Predicting Low-Level Childhood Lead Exposure in Metro Atlanta Using Ensemble Machine Learning of High-Resolution Raster Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Study Sample Raster Grid Creation and Outcomes
2.3. Study Sample Raster Grid Creation and Outcomes
2.3.1. Predictors from the U.S. Census
2.3.2. Environmental Protection Agency (EPA) Toxics Release Inventory (TRI)
2.3.3. Crime Index and Road Network Density
2.4. Study Sample
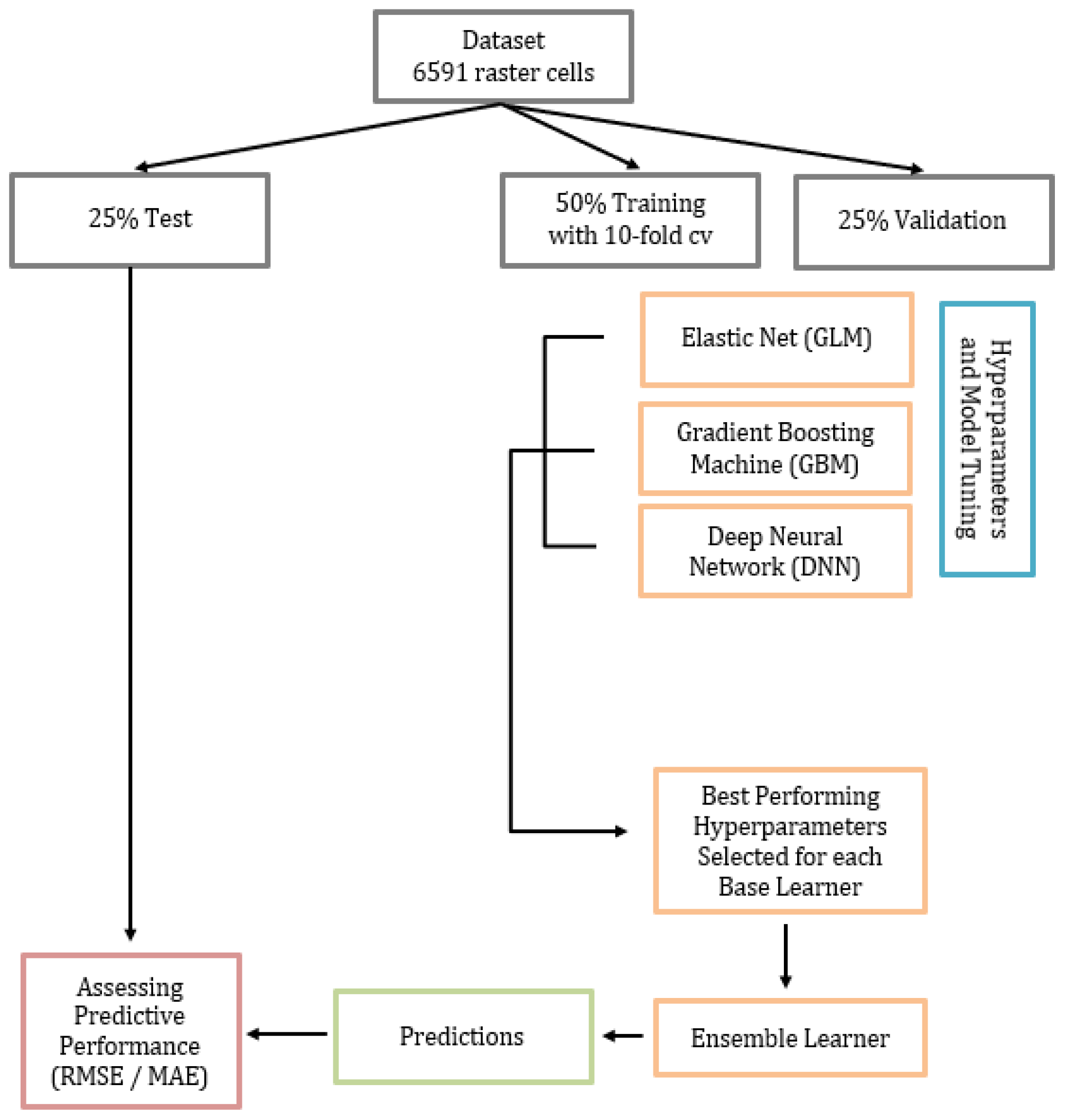
2.4.1. Training, Validation, and Test Datasets
2.4.2. Base Learners and Stacked Ensemble Model
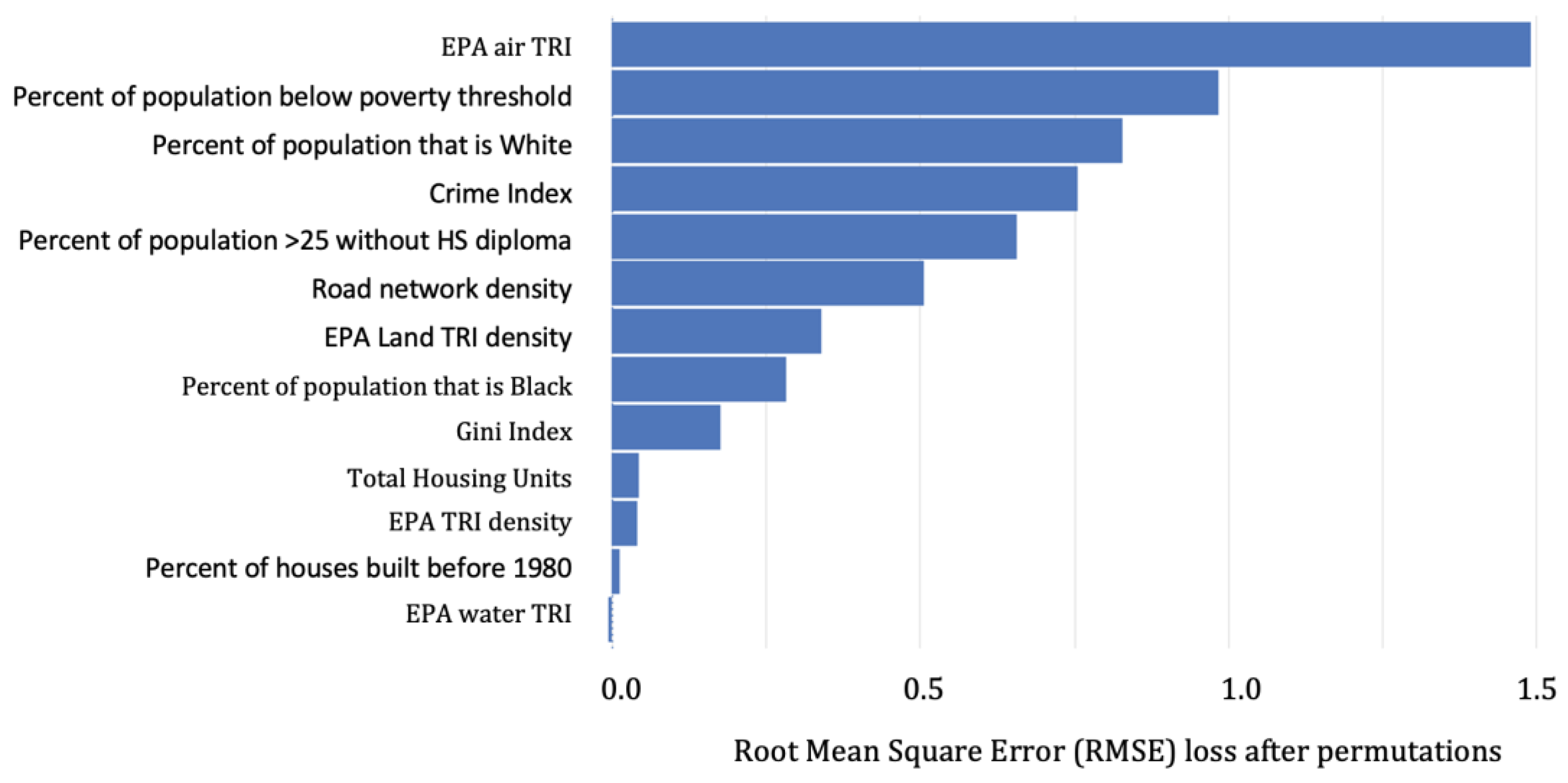
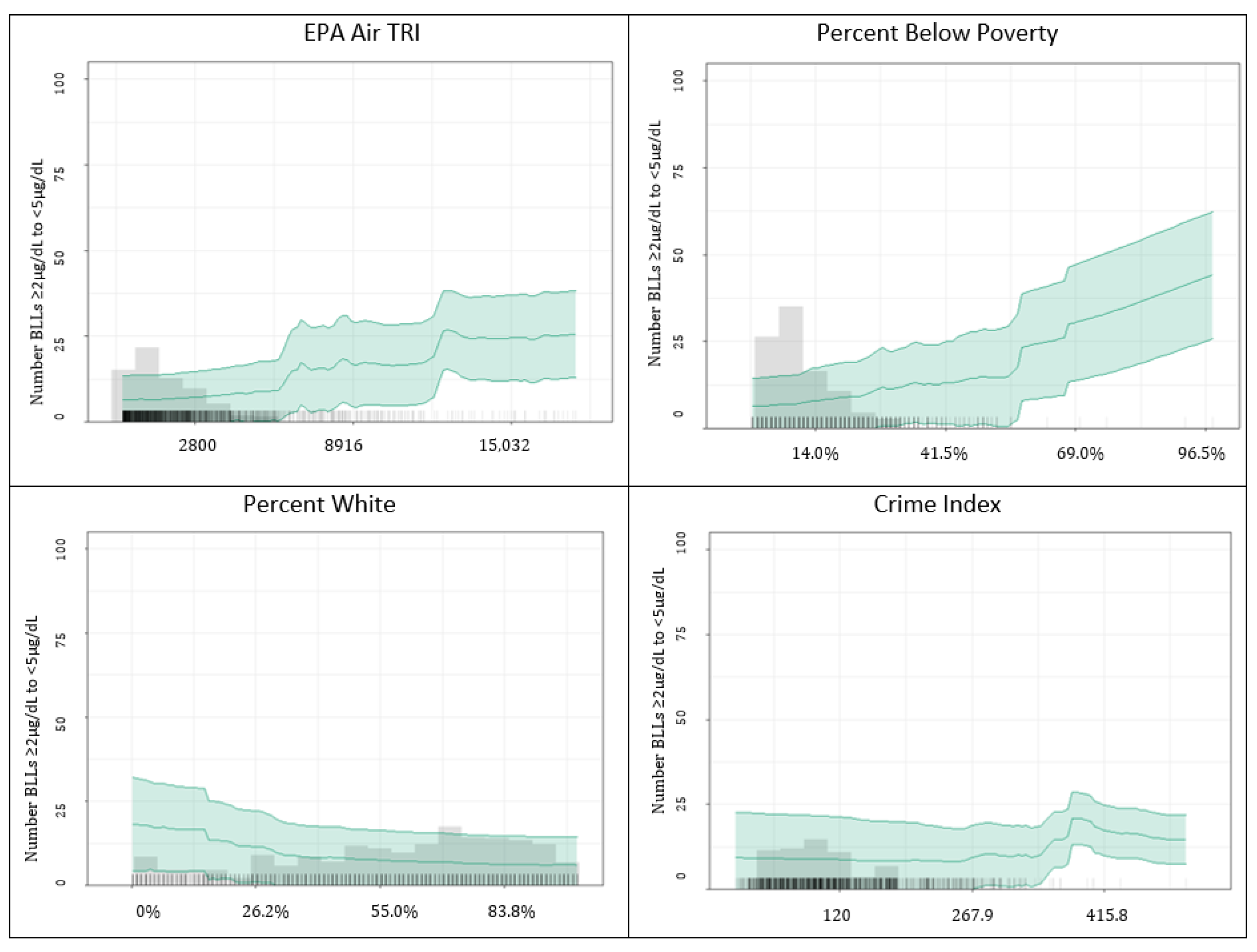
2.4.3. Model Performance, Predictor Importance, and Partial Dependence Plots
3. Results
3.1. Model Building, Performance, and Interpretation
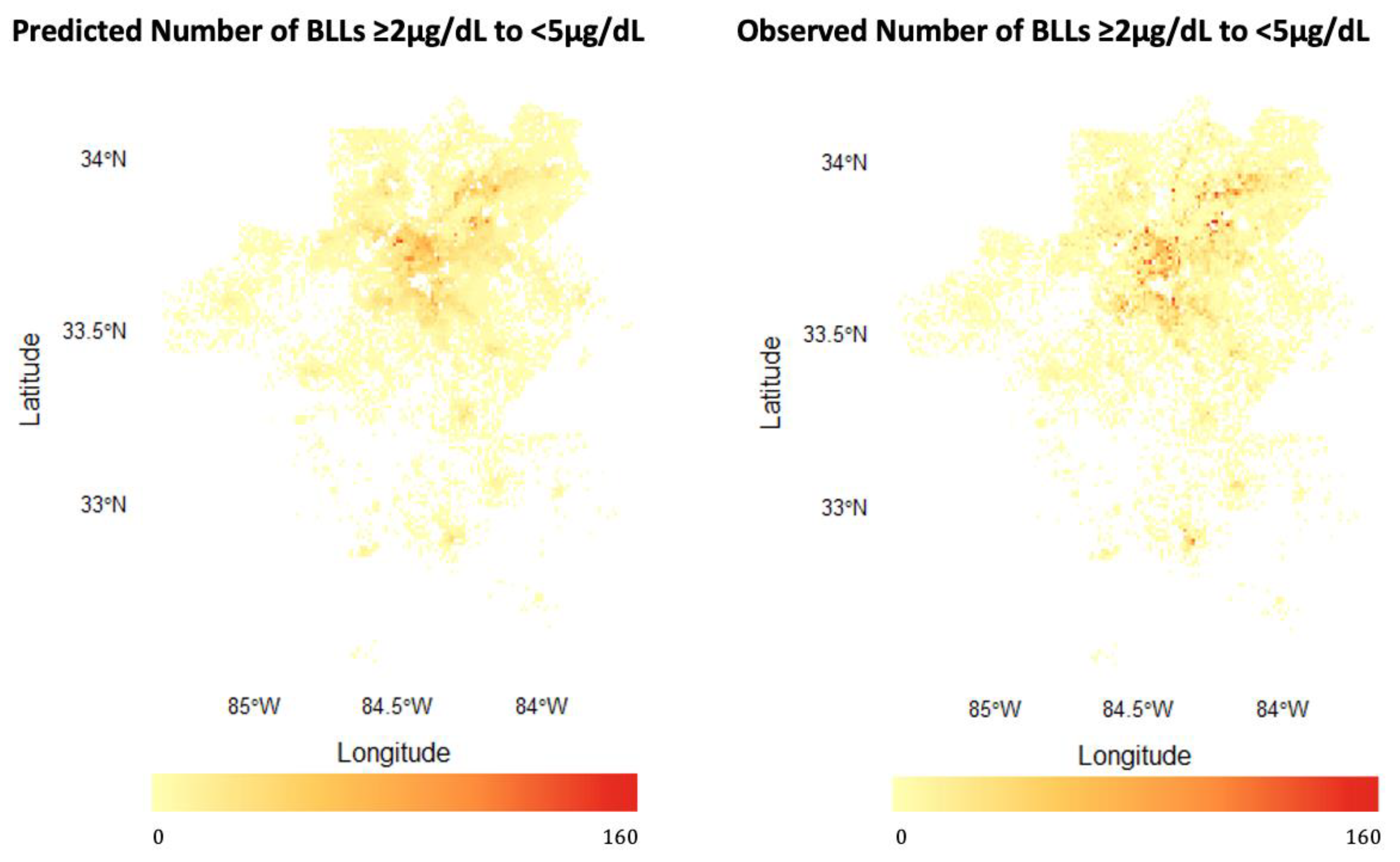
3.1.1. Predicting BLLs ≥2–<5 µg/dL
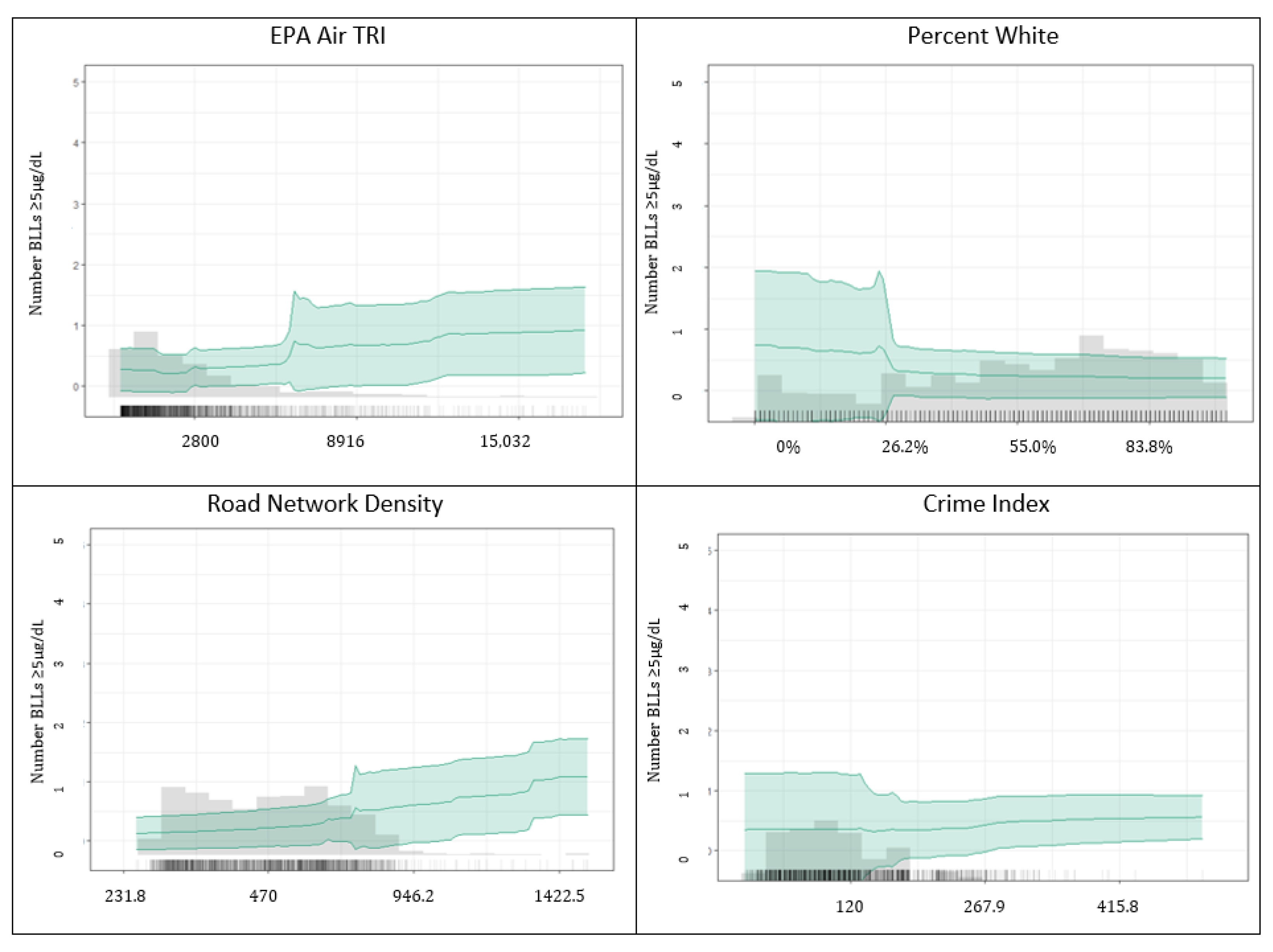
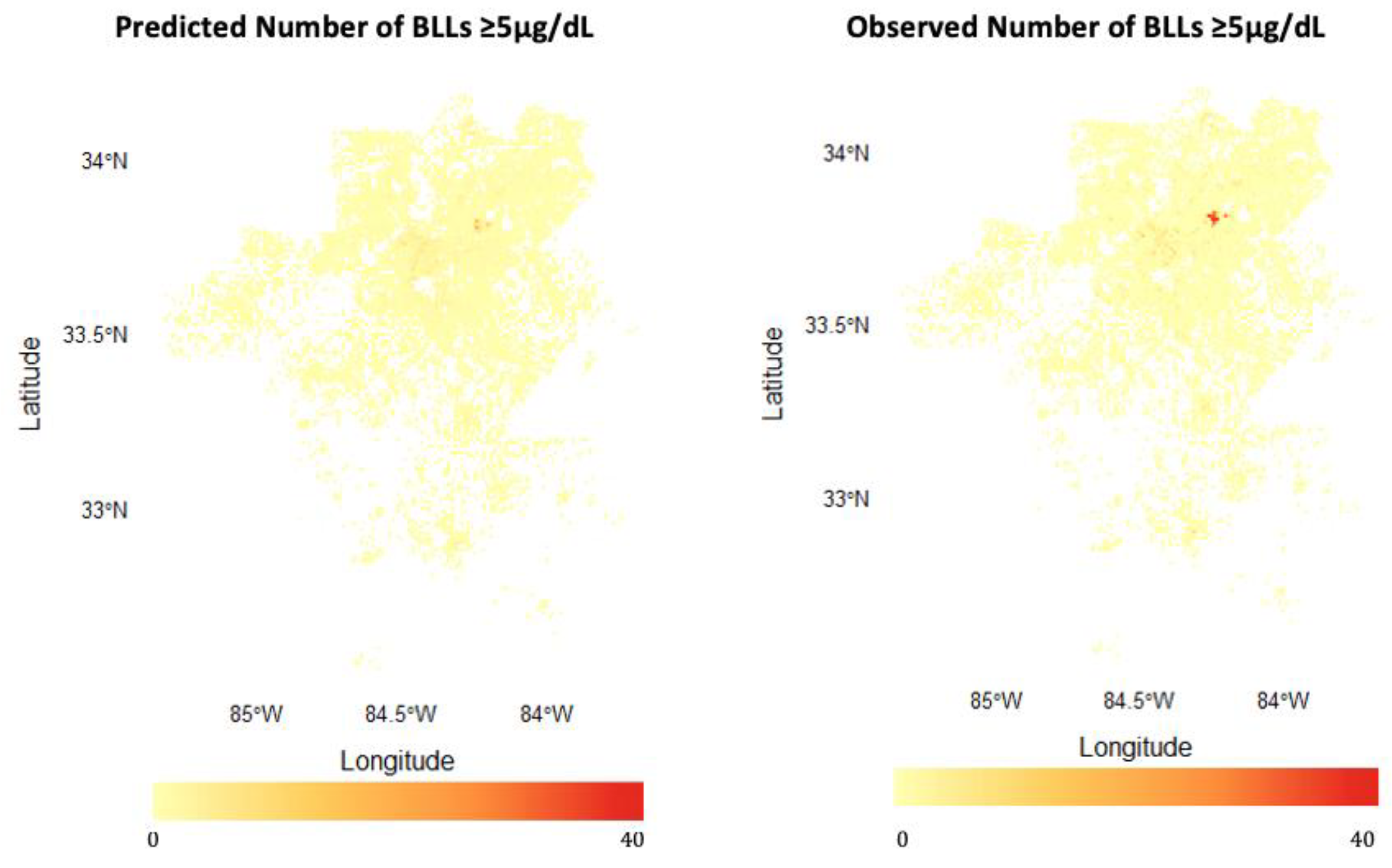
3.1.2. Predicting BLLs ≥5 µg/dL
4. Discussion
4.1. Model Performance: Strengths and Limitations
4.2. Important Predictors among BLLs ≥2–<5 µg/dL vs. BLLs ≥5 µg/dL
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nigg, J.T.; Knottnerus, G.M.; Martel, M.M.; Nikolas, M.; Cavanagh, K.; Karmaus, W.; Rappley, M.D. Low Blood Lead Levels Associated with Clinically Diagnosed Attention-Deficit/Hyperactivity Disorder and Mediated by Weak Cognitive Control. Biol. Psychiatry 2008, 63, 325–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanphear, B.P.; Dietrich, K.; Auinger, P.; Cox, C. Cognitive Deficits Associated with Blood Lead Concentrations <10 Microg/DL in US Children and Adolescents. Public Health Rep. 2000, 115, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Surkan, P.J.; Zhang, A.; Trachtenberg, F.; Daniel, D.B.; McKinlay, S.; Bellinger, D.C. Neuropsychological Function in Children with Blood Lead Levels <10 Microg/DL. Neurotoxicology 2007, 28, 1170–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vorvolakos, T.; Arseniou, S.; Samakouri, M. There is no safe threshold for lead exposure: A literature review. Psychiatriki 2016, 27, 204–214. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, S.G.; Weiss, B. A rationale for lowering the blood lead action level from 10 to 2 μg/dL. Neurotoxicology 2006, 27, 693–701. [Google Scholar] [CrossRef] [Green Version]
- Ruckart, P.Z.; Jones, R.L.; Courtney, J.G.; LeBlanc, T.T.; Jackson, W.; Karwowski, M.P.; Cheng, P.-Y.; Allwood, P.; Svendsen, E.R.; Breysse, P.N. Update of the Blood Lead Reference Value—United States, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1509–1512. [Google Scholar] [CrossRef]
- Pirkle, J.L.; Brody, D.J.; Gunter, E.W.; Kramer, R.A.; Paschal, D.C.; Flegal, K.M.; Matte, T.D. The Decline in Blood Lead Levels in the United States. The National Health and Nutrition Examination Surveys (NHANES). JAMA 1994, 272, 284–291. [Google Scholar] [CrossRef]
- Egan, K.B.; Cornwell, C.R.; Courtney, J.G.; Ettinger, A.S. Blood Lead Levels in U.S. Children Ages 1–11 Years, 1976–2016. Environ. Health Perspect. 2021, 129, 37003. [Google Scholar] [CrossRef]
- Woolf, A.D.; Brown, S.M.J. Old Adversary, New Challenges: Childhood Lead Exposure and Testing. Pediatrics 2022, 149, e2021055944. [Google Scholar] [CrossRef]
- Council on Environmental Health, American Academy of Pediatrics. Prevention of Childhood Lead Toxicity. Pediatrics 2016, 138, e20161493. [Google Scholar] [CrossRef] [Green Version]
- Bellinger, D.C. A Strategy for Comparing the Contributions of Environmental Chemicals and Other Risk Factors to Neurodevelopment of Children. Environ. Health Perspect. 2012, 120, 501–507. [Google Scholar] [CrossRef]
- Rogan, W.J.; Dietrich, K.N.; Ware, J.H.; Dockery, D.W.; Salganik, M.; Radcliffe, J.; Jones, R.L.; Ragan, N.B.; Chisolm, J.J.; Rhoads, G.G. The Effect of Chelation Therapy with Succimer on Neuropsychological Development in Children Exposed to Lead. N. Engl. J. Med. 2001, 344, 1421–1426. [Google Scholar] [CrossRef] [Green Version]
- Honsberger, K.; McCaman, L.; VanLandeghem, K. State Strategies to Improve Childhood Lead Screening and Treatment Services under Medicaid and CHIP. In A Publication of the National Academy for State Health Policy; The National Academies Press: Washington, DC, USA, 2018. [Google Scholar]
- Beidinger-Burnett, H.; Ahern, L.; Ngai, M.; Filippelli, G.; Sisk, M. Inconsistent Screening for Lead Endangers Vulnerable Children: Policy Lessons from South Bend and Saint Joseph County, Indiana, USA. J. Public Health Policy 2019, 40, 103–113. [Google Scholar] [CrossRef] [PubMed]
- New York State Department of Health. Guidelines for Health Care Providers for the Prevention, Identification, and Management of Lead Exposure in Children. Available online: https://www.health.ny.gov/publications/6671.pdf (accessed on 31 December 2022).
- Healthy Homes and Lead Poisoning Prevention. Available online: https://dph.georgia.gov/environmental-health/healthy-homes-and-lead-poisoning-prevention (accessed on 26 December 2022).
- Erie County Environmental Health Department. Lead Poisoning Primary Prevention & Primary Initiative Programs. Available online: https://www3.erie.gov/envhealth/lead-poisoning-prevention (accessed on 31 December 2022).
- Potash, E.; Ghani, R.; Walsh, J.; Jorgensen, E.; Lohff, C.; Prachand, N.; Mansour, R. Validation of a Machine Learning Model to Predict Childhood Lead Poisoning. JAMA Netw. Open 2020, 3, e2012734. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Taylor, M.P.; Aelion, C.M.; Dong, C. Novel Application of Machine Learning Algorithms and Model-Agnostic Methods to Identify Factors Influencing Childhood Blood Lead Levels. Environ. Sci. Technol. 2021, 55, 13387–13399. [Google Scholar] [CrossRef] [PubMed]
- Lobo, G.; Kalyan, B.; Gadgil, A. Predicting Childhood Lead Exposure at an Aggregated Level Using Machine Learning. Int. J. Hyg. Environ. Health 2021, 238, 113862. [Google Scholar] [CrossRef]
- Dickinson-Copeland, C.; Immergluck, L.; Britez, M.; Yan, F.; Geng, R.; Edelson, M.; Kendrick-Allwood, S.; Kordas, K. Increased Risk of Sub-Clinical Blood Lead Levels in the 20-County Metro Atlanta, Georgia Area—A Laboratory Surveillance-Based Study. Int. J. Environ. Res. Public Health 2021, 18, 5163. [Google Scholar] [CrossRef]
- Hauptman, M.; Bruccoleri, R.; Woolf, A.D. An Update on Childhood Lead Poisoning. Clin. Pediatr. Emerg. Med. 2017, 18, 181–192. [Google Scholar] [CrossRef]
- Aelion, C.; Davis, H. Blood lead levels in children in urban and rural areas: Using multilevel modeling to investigate impacts of gender, race, poverty, and the environment. Sci. Total. Environ. 2019, 694, 133783. [Google Scholar] [CrossRef]
- Kamai, E.M.; Daniels, J.L.; Delamater, P.L.; Lanphear, B.P.; MacDonald Gibson, J.; Richardson, D.B. Patterns of Children’s Blood Lead Screening and Blood Lead Levels in North Carolina, 2011–2018—Who Is Tested, Who Is Missed? Environ. Health Perspect. 2022, 130, 67002. [Google Scholar] [CrossRef]
- Brink, L.L.; Talbott, E.O.; Sharma, R.K.; Marsh, G.M.; Wu, W.C.; Rager, J.R.; Strosnider, H.M. Do US Ambient Air Lead Levels Have a Significant Impact on Childhood Blood Lead Levels: Results of a National Study. J. Environ. Public Health 2013, 2013, 278042. [Google Scholar] [CrossRef] [Green Version]
- Urban Institute. Simulating the 2020 Census: Miscounts and the Fairness of Outcomes. Available online: https://www.urban.org/research/publication/simulating-2020-census-miscounts-and-fairness-outcomes (accessed on 26 December 2022).
- Wiese, D.; Escalante, A.; Murphy, H.; Henry, K.A.; Gutierrez-Velez, V.H. Integrating Environmental and Neighborhood Factors in MaxEnt Modeling to Predict Species Distributions: A Case Study of Aedes Albopictus in Southeastern Pennsylvania. PLoS ONE 2019, 14, e0223821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toole, J.L.; Colak, S.; Alhasoun, F.; Evsukoff, A.; Gonzalez, M.C. The Path Most Travelled: Mining Road Usage Patterns from Massive Call Data. arXiv 2014, arXiv:1403.0636. [Google Scholar]
- Fernández, I.C.; Wu, J. Assessing environmental inequalities in the city of Santiago (Chile) with a hierarchical multiscale approach. Appl. Geogr. 2016, 74, 160–169. [Google Scholar] [CrossRef]
- Han, I.; Whitworth, K.W.; Zhang, X.; Afshar, M.; Berens, P.D.; Symanski, E. Characterization of Urinary Concentrations of Heavy Metals among Socioeconomically Disadvantaged Black Pregnant Women. Environ. Monit. Assess. 2020, 192, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masri, S.; LeBrón, A.; Logue, M.; Valencia, E.; Ruiz, A.; Reyes, A.; Lawrence, J.M.; Wu, J. Social and Spatial Distribution of Soil Lead Concentrations in the City of Santa Ana, California: Implications for Health Inequities. Sci. Total Environ. 2020, 743, 140764. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.E.; Wolfe, S.E. Lead Exposure, Concentrated Disadvantage, and Violent Crime Rates. Justice Q. 2020, 37, 1–24. [Google Scholar] [CrossRef]
- Yeter, D.; Banks, E.C.; Aschner, M. Disparity in Risk Factor Severity for Early Childhood Blood Lead among Predominantly African-American Black Children: The 1999 to 2010 US NHANES. Int. J. Environ. Res. Public Health 2020, 17, 1552. [Google Scholar] [CrossRef] [Green Version]
- Income Inequality in, U.S. Is at a Five-Decade High, Census Data Show. The Washington Post. 26 September 2019. Available online: https://www.washingtonpost.com/business/2019/09/26/income-inequality-america-highest-its-been-since-census-started-tracking-it-data-show/ (accessed on 26 December 2022).
- US EPA. Toxics Release Inventory (TRI) Program. Available online: https://www.epa.gov/toxics-release-inventory-tri-program (accessed on 26 December 2022).
- Boutwell, B.B.; Nelson, E.J.; Emo, B.; Vaughn, M.G.; Schootman, M.; Rosenfeld, R.; Lewis, R. The Intersection of Aggregate-Level Lead Exposure and Crime. Environ. Res. 2016, 148, 79–85. [Google Scholar] [CrossRef]
- Zhang, Y.; O’Connor, D.; Xu, W.; Hou, D. Blood Lead Levels among Chinese Children: The Shifting Influence of Industry, Traffic, and e-Waste over Three Decades. Environ. Int. 2020, 135, 105379. [Google Scholar] [CrossRef]
- Bowling Green State University; Merkley, B.; Farver, J.R. Sources and Pathways of Lead Contamination in Urban Community Gardens in Toledo, Ohio. Ph.D. Thesis, Bowling Green State University, Bowling Green, OH, USA, 2018. [Google Scholar]
- Ahmed, Z.U.; Sun, K.; Shelly, M.; Mu, L. Explainable Artificial Intelligence (XAI) for Exploring Spatial Variability of Lung and Bronchus Cancer (LBC) Mortality Rates in the Contiguous USA. Sci. Rep. 2021, 11, 24090. [Google Scholar] [CrossRef] [PubMed]
- van der Laan, M.J.; Polley, E.C.; Hubbard, A.E. Super Learner. Stat. Appl. Genet. Mol. Biol. 2007, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Stacked Regressions. Mach. Learn. 1996, 24, 49–64. [Google Scholar] [CrossRef] [Green Version]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning: With Applications in R. In Springer Texts in Statistics, 2nd ed.; Springer: New York, NY, USA, 2021. [Google Scholar]
- Kuhn, M.; Johnson, K. Applied Predictive Modeling; Springer: New York, NY, USA, 2013. [Google Scholar]
- LeDell, E.; Gill, N.; Aiello, S.; Fu, A.; Candel, A.; Click, C.; Kraljevic, T.; Nykodym, T.; Aboyoun, P.; Kurka, M.; et al. Package ′h2o′; R Foundation for Statistical Computing: Vienna, Austria, 2018; p. 17. [Google Scholar]
- 2500 Georgia Children Test Positive for Lead Exposure. Available online: https://www.wfaa.com/article/news/local/2500-georgia-children-test-positive-for-lead-exposure/85-159845471 (accessed on 26 December 2022).
- Pavilonis, B.; Maroko, A.; Cheng, Z. Lead in New York City’s Soils: Population Growth, Land Use, and Contamination. Int. J. Hyg. Environ. Health 2020, 229, 113564. [Google Scholar] [CrossRef]
- Castro, I.E.; Larsen, D.A.; Hruska, B.; Parsons, P.J.; Palmer, C.D.; Gump, B.B. Variability in the Spatial Density of Vacant Properties Contributes to Background Lead (Pb) Exposure in Children. Environ. Res. 2019, 170, 463–471. [Google Scholar] [CrossRef]
- Dogic, S. Snapshot: Georgia’s Medicaid Enrollment and Spending. 2022. Available online: https://cslf.gsu.edu/interactive-tableau-medicaid/ (accessed on 1 October 2022).

| <2 µg/dL (N = 38,124) | ≥2 to <5 µg/dL (N = 52,522) | ≥5 µg/dL (N = 2146) | Overall (N = 92,792) | |
|---|---|---|---|---|
| Child age in months | ||||
| Mean (SD) | 24.5 (14.0) | 26.7 (15.3) | 29.4 (16.3) | 25.9 (14.8) |
| Median [Min, Max] | 24.0 [0, 72.0] | 24.0 [0, 72.0] | 25.0 [0, 72.0] | 24.0 [0, 72.0] |
| Child Sex | ||||
| Female | 18,600 (48.8%) | 25,468 (48.5%) | 1020 (47.5%) | 45,088 (48.4%) |
| Missing | 36 (0.1%) | 69 (0.1%) | 6 (0.3%) | 545 (0.6%) |
| Child Race | ||||
| Black | 18,985 (49.8%) | 12,006 (22.9%) | 604 (28.1%) | 31,595 (33.9%) |
| White | 5272 (13.8%) | 3507 (6.7%) | 189 (8.8%) | 8968 (9.6%) |
| Other | 6288 (16.5%) | 3536 (6.7%) | 403 (18.8%) | 10,227 (11.0%) |
| Not Reported | 7491 (19.6%) | 32,122 (61.2%) | 927 (43.2%) | 40,540 (43.5%) |
| Missing | 88 (0.2%) | 1351 (2.6%) | 23 (1.1%) | 1896 (2.0%) |
| Receiving Medicaid | ||||
| Yes | 27,526 (72.2%) | 39,413 (75.0%) | 1456 (67.8%) | 68,395 (73.4%) |
| Urban Zip Code | ||||
| Yes | 7944 (20.8%) | 12,519 (23.8%) | 387 (18.0%) | 20,957 (22.5%) |
| Predictors | Mean, Median (Min–Max) |
|---|---|
| Number of children with BLLs >= 5 µg/dL | 0.29, 0.0 (0.0–36) |
| Number of children with BLLs >2 to <5 µg/dL | 7.2, 2.0 (0.0–63) |
| Percentage of the population below the poverty threshold | 0.14, 0.11 (0.0–1.0) |
| Percentage of the population that is Black | 0.36, 0.27 (0.0–1.0) |
| Percentage of the population that is White | 0.55, 0.59 (0–1.0) |
| Percentage of houses built before 1980 | 0.31, 0.24 (0–1.0) |
| Percentage of the population >25 years old without an HS diploma | 0.12, 0.10 (0–0.74) |
| Gini index | 0.41, 0.41 (0.30–0.66) |
| Crime index | 120, 98 (5.0–710) |
| Road network density | 470, 470 (31–1600) |
| EPA TRI density | 840, 380 (0–7100) |
| EPA Water TRI density | 46,000, 32,000 (0–400,000) |
| EPA Air TRI density | 2800, 1700 (0–18,000) |
| EPA Land TRI density | 8200, 3000 (0–100,000) |
| Total Housing Units | 1100, 980 (94–4200) |
| BLLs ≥2–<5 µg/dL | BLL ≥5 µg/dL | |||
|---|---|---|---|---|
| RMSE | MAE | RMSE | MAE | |
| Gradient Boosting Machine (GBM) | ||||
| Training | 8.55 | 2.92 | 0.38 | 0.19 |
| Validation | 13.04 | 5.48 | 1.32 | 0.42 |
| Test | 15.51 | 6.03 | 1.57 | 0.46 |
| Elastic Net Generalized Linear Model (GLM) | ||||
| Training | 15.81 | 7.41 | 1.21 | 0.43 |
| Validation | 13.79 | 7.20 | 1.48 | 0.45 |
| Test | 17.07 | 7.60 | 1.65 | 0.45 |
| Deep Neural Network (DNN) | ||||
| Training | 11.32 | 5.42 | 1.19 | 0.37 |
| Validation | 12.96 | 5.62 | 1.44 | 0.39 |
| Test | 16.00 | 6.05 | 1.66 | 0.40 |
| Ensemble Learner [Final Model] | ||||
| Training | 9.11 | 3.93 | 0.62 | 0.23 |
| Validation | 12.74 | 5.53 | 1.33 | 0.38 |
| Test | 15.51 | 6.08 | 1.59 | 0.41 |
| Simple Median [Comparison] | ||||
| Training | 19.63 | 7.32 | 1.31 | 0.29 |
| Validation | 17.70 | 6.83 | 1.58 | 0.31 |
| Test | 20.29 | 6.93 | 1.72 | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frndak, S.; Yan, F.; Edelson, M.; Immergluck, L.C.; Kordas, K.; Idris, M.Y.; Dickinson-Copeland, C.M. Predicting Low-Level Childhood Lead Exposure in Metro Atlanta Using Ensemble Machine Learning of High-Resolution Raster Cells. Int. J. Environ. Res. Public Health 2023, 20, 4477. https://doi.org/10.3390/ijerph20054477
Frndak S, Yan F, Edelson M, Immergluck LC, Kordas K, Idris MY, Dickinson-Copeland CM. Predicting Low-Level Childhood Lead Exposure in Metro Atlanta Using Ensemble Machine Learning of High-Resolution Raster Cells. International Journal of Environmental Research and Public Health. 2023; 20(5):4477. https://doi.org/10.3390/ijerph20054477
Chicago/Turabian StyleFrndak, Seth, Fengxia Yan, Mike Edelson, Lilly Cheng Immergluck, Katarzyna Kordas, Muhammed Y. Idris, and Carmen M. Dickinson-Copeland. 2023. "Predicting Low-Level Childhood Lead Exposure in Metro Atlanta Using Ensemble Machine Learning of High-Resolution Raster Cells" International Journal of Environmental Research and Public Health 20, no. 5: 4477. https://doi.org/10.3390/ijerph20054477
APA StyleFrndak, S., Yan, F., Edelson, M., Immergluck, L. C., Kordas, K., Idris, M. Y., & Dickinson-Copeland, C. M. (2023). Predicting Low-Level Childhood Lead Exposure in Metro Atlanta Using Ensemble Machine Learning of High-Resolution Raster Cells. International Journal of Environmental Research and Public Health, 20(5), 4477. https://doi.org/10.3390/ijerph20054477







