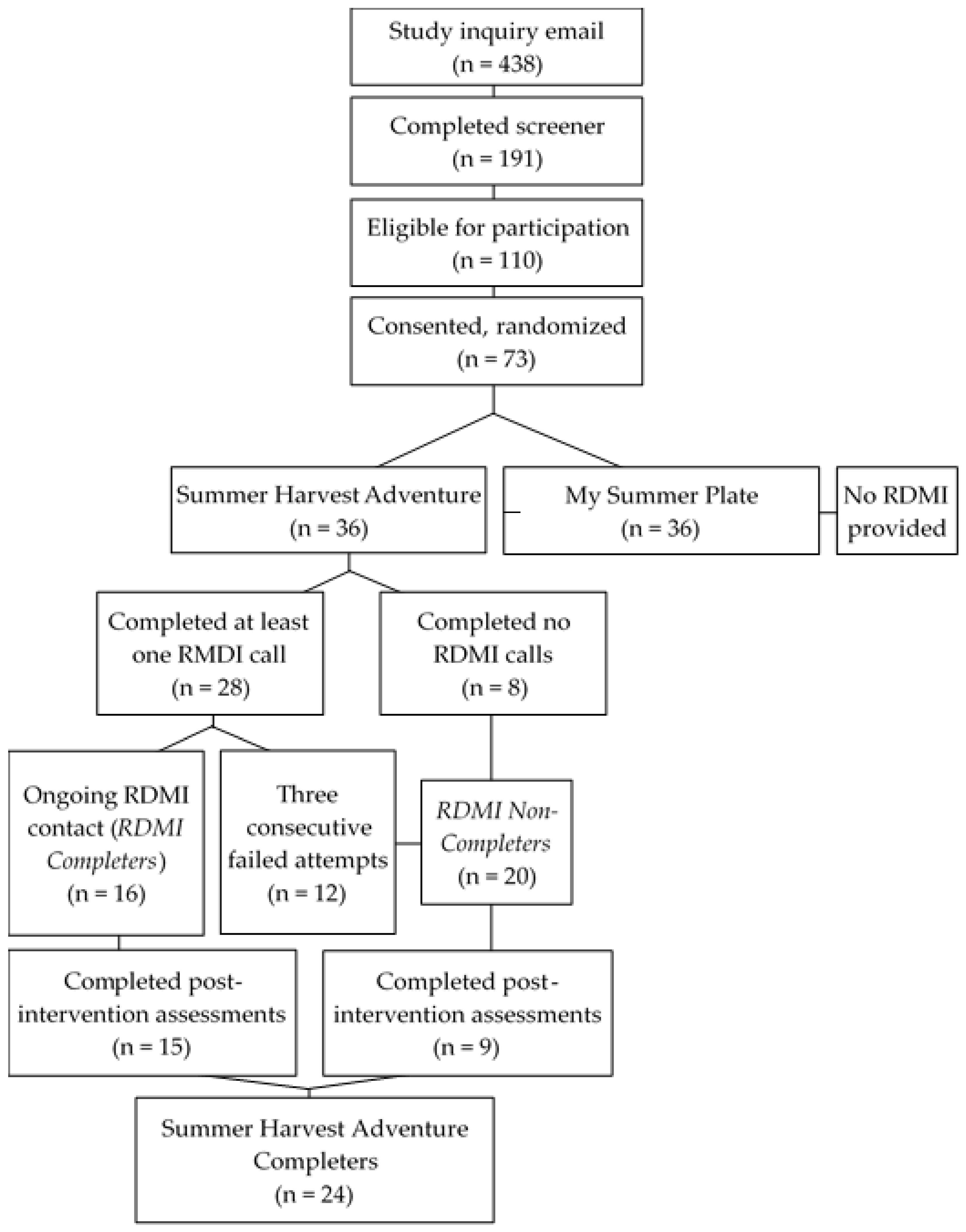
2.1. Participants and Recruitment
2.2. Study Design
2.3. Weekly Intervention Sessions
2.4. Remote Motivational Interviewing from a Registered Dietitian (RDMI)
2.5. Data Collection
2.6. Survey Measurements
2.7. Dietary Patterns and Diet Quality
2.8. Anthropometric and Clinical Measurements
2.9. Interaction and Process Data
2.10. Attendance and Participation
2.11. Fidelity to MI
2.12. Statistical Analyses
3. Results
3.1. Baseline Characteristics and Measures of RDMI Use
3.2. Ambivalence
3.3. PAC Clinical Outcomes and Diet Quality
3.4. Child and Home-Related Outcomes
3.5. Programmatic Evaluations and Autonomy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Continue Update Project Expert Report. 2018. Available online: https://dietandcancerreport.org (accessed on 1 January 2023).
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Vucenik, I.; Stains, J.P. Obesity and cancer risk: Evidence, mechanisms, and recommendations. Ann. N. Y. Acad. Sci. 2012, 1271, 37–43. [Google Scholar] [CrossRef] [PubMed]
- National Center for Health Statistics. Health, United States, 2018; National Center for Health Statistics: Hyattsville, MD, USA, 2019. Available online: https://www.cdc.gov/nchs/data/hus/hus18.pdf (accessed on 1 January 2023).
- Hales, C.M.; Carroll, M.D.; Fryar, C.D. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS Data Brief 2017, 288, 1–8. [Google Scholar]
- Lange, S.J.; Moore, L.V.; Harris, D.M.; Merlo, C.L.; Lee, S.H.; Demissie, Z.; Galuska, D.A. Percentage of Adolescents Meeting Federal Fruit and Vegetable Intake Recommendations—Youth Risk Behavior Surveillance System, United States, 2017. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Moore, L.V.; Park, S.; Harris, D.M.; Blanck, H.M. Adults Meeting Fruit and Vegetable Intake Recommendations—United States, 2019. Morb. Mortal. Wkly. Rep. 2022, 71, 1–9. [Google Scholar] [CrossRef]
- Livingston, E.H. Reimagining Obesity in 2018: A JAMA Theme Issue on Obesity. JAMA 2018, 319, 238–240. [Google Scholar] [CrossRef]
- Faith, M.S.; Van Horn, L.; Appel, L.J.; Burke, L.E.; Carson, J.A.S.; Franch, H.A.; Jakicic, J.M.; Kral, T.V.E.; Odoms-Young, A.; Wansink, B.; et al. Evaluating Parents and Adult Caregivers as “Agents of Change” for Treating Obese Children: Evidence for Parent Behavior Change Strategies and Research Gaps. Circulation 2012, 125, 1186–1207. [Google Scholar] [CrossRef] [PubMed]
- Coto, J.; Pulgaron, E.R.; Graziano, P.A.; Bagner, D.M.; Villa, M.; Malik, J.A.; Delamater, A.M. Parents as Role Models: Associations Between Parent and Young Children’s Weight, Dietary Intake, and Physical Activity in a Minority Sample. Matern. Child Health J. 2019, 23, 943–950. [Google Scholar] [CrossRef]
- Bahia, L.; Schaan, C.W.; Sparrenberger, K.; Abreu, G.A.; Barufaldi, L.A.; Coutinho, W.; Schaan, B.D. Overview of meta-analysis on prevention and treatment of childhood obesity. J. Pediatr. (Rio J.) 2019, 95, 385–400. [Google Scholar] [CrossRef]
- Weihrauch-Blüher, S.; Kromeyer-Hauschild, K.; Graf, C.; Widhalm, K.; Korsten-Reck, U.; Jödicke, B.; Markert, J.; Müller, M.J.; Moss, A.; Wabitsch, M.; et al. Current Guidelines for Obesity Prevention in Childhood and Adolescence. Obes. Facts 2018, 11, 263–276. [Google Scholar] [CrossRef]
- Endalifer, M.L.; Diress, G. Epidemiology, Predisposing Factors, Biomarkers, and Prevention Mechanism of Obesity: A Systematic Review. J. Obes. 2020, 2020, 6134362. [Google Scholar] [CrossRef]
- Teixeira, P.J.; Silva, M.N.; Mata, J.; Palmeira, A.L.; Markland, D. Motivation, self-determination, and long-term weight control. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 22. [Google Scholar] [CrossRef]
- Bean, M.K.; Thornton, L.M.; Jeffers, A.J.; Gow, R.W.; Mazzeo, S.E. Impact of motivational interviewing on engagement in a parent-exclusive paediatric obesity intervention: Randomized controlled trial of NOURISH+MI. Pediatr. Obes. 2019, 14, e12484. [Google Scholar] [CrossRef]
- Wong, E.M.; Cheng, M.M. Effects of motivational interviewing to promote weight loss in obese children. J. Clin. Nurs. 2013, 22, 2519–2530. [Google Scholar] [CrossRef]
- Resnicow, K.; McMaster, F.; Bocian, A.; Harris, D.; Zhou, Y.; Snetselaar, L.; Schwartz, R.; Myers, E.; Gotlieb, J.; Foster, J.; et al. Motivational Interviewing and Dietary Counseling for Obesity in Primary Care: An RCT. Pediatrics 2015, 135, 649–657. [Google Scholar] [CrossRef]
- Schwartz, R.P.; Hamre, R.; Dietz, W.H.; Wasserman, R.C.; Slora, E.J.; Myers, E.F.; Sullivan, S.; Rockett, H.; Thoma, K.A.; Dumitru, G.; et al. Office-Based Motivational Interviewing to Prevent Childhood Obesity: A Feasibility Study. Arch. Pediatr. Adolesc. Med. 2007, 161, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Christison, A.L.; Daley, B.M.; Asche, C.V.; Ren, J.; Aldag, J.C.; Ariza, A.J.; Lowry, K.W. Pairing Motivational Interviewing with a Nutrition and Physical Activity Assessment and Counseling Tool in Pediatric Clinical Practice: A Pilot Study. Child. Obes. 2014, 10, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.; Mendelsohn, A.; Bennett, G.; Taveras, E.M.; Kimberg, A.; Kemper, A.R. Texting Motivational Interviewing: A Randomized Controlled Trial of Motivational Interviewing Text Messages Designed to Augment Childhood Obesity Treatment. Child. Obes. Print 2018, 14, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Draxten, M.; Flattum, C.; Fulkerson, J. An example of how to supplement goal setting to promote behavior change for families using motivational interviewing. Health Commun. 2016, 31, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Dalton, W.T.; Schetzina, K.E.; McBee, M.T.; Maphis, L.; Fulton-Robinson, H.; Ho, A.-L.; Tudiver, F.; Wu, T. Parent Report of Child’s Health-Related Quality of Life after a Primary-Care-Based Weight Management Program. Child. Obes. 2013, 9, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Davoli, A.M.; Broccoli, S.; Bonvicini, L.; Fabbri, A.; Ferrari, E.; D’Angelo, S.; Buono, A.D.; Montagna, G.; Panza, C.; Pinotti, M.; et al. Pediatrician-led Motivational Interviewing to Treat Overweight Children: An RCT. Pediatrics 2013, 132, e1236–e1246. [Google Scholar] [CrossRef]
- Tripp, S.B.; Perry, J.T.; Romney, S.; Blood-Siegfried, J. Providers as Weight Coaches: Using Practice Guides and Motivational Interview to Treat Obesity in the Pediatric Office. J. Pediatr. Nurs. 2011, 26, 474–479. [Google Scholar] [CrossRef]
- Tucker, S.J.; Ytterberg, K.L.; Lenoch, L.M.; Schmit, T.L.; Mucha, D.I.; Wooten, J.A.; Lohse, C.M.; Austin, C.M.; Mongeon Wahlen, K.J. Reducing Pediatric Overweight: Nurse-Delivered Motivational Interviewing in Primary Care. J. Pediatr. Nurs. 2013, 28, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Tyler, D.O.; Horner, S.D. Collaborating With Low-Income Families and Their Overweight Children to Improve Weight-Related Behaviors: An Intervention Process Evaluation. J. Spec. Pediatr. Nurs. 2008, 13, 263–274. [Google Scholar] [CrossRef]
- Döring, N.; Ghaderi, A.; Bohman, B.; Heitmann, B.L.; Larsson, C.; Berglind, D.; Hansson, L.; Sundblom, E.; Magnusson, M.; Blennow, M.; et al. Motivational Interviewing to Prevent Childhood Obesity: A Cluster RCT. Pediatrics 2016, 137, e20153104. [Google Scholar] [CrossRef] [PubMed]
- Suire, K.B.; Kavookjian, J.; Wadsworth, D.D. Motivational Interviewing for Overweight Children: A Systematic Review. Pediatrics 2020, 146, e20200193. [Google Scholar] [CrossRef]
- Michie, S.; van Stralen, M.M.; West, R. The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implement. Sci. IS 2011, 6, 42. [Google Scholar] [CrossRef]
- Söderlund, L.L.; Nordqvist, C.; Angbratt, M.; Nilsen, P. Applying motivational interviewing to counselling overweight and obese children. Health Educ. Res. 2009, 24, 442–449. [Google Scholar] [CrossRef]
- Pakpour, A.H.; Gellert, P.; Dombrowski, S.U.; Fridlund, B. Motivational Interviewing with Parents for Obesity: An RCT. PEDIATRICS 2015, 135, e644–e652. [Google Scholar] [CrossRef]
- Dawson, A.M.; Brown, D.A.; Cox, A.; Williams, S.M.; Treacy, L.; Haszard, J.; Meredith-Jones, K.; Hargreaves, E.; Taylor, B.J.; Ross, J.; et al. Using motivational interviewing for weight feedback to parents of young children. J. Paediatr. Child Health 2014, 50, 461–470. [Google Scholar] [CrossRef]
- Berkel, C.; Mauricio, A.M.; Rudo-Stern, J.; Dishion, T.J.; Smith, J.D. Motivational Interviewing and Caregiver Engagement in the Family Check-Up 4 Health. Prev. Sci. 2020, 22, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Bean, M.K.; Caccavale, L.J.; Adams, E.L.; Burnette, C.B.; LaRose, J.G.; Raynor, H.A.; Wickham, E.P.; Mazzeo, S.E. Parent Involvement in Adolescent Obesity Treatment: A Systematic Review. Pediatrics 2020, 146, e20193315. [Google Scholar] [CrossRef] [PubMed]
- Niemeier, B.S.; Hektner, J.M.; Enger, K.B. Parent participation in weight-related health interventions for children and adolescents: A systematic review and meta-analysis. Prev. Med. 2012, 55, 3–13. [Google Scholar] [CrossRef]
- Young, K.M.; Northern, J.J.; Lister, K.M.; Drummond, J.A.; O’Brien, W.H. A meta-analysis of family-behavioral weight-loss treatments for children. Clin. Psychol. Rev. 2007, 27, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Ewald, H.; Kirby, J.; Rees, K.; Robertson, W. Parent-only interventions in the treatment of childhood obesity: A systematic review of randomized controlled trials. J. Public Health Oxf. Engl. 2014, 36, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Campbell-Voytal, K.D.; Hartlieb, K.B.; Cunningham, P.B.; Jacques-Tiura, A.J.; Ellis, D.A.; Jen, K.-L.C.; Naar-King, S. African American Adolescent-Caregiver Relationships in a Weight Loss Trial. J. Child Fam. Stud. 2018, 27, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.K.; Collins, C.; May, C.; Brain, K.; Wong See, D.; Burrows, T. Effectiveness of family-based weight management interventions for children with overweight and obesity: An umbrella review. JBI Database Syst. Rev. Implement. Rep. 2019, 17, 1341–1427. [Google Scholar] [CrossRef] [PubMed]
- Schuster, R.C.; Szpak, M.; Klein, E.; Sklar, K.; Dickin, K.L. “I try, I do”: Child feeding practices of motivated, low-income parents reflect trade-offs between psychosocial and nutrition goals. Appetite 2019, 136, 114–123. [Google Scholar] [CrossRef]
- Janicke, D.M.; Steele, R.G.; Gayes, L.A.; Lim, C.S.; Clifford, L.M.; Schneider, E.M.; Carmody, J.K.; Westen, S. Systematic Review and Meta-Analysis of Comprehensive Behavioral Family Lifestyle Interventions Addressing Pediatric Obesity. J. Pediatr. Psychol. 2014, 39, 809–825. [Google Scholar] [CrossRef]
- Hammersley, M.L.; Okely, A.D.; Batterham, M.J.; Jones, R.A. An Internet-Based Childhood Obesity Prevention Program (Time2bHealthy) for Parents of Preschool-Aged Children: Randomized Controlled Trial. J. Med. Internet Res. 2019, 21, e11964. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.; Harris, J.; Craven, K.; Sastre, L. Sharing the ‘weight’ of obesity management in primary care: Integration of registered dietitian nutritionists to provide intensive behavioural therapy for obesity for Medicare patients. Fam. Pract. 2021, 38, 18–24. [Google Scholar] [CrossRef]
- Kramer Schmidt, L.; Moyers, T.B.; Nielsen, A.S.; Andersen, K. Is fidelity to motivational interviewing associated with alcohol outcomes in treatment-seeking 60+ year-old citizens? J. Subst. Abuse Treat. 2019, 101, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; Carey, R.N.; Johnston, M.; Rothman, A.J.; de Bruin, M.; Kelly, M.P.; Connell, L.E. From Theory-Inspired to Theory-Based Interventions: A Protocol for Developing and Testing a Methodology for Linking Behaviour Change Techniques to Theoretical Mechanisms of Action. Ann. Behav. Med. 2018, 52, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Moyers, T.B.; Rowell, L.N.; Manuel, J.K.; Ernst, D.; Houck, J.M. The Motivational Interviewing Treatment Integrity Code (MITI 4): Rationale, Preliminary Reliability and Validity. J. Subst. Abuse Treat. 2016, 65, 36–42. [Google Scholar] [CrossRef]
- Bellg, A.J.; Borrelli, B.; Resnick, B.; Hecht, J.; Minicucci, D.S.; Ory, M.; Ogedegbe, G.; Orwig, D.; Ernst, D.; Czajkowski, S.; et al. Enhancing Treatment Fidelity in Health Behavior Change Studies: Best Practices and Recommendations from the NIH Behavior Change Consortium. Health Psychol. Off. J. Div. Health Psychol. Am. Psychol. Assoc. 2004, 23, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Ory, M.G.; Lee Smith, M.; Mier, N.; Wernicke, M.M. The Science of Sustaining Health Behavior Change: The Health Maintenance Consortium. Am. J. Health Behav. 2010, 34, 647–659. [Google Scholar] [CrossRef]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J. Am. Coll. Cardiol. 2014, 129, S102–S138. [Google Scholar] [CrossRef]
- Moyers, T.B.; Houck, J. Combining Motivational Interviewing With Cognitive-Behavioral Treatments for Substance Abuse: Lessons From the COMBINE Research Project. Cogn. Behav. Pract. 2011, 18, 38–45. [Google Scholar] [CrossRef]
- Brug, J.; Spikmans, F.; Aartsen, C.; Breedveld, B.; Bes, R.; Fereira, I. Training Dietitians in Basic Motivational Interviewing Skills Results in Changes in Their Counseling Style and in Lower Saturated Fat Intakes in Their Patients. J. Nutr. Educ. Behav. 2007, 39, 8–12. [Google Scholar] [CrossRef]
- Resnicow, K.; McMaster, F. Motivational Interviewing: Moving from why to how with autonomy support. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 19. [Google Scholar] [CrossRef]
- Hoffmann, T.C.; Glasziou, P.P.; Boutron, I.; Milne, R.; Perera, R.; Moher, D.; Altman, D.G.; Barbour, V.; Macdonald, H.; Johnston, M.; et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014, 348, g1687. [Google Scholar] [CrossRef] [PubMed]
- Adams, I.; Braun, A.; Hill, E.; Al-Muhanna, K.; Stigall, N.; Lobb, J.; Rausch, J.; Portner, J.; Evans, K.; Spees, C. Garden-Based Intervention for Youth Improves Dietary and Physical Activity Patterns, Quality of Life, Family Relationships, and Indices of Health. J. Nutr. Educ. Behav. 2019, 51, S20–S21. [Google Scholar] [CrossRef]
- Spees, C.; Lobb, J.; Portner, J.; Braun, A.; Adams, I. Summer Harvest Adventure: A Garden-Based Obesity Prevention Program for Children Residing in Low-Resource Communities. J. Nutr. Educ. Behav. 2018, 50, S121. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th ed. 2015. Available online: http://health.gov/dietaryguidelines/2015/ (accessed on 8 August 2020).
- US Department of Agriculture and Nutrition Services. The Great Garden Detective Adventure: A Standards-Based Gardening Nutrition Curriculum for Grades 3 and 4; US Department of Agriculture Food and Nutrition Service: Washington, DC, USA, 2013. [Google Scholar]
- Jiang, L.; Smith, M.L.; Chen, S.; Ahn, S.; Kulinski, K.P.; Lorig, K.; Ory, M.G. The Role of Session Zero in Successful Completion of Chronic Disease Self-Management Program Workshops. Front. Public Health 2015, 2, 205. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.M.; Deci, E.L. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am. Psychol. 2000, 55, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Rubak, S.; Sandbæk, A.; Lauritzen, T.; Christensen, B. Motivational interviewing: A systematic review and meta-analysis. Br. J. Gen. Pract. 2005, 55, 305–312. [Google Scholar]
- Carpenter, K.M.; Lovejoy, J.C.; Lange, J.M.; Hapgood, J.E.; Zbikowski, S.M. Outcomes and Utilization of a Low Intensity Workplace Weight Loss Program. J. Obes. 2014, 2014, 414987. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- BRFSS Questionnaires. Available online: http://www.cdc.gov/brfss/questionnaires/index.htm (accessed on 4 September 2016).
- Vaughn, A.E.; Dearth-Wesley, T.; Tabak, R.G.; Bryant, M.; Ward, D.S. Development of a Comprehensive Assessment of Food Parenting Practices: The Home Self-Administered Tool for Environmental Assessment of Activity and Diet Family Food Practices Survey. J. Acad. Nutr. Diet. 2017, 117, 214–227. [Google Scholar] [CrossRef]
- Gaume, J.; Bertholet, N.; Daeppen, J.-B.; Gmel, G. The Change Questionnaire predicts change in hazardous tobacco and alcohol use. Addict. Behav. 2013, 38, 2718–2723. [Google Scholar] [CrossRef]
- Miller, W.R.; Johnson, W.R. A natural language screening measure for motivation to change. Addict. Behav. 2008, 33, 1177–1182. [Google Scholar] [CrossRef]
- National Cancer Institute Division of Cancer Control & Population Sciences Diet History Questionnaire III. Available online: https://epi.grants.cancer.gov/dhq3/ (accessed on 22 July 2020).
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef]
- Williams, G.C.; Grow, V.M.; Freedman, Z.R.; Ryan, R.M.; Deci, E.L. Motivational Predictors of Weight Loss and Weight-Loss Maintenance. J. Pers. Soc. Psychol. 1996, 70, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.C.; Deci, E.L. Activating Patients for Smoking Cessation through Physician Autonomy Support. Med. Care 2001, 39, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Bandura, A. Self-efficacy: Toward a unifying theory of behavioral change. Psychol. Rev. 1977, 84, 191–215. [Google Scholar] [CrossRef] [PubMed]
- Grobe, J.E.; Goggin, K.; Harris, K.J.; Richter, K.P.; Resnicow, K.; Catley, D. Race moderates the effects of Motivational Interviewing on smoking cessation induction. Patient Educ. Couns. 2020, 103, 350–358. [Google Scholar] [CrossRef]
- Boutelle, K.N.; Cafri, G.; Crow, S.J. Parent Predictors of Child Weight Change in Family Based Behavioral Obesity Treatment. Obesity 2012, 20, 1539–1543. [Google Scholar] [CrossRef]
- Bean, M.K.; Powell, P.; Quinoy, A.; Ingersoll, K.; Wickham, E.P.; Mazzeo, S.E. Motivational interviewing targeting diet and physical activity improves adherence to paediatric obesity treatment: Results from the MI Values randomized controlled trial. Pediatr. Obes. 2015, 10, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, B.; Tooley, E.M.; Scott-Sheldon, L.A.J. Motivational Interviewing for Parent-child Health Interventions: A Systematic Review and Meta-Analysis. Pediatr. Dent. 2015, 37, 254–265. [Google Scholar] [PubMed]
- Braun, A.; Portner, J.; Grainger, E.M.; Hill, E.B.; Young, G.S.; Clinton, S.K.; Spees, C.K. Tele-Motivational Interviewing for Cancer Survivors: Feasibility, Preliminary Efficacy, and Lessons Learned. J. Nutr. Educ. Behav. 2018, 50, 19–32.e1. [Google Scholar] [CrossRef]
- Befort, C.A.; Nollen, N.; Ellerbeck, E.F.; Sullivan, D.K.; Thomas, J.L.; Ahluwalia, J.S. Motivational interviewing fails to improve outcomes of a behavioral weight loss program for obese African American women: A pilot randomized trial. J. Behav. Med. 2008, 31, 367–377. [Google Scholar] [CrossRef]
- Vansteenkiste, M.; Sheldon, K.M. There’s nothing more practical than a good theory: Integrating motivational interviewing and self-determination theory. Br. J. Clin. Psychol. 2006, 45, 63–82. [Google Scholar] [CrossRef]
- Heerman, W.J.; JaKa, M.M.; Berge, J.M.; Trapl, E.S.; Sommer, E.C.; Samuels, L.R.; Jackson, N.; Haapala, J.L.; Kunin-Batson, A.S.; Olson-Bullis, B.A.; et al. The dose of behavioral interventions to prevent and treat childhood obesity: A systematic review and meta-regression. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 157. [Google Scholar] [CrossRef]
- VanBuskirk, K.A.; Wetherell, J.L. Motivational interviewing with primary care populations: A systematic review and meta-analysis. J. Behav. Med. 2014, 37, 768–780. [Google Scholar] [CrossRef]
- Reedy, J.; Krebs-Smith, S.M.; Miller, P.E.; Liese, A.D.; Kahle, L.L.; Park, Y.; Subar, A.F. Higher Diet Quality Is Associated with Decreased Risk of All-Cause, Cardiovascular Disease, and Cancer Mortality among Older Adults. J. Nutr. 2014, 144, 881–889. [Google Scholar] [CrossRef]
- Stolley, M.R.; Fitzgibbon, M.L.; Schiffer, L.; Sharp, L.K.; Singh, V.; Horn, L.V.; Dyer, A. Obesity Reduction Black Intervention Trial (ORBIT): Six-month Results. Obesity 2009, 17, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Draxten, M.; Fulkerson, J.A.; Friend, S.; Flattum, C.F.; Schow, R. Parental role modeling of fruits and vegetables at meals and snacks is associated with children’s adequate consumption. Appetite 2014, 78, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Balantekin, K.N. The Influence of Parental Dieting Behavior on Child Dieting Behavior and Weight Status. Curr. Obes. Rep. 2019, 8, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Mulgrew, K.W.; Shaikh, U.; Nettiksimmons, J. Comparison of Parent Satisfaction with Care for Childhood Obesity Delivered Face-to-Face and by Telemedicine. Telemed. e-Health 2011, 17, 383–387. [Google Scholar] [CrossRef]
- Bala, N.; Price, S.N.; Horan, C.M.; Gerber, M.W.; Taveras, E.M. Use of Telehealth to Enhance Care in a Family-Centered Childhood Obesity Intervention. Clin. Pediatr. 2019, 58, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Nepper, M.J.; Chai, W. Parents’ barriers and strategies to promote healthy eating among school-age children. Appetite 2016, 103, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Puhl, R.; Suh, Y. Health Consequences of Weight Stigma: Implications for Obesity Prevention and Treatment. Curr. Obes. Rep. 2015, 4, 182–190. [Google Scholar] [CrossRef]
- Thomas, J.L.; Stewart, D.W.; Lynam, I.M.; Daley, C.M.; Befort, C.; Scherber, R.M.; Mercurio, A.E.; Okuyemi, K.S.; Ahluwalia, J.S. Support Needs of Overweight African American Women for Weight Loss. Am. J. Health Behav. 2009, 33, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Croghan, I.T.; Ebbert, J.O.; Njeru, J.W.; Rajjo, T.I.; Lynch, B.A.; DeJesus, R.S.; Jensen, M.D.; Fischer, K.M.; Phelan, S.; Kaufman, T.K.; et al. Identifying Opportunities for Advancing Weight Management in Primary Care. J. Prim. Care Community Health 2019, 10, 2150132719870879. [Google Scholar] [CrossRef]
- Miller, W.R.; Rollnick, S. Motivational Interviewing: Helping People Change, 3rd ed.; The Guilford Press: New York, NY, USA, 2013. [Google Scholar]
- Butler, C.C.; Simpson, S.A.; Hood, K.; Cohen, D.; Pickles, T.; Spanou, C.; McCambridge, J.; Moore, L.; Randell, E.; Alam, M.F.; et al. Training practitioners to deliver opportunistic multiple behaviour change counselling in primary care: A cluster randomised trial. BMJ 2013, 346, f1191. [Google Scholar] [CrossRef]
- McCambridge, J.; Strang, J. The efficacy of single-session motivational interviewing in reducing drug consumption and perceptions of drug-related risk and harm among young people: Results from a multi-site cluster randomized trial. Addiction 2004, 99, 39–52. [Google Scholar] [CrossRef] [PubMed]

| Variable | Levels | RDMI Completers (n = 16) % (n) | SHA Completers (n = 24) % (n) | Entire Cohort (n = 36) % (n) |
|---|---|---|---|---|
| Age (years, mean (SD)) | - | 38 (6) | 37 (5) | 36 (5) |
| Sex | Female | 81 (13) | 83 (20) | 89 (32) |
| Male | 19 (3) | 17 (4) | 11 (4) | |
| Race | Black/African American | 44 (7) | 46 (11) | 50 (18) |
| White | 44 (7) | 42 (10) | 36 (13) | |
| Mixed Race | 6 (1) | 8 (2) | 8 (3) | |
| Prefer not to answer | 6 (1) | 4 (1) | 6 (2) | |
| Marital Status | Married | 44 (7) | 42 (10) | 39 (14) |
| Never Married | 19 (3) | 29 (7) | 25 (9) | |
| Member of an Unmarried Couple | 13 (2) | 13 (3) | 19 (7) | |
| Divorced | 19 (3) | 13 (3) | 11 (4) | |
| Separated | 6 (1) | 4 (1) | 6 (2) | |
| Education Level | Grade 12 or Higher | 56 (9) | 4 (1) | 6 (2) |
| 1–3 Years of College | 44 (7) | 50 (12) | 50 (18) | |
| 4 Years or More of College | 0 (0) | 46 (11) | 44 (16) | |
| Employment Status | Employed or Self-Employed | 94 (15) | 92 (22) | 83 (30) |
| Unemployed | 6 (1) | 4 (1) | 8 (3) | |
| Unable to Work | 0 (0) | 4 (1) | 6 (2) | |
| Student | 0 (0) | 0 (0) | 3 (1) | |
| BMI (kg/m2, mean (SD)) | - | 31.1 (6.11) | 30.4 (6.04) | 30.3 (5.67) |
| HEI Scores a | - | 61.5 (9.93) | 62.3 (9.98) | 62.9 (9.6) |
| Change Scores (i.e., ambivalence) b | - | 8.8 (1.30) | 9.1 (1.13) | 9.0 (1.1) |
| Variables (n = 24) b | B c | SE B | Β d | p | 95% CI |
|---|---|---|---|---|---|
| RI Dose Received (Total Model Adjusted R2 = 0.002; f2 = 0.096) | |||||
| RI Dose Received | 0.571 | 0.894 | 0.145 | 0.530 | −1.289, 2.431 |
| SHA Attendance | 1.986 | 2.184 | 0.207 | 0.374 | −2.556, 6.528 |
| RDMI Dose Received (Total Model Adjusted R2 = 0.055; f2 = 0.159) | |||||
| RDMI Dose Received | 0.980 | 0.773 | 0.271 | 0.219 | −0.629, 2.588 |
| SHA Attendance | 1.708 | 2.054 | 0.178 | 0.415 | −2.564, 5.979 |
| RDMI Time Received (Total Model Adjusted R2 = 0.089; f2 = 0.202) | |||||
| RDMI Time Received | 0.055 | 0.035 | 0.344 | 0.131 | −0.018, 0.129 |
| SHA Attendance | 1.169 | 2.101 | 0.122 | 0.584 | −3.199, 5.537 |
| RDMI Engagement (Total Model Adjusted R2 = 0.095; f2 = 0.211) | |||||
| RDMI Engagement | 0.071 | 0.044 | 0.328 | 0.121 | −0.020, 0.163 |
| SHA Attendance | 1.877 | 1.947 | 0.196 | 0.346 | −2.171, 5.925 |
| Variables (n = 24) b | B c | SE B | Β d | p | 95% CI |
|---|---|---|---|---|---|
| RI Dose Received (Total Model Adjusted R2 = −0.055; f2 = 0.037) | |||||
| RI Dose Received | 1.315 | 2.416 | 0.128 | 0.592 | −3.710, 6.340 |
| SHA Attendance | −5.096 | 5.901 | −0.202 | 0.398 | −17.368, 7.176 |
| RDMI Dose Received (Total Model Adjusted R2 = −0.067; f2 = 0.027) | |||||
| RDMI Dose Received | 0.591 | 2.158 | 0.062 | 0.787 | −3.898, 5.079 |
| SHA Attendance | −4.303 | 5.733 | −0.171 | 0.461 | −16.225, 7.619 |
| RDMI Time Received (Total Model Adjusted R2 = −0.059; f2 = 0.034) | |||||
| RDMI Time Received | 0.047 | 0.100 | 0.111 | 0.643 | −0.161, 0.255 |
| SHA Attendance | −4.966 | 5.952 | −0.197 | 0.414 | −17.345, 7.413 |
| RDMI Engagement (Total Model Adjusted R2 = −0.068; f2 = 0.026) | |||||
| RDMI Engagement | −0.027 | 0.126 | −0.047 | 0.823 | −0.288, 0.234 |
| SHA Attendance | −3.541 | 5.556 | −0.141 | 0.531 | −15.096, 8.014 |
| Variables (n = 24) b | B c | SE B | Β d | p | 95% CI |
|---|---|---|---|---|---|
| RI Dose Received (Total Model Adjusted R2 = 0.144; f2 = 0.279) | |||||
| RI Dose Received | 3.559 | 1.484 | 0.506 | 0.026 * | 0.471, 6.646 |
| SHA Attendance | −2.418 | 3.625 | −0.141 | 0.512 | −9.957, 5.121 |
| RDMI Dose Received (Total Model Adjusted R2 = 0.036; f2 = 0.136) | |||||
| RDMI Dose Received | 2.325 | 1.400 | 0.359 | 0.112 | −0.586, 5.236 |
| SHA Attendance | −0.898 | 3.717 | −0.052 | 0.811 | −8.628, 6.832 |
| RDMI Time Received (Total Model Adjusted R2 = 0.016; f2 = 0.124) | |||||
| RDMI Time Received | 0.099 | 0.066 | 0.344 | 0.146 | −0.038, 0.236 |
| SHA Attendance | −1.371 | 3.913 | −0.080 | 0.730 | −9.508, 6.767 |
| RDMI Engagement (Total Model Adjusted R2 = −0.037; f2 = 0.057) | |||||
| RDMI Engagement | 0.088 | 0.084 | 0.228 | 0.307 | −0.087, 0.264 |
| SHA Attendance | 0.265 | 3.733 | 0.015 | 0.944 | −7.499, 8.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braun, A.; Portner, J.; Xu, M.; Weaver, L.; Pratt, K.; Darragh, A.; Spees, C.K. Preliminary Support for the Use of Motivational Interviewing to Improve Parent/Adult Caregiver Behavior for Obesity and Cancer Prevention. Int. J. Environ. Res. Public Health 2023, 20, 4726. https://doi.org/10.3390/ijerph20064726
Braun A, Portner J, Xu M, Weaver L, Pratt K, Darragh A, Spees CK. Preliminary Support for the Use of Motivational Interviewing to Improve Parent/Adult Caregiver Behavior for Obesity and Cancer Prevention. International Journal of Environmental Research and Public Health. 2023; 20(6):4726. https://doi.org/10.3390/ijerph20064726
Chicago/Turabian StyleBraun, Ashlea, James Portner, Menglin Xu, Lindy Weaver, Keeley Pratt, Amy Darragh, and Colleen K. Spees. 2023. "Preliminary Support for the Use of Motivational Interviewing to Improve Parent/Adult Caregiver Behavior for Obesity and Cancer Prevention" International Journal of Environmental Research and Public Health 20, no. 6: 4726. https://doi.org/10.3390/ijerph20064726
APA StyleBraun, A., Portner, J., Xu, M., Weaver, L., Pratt, K., Darragh, A., & Spees, C. K. (2023). Preliminary Support for the Use of Motivational Interviewing to Improve Parent/Adult Caregiver Behavior for Obesity and Cancer Prevention. International Journal of Environmental Research and Public Health, 20(6), 4726. https://doi.org/10.3390/ijerph20064726







