Spatial Distribution of COVID-19 Hospitalizations and Associated Risk Factors in Health Insurance Data Using Bayesian Spatial Modelling
Abstract
1. Introduction
2. Methods
2.1. Data
2.2. Statistical Analysis
2.3. Regression Analysis
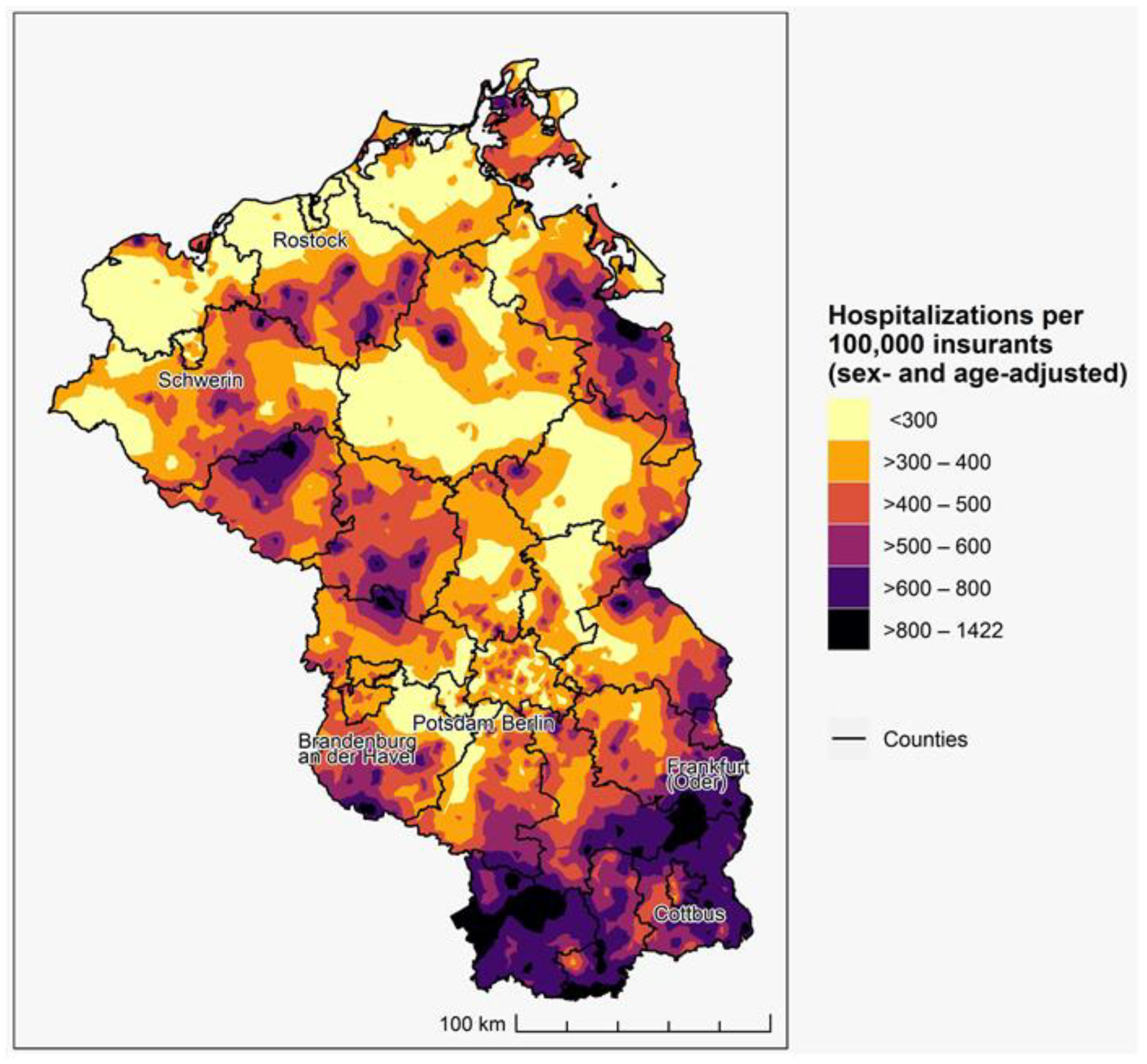
3. Results
3.1. Spatial Distribution of Accumulated COVID-19 Incidence 2021
3.2. Risk Factors for COVID-19 Hospitalizations
4. Discussion
5. Limitations
- The database of AOK Nordost does not contain any information on vaccination status of its insurants. Logically, the positive effect of vaccination could not be quantified. It would have been interesting to quantify the effect of vaccination with regards to date of vaccination, number of doses, and pre-existing conditions on COVID-19 hospitalizations. Such an approach could help to determine in which groups with specific underlying medical conditions vaccination is more effective than in others.
- Although as cases we selected only those persons who have a laboratory-confirmed diagnosis of COVID-19 as the primary code in addition to a secondary diagnosis of viral pneumonia or respiratory syndrome, it is not clear how high the quality of diagnosis actually is, e.g., COVID-19 being detected as a by-product of another reason for hospital admission.
- AOK Nordost is northeast Germany‘s largest health insurance provider, covering appr. 25% of the inhabitants. However, large sociodemographic differences of members of different health insurance providers exist, with the AOK Nordost having a higher proportion of elderly and chronically ill persons. As a result, our analysis may not be representative of the whole population. While the prevalence rates may be slightly higher than for all statutory health insurants, the regional distribution of diseases is generally comparable to those of all statutory health insurants [26,44,45,46]. As a result, the general distribution of COVID-19 hospitalizations may be slightly higher than for all statutory health insurants, but the regional distribution is expected to still be comparable.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sohrabi, C.; Alsafi, Z.; O’Neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef]
- United Nations. UN Response to COVID-19. Available online: https://www.un.org/en/coronavirus/UN-response (accessed on 8 December 2022).
- Aral, N.; Bakir, H. Spatiotemporal Analysis of Covid-19 in Turkey. Sustain. Cities Soc. 2022, 76, 103421. [Google Scholar] [CrossRef]
- Ärzteblatt.de Rückblick 2020: Die Welt im Griff des Virus. Available online: https://www.aerzteblatt.de/nachrichten/119821/Rueckblick-2020-Die-Welt-im-Griff-des-Virus (accessed on 29 November 2022).
- Felbermayr, G.; Hinz, J.; Chowdhry, S. Après-ski: The spread of coronavirus from Ischgl through Germany. Ger. Econ. Rev. 2021, 22, 415–446. [Google Scholar] [CrossRef]
- Steiger, E.; Mussgnug, T.; Kroll, L.E. Causal graph analysis of COVID-19 observational data in German districts reveals effects of determining factors on reported case numbers. PLoS ONE 2021, 16, e0237277. [Google Scholar] [CrossRef]
- Scarpone, C.; Brinkmann, S.T.; Große, T.; Sonnenwald, D.; Fuchs, M.; Walker, B.B. A multimethod approach for county-scale geospatial analysis of emerging infectious diseases: A cross-sectional case study of COVID-19 incidence in Germany. Int. J. Health Geogr. 2020, 19, 32. [Google Scholar] [CrossRef]
- Kuebart, A.; Stabler, M. Infectious Diseases as Socio-Spatial Processes: The COVID-19 Outbreak In Germany. Tijdschr. Econ. Soc. Geogr. 2020, 111, 482–496. [Google Scholar] [CrossRef]
- Plümper, T.; Neumayer, E. The pandemic predominantly hits poor neighbourhoods? SARS-CoV-2 infections and COVID-19 fatalities in German districts. Eur. J. Public Health 2020, 30, 1176–1180. [Google Scholar] [CrossRef]
- Wachtler, B.; Michalski, N.; Nowossadeck, E.; Diercke, M.; Wahrendorf, M.; Santos-Hövener, C.; Lampert, T.; Hoebel, J. Sozioökonomische Ungleichheit im Infektionsrisiko mit SARS-CoV-2—Erste Ergebnisse einer Analyse der Meldedaten für Deutschland; The Robert Koch Institute: Berlin, Germany, 2020. [Google Scholar] [CrossRef]
- Siljander, M.; Uusitalo, R.; Pellikka, P.; Isosomppi, S.; Vapalahti, O. Spatiotemporal clustering patterns and sociodemographic determinants of COVID-19 (SARS-CoV-2) infections in Helsinki, Finland. Spat. Spatiotemporal. Epidemiol. 2022, 41, 100493. [Google Scholar] [CrossRef]
- Nazia, N.; Law, J.; Butt, Z.A. Spatiotemporal clusters and the socioeconomic determinants of COVID-19 in Toronto neighbourhoods, Canada. Spat. Spatiotemporal. Epidemiol. 2022, 43, 100534. [Google Scholar] [CrossRef]
- Lu, Y.; Cai, G.; Hu, Z.; He, F.; Jiang, Y.; Aoyagi, K. Exploring spatiotemporal patterns of COVID-19 infection in Nagasaki Prefecture in Japan using prospective space-time scan statistics from April 2020 to April 2022. Arch. Public Health 2022, 80, 176. [Google Scholar] [CrossRef]
- Iyanda, A.E.; Boakye, K.A.; Lu, Y.; Oppong, J.R. Racial/Ethnic Heterogeneity and Rural-Urban Disparity of COVID-19 Case Fatality Ratio in the USA: A Negative Binomial and GIS-Based Analysis. J. Racial Ethn. Health Disparities 2022, 9, 708–721. [Google Scholar] [CrossRef]
- Lee, J.; Ramírez, I.J. Geography of Disparity: Connecting COVID-19 Vulnerability and Social Determinants of Health in Colorado. Behav. Med. 2022, 48, 72–84. [Google Scholar] [CrossRef]
- Rohleder, S.; Bozorgmehr, K. Monitoring the spatiotemporal epidemiology of Covid-19 incidence and mortality: A small-area analysis in Germany. Spat. Spatiotemporal. Epidemiol. 2021, 38, 100433. [Google Scholar] [CrossRef] [PubMed]
- Adin, A.; Congdon, P.; Santafé, G.; Ugarte, M.D. Identifying extreme COVID-19 mortality risks in English small areas: A disease cluster approach. Stoch. Environ. Res. Risk Assess. 2022, 36, 2995–3010. [Google Scholar] [CrossRef] [PubMed]
- Dhewantara, P.W.; Puspita, T.; Marina, R.; Lasut, D.; Riandi, M.U.; Wahono, T.; Ridwan, W.; Ruliansyah, A. Geo-clusters and socio-demographic profiles at village-level associated with COVID-19 incidence in the metropolitan city of Jakarta: An ecological study. Transbound. Emerg. Dis. 2022, 69, e362–e373. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.K.; Peterson, E.R.; Balan, D.; Jones, L.; Culp, G.M.; Fine, A.D.; Kulldorff, M. Detecting COVID-19 Clusters at High Spatiotemporal Resolution, New York City, New York, USA, June–July 2020. Emerg. Infect. Dis. 2021, 27, 1500–1504. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.; O’Keefe, K.J.; Wei, W.; Arshad, S.; Gruebner, O. Geospatial Analysis of COVID-19: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 2336. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.; Reed, A.B.; Ponzo, S.; Yassaee, A.; Aral, M.; Plans, D.; Labrique, A.; Mohan, D. Population risk factors for severe disease and mortality in COVID-19: A global systematic review and meta-analysis. PLoS ONE 2021, 16, e0247461. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, M.; Nguyen, D.T.; Vahidy, F.S.; Graviss, E.A. Risk factors for severity of COVID-19 in hospital patients age 18–29 years. PLoS ONE 2021, 16, e0255544. [Google Scholar] [CrossRef]
- Meurisse, M.; Lajot, A.; Devleesschauwer, B.; van Cauteren, D.; van Oyen, H.; van den Borre, L.; Brondeel, R. The association between area deprivation and COVID-19 incidence: A municipality-level spatio-temporal study in Belgium, 2020–2021. Arch. Public Health 2022, 80, 109. [Google Scholar] [CrossRef] [PubMed]
- Madhav, K.C.; Oral, E.; Straif-Bourgeois, S.; Rung, A.L.; Peters, E.S. The effect of area deprivation on COVID-19 risk in Louisiana. PLoS ONE 2020, 15, e0243028. [Google Scholar] [CrossRef]
- Maier, W.; Fairburn, J.; Mielck, A. Regionale Deprivation und Mortalität in Bayern. Entwicklung eines ’Index Multipler Deprivation’ auf Gemeindeebene. Gesundheitswesen 2012, 74, 416–425. [Google Scholar] [CrossRef]
- Kauhl, B.; Maier, W.; Schweikart, J.; Keste, A.; Moskwyn, M. Who is where at risk for Chronic Obstructive Pulmonary Disease? A spatial epidemiological analysis of health insurance claims for COPD in Northeastern Germany. PLoS ONE 2018, 13, e0190865. [Google Scholar] [CrossRef] [PubMed]
- Odoi, A.; Busingye, D. Neighborhood geographic disparities in heart attack and stroke mortality: Comparison of global and local modeling approaches. Spat. Spatiotemporal. Epidemiol. 2014, 11, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Lawson, A.B.; Biggeri, A.B.; Boehning, D.; Lesaffre, E.; Viel, J.F.; Clark, A.; Schlattmann, P.; Divino, F. Disease mapping models: An empirical evaluation. Disease Mapping Collaborative Group. Stat. Med. 2000, 19, 2217–2241. [Google Scholar] [CrossRef]
- Dwyer-Lindgren, L.; Cork, M.A.; Sligar, A.; Steuben, K.M.; Wilson, K.F.; Provost, N.R.; Mayala, B.K.; VanderHeide, J.D.; Collison, M.L.; Hall, J.B.; et al. Mapping HIV prevalence in sub-Saharan Africa between 2000 and 2017. Nature 2019, 570, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Kauhl, B.; Vietzke, M.; König, J.; Schönfelder, M. Exploring regional and sociodemographic disparities associated with unenrollment for the disease management program for type 2 Diabetes Mellitus using Bayesian spatial modelling. Res. Health Serv. Reg 2022, 1, 7. [Google Scholar] [CrossRef]
- Lindgren, F.; Rue, H. Bayesian Spatial Modelling with R—INLA. J. Stat. Soft. 2015, 63, 1–25. [Google Scholar] [CrossRef]
- Wickham, H.; Winston, C.; Henry, L.; Lin Pedersen, T. Package ‘ggplot2’. Create Elegant Data Visualisations using the Grammar of Graphics. Version 2.1. 2016. Available online: https://cran.r-project.org/package=ggplot2/ggplot2.pdf (accessed on 5 December 2022).
- Bland, J.M.; Altman, D.G. Statistics notes. The odds ratio. BMJ 2000, 320, 1468. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.P.; Jin, R.; Grunkemeier, G.L. Understanding logistic regression analysis in clinical reports: An introduction. Ann. Thorac. Surg. 2003, 75, 753–757. [Google Scholar] [CrossRef]
- Heiberger, R.M. Package ‘HH’. Statistival Analysis and Data Display: Heidberger and Holland. Available online: https://cran.r-project.org/web/packages/HH/HH.pdf (accessed on 5 December 2022).
- Schüler, L.; Calabrese, J.M.; Attinger, S. Data driven high resolution modeling and spatial analyses of the COVID-19 pandemic in Germany. PLoS ONE 2021, 16, e0254660. [Google Scholar] [CrossRef]
- Dragano, N.; Rupprecht, C.J.; Dortmann, O.; Scheider, M.; Wahrendorf, M. Higher risk of COVID-19 hospitalization for unemployed: An analysis of health insurance data from 1.28 million insured individuals in Germany. Bundesgesundheitsblatt Gesundh. Gesundh. 2021, 64, 314–321. [Google Scholar]
- Mena, G.E.; Martinez, P.P.; Mahmud, A.S.; Marquet, P.A.; Buckee, C.O.; Santillana, M. Socioeconomic status determines COVID-19 incidence and related mortality in Santiago, Chile. Science 2021, 372, eabg5298. [Google Scholar] [CrossRef]
- Doblhammer, G.; Kreft, D.; Reinke, C. Regional Characteristics of the Second Wave of SARS-CoV-2 Infections and COVID-19 Deaths in Germany. Int. J. Environ. Res. Public Health 2021, 18, 10663. [Google Scholar] [CrossRef]
- Hayward, S.E.; Deal, A.; Cheng, C.; Crawshaw, A.; Orcutt, M.; Vandrevala, T.F.; Norredam, M.; Carballo, M.; Ciftci, Y.; Requena-Méndez, A.; et al. Clinical outcomes and risk factors for COVID-19 among migrant populations in high-income countries: A systematic review. J. Migr. Health 2021, 3, 100041. [Google Scholar] [CrossRef]
- Said, D.; Suwono, B.; Schweickert, B.; Schönfeld, V.; Eckmanns, T.; Haller, S. SARS-CoV-2 Outbreaks in Care Homes for the Elderly and Disabled in Germany. Dtsch. Arztebl. Int. 2022, 119, 486–487. [Google Scholar] [CrossRef] [PubMed]
- McGowan, V.J.; Bambra, C. COVID-19 mortality and deprivation: Pandemic, syndemic, and endemic health inequalities. Lancet Public Health 2022, 7, e966–e975. [Google Scholar] [CrossRef] [PubMed]
- Salkeld, D.J.; Antolin, M.F. Ecological Fallacy and Aggregated Data: A Case Study of Fried Chicken Restaurants, Obesity and Lyme Disease. Ecohealth 2020, 17, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Kauhl, B.; Schweikart, J.; Krafft, T.; Keste, A.; Moskwyn, M. Do the risk factors for type 2 diabetes mellitus vary by location? A spatial analysis of health insurance claims in Northeastern Germany using kernel density estimation and geographically weighted regression. Int. J. Health. Geogr. 2016, 15, 1–12. [Google Scholar] [CrossRef]
- Goffrier, B.; Schulz, M.; Bätzing-Feigenbaum, J. Administrative Prevalence and Incidence of Diabetes Mellitus in Germany, 2009–2015. Available online: https://www.versorgungsatlas.de/fileadmin/ziva_docs/79/VA-79-Abstract_EN_Final.pdf (accessed on 13 December 2022).
- Akmatov, M.K.; Steffen, A.; Holstiege, J.; Bätzing, J. Die Chronisch Obstruktive Lungenerkrankung (COPD) in der Ambulanten Versorgung in Deutschland–Zeitliche Trends und Kleinräumige Unterschiede. 2019. Available online: https://www.versorgungsatlas.de/fileadmin/ziva_docs/99/VA_19-06_Bericht-COPD_2019-08-20_V2_1.pdf (accessed on 13 December 2022).
| Variable | Coefficient | 2.5% CI | 97.5% CI |
|---|---|---|---|
| Intercept | 0.000 | 0.000 | 0.000 |
| Sex: male (Ref. female) | 1.677 | 1.603 | 1.754 |
| Age (in years) | 1.039 | 1.037 | 1.041 |
| Foreign citizensip (Ref. German) | 2.502 | 2.340 | 2.675 |
| Unemployed (Ref. not unemployed) | 1.296 | 1.196 | 1.404 |
| Nursing home (Ref. not in nursing home) | 1.759 | 1.634 | 1.893 |
| I Certain infectious and parasitic diseases | 1.236 | 1.165 | 1.311 |
| II Neoplasms | 0.964 | 0.913 | 1.016 |
| III Diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism | 1.293 | 1.224 | 1.368 |
| IV Endocrine, nutritional and metabolic diseases | 1.355 | 1.269 | 1.449 |
| V Mental and behavioural disorders | 1.007 | 0.959 | 1.058 |
| VI Diseases of the nervous system | 1.284 | 1.223 | 1.350 |
| VII Diseases of the eye and adnexa | 0.947 | 0.900 | 0.997 |
| VIII Diseases of the ear and mastoid process | 0.942 | 0.890 | 0.997 |
| IX Diseases of the circulatory system | 1.214 | 1.124 | 1.313 |
| X Diseases of the respiratory system | 1.232 | 1.175 | 1.293 |
| XI Diseases of the digestive system | 1.012 | 0.963 | 1.063 |
| XII Diseases of the skin and subcutaneous tissue | 1.033 | 0.979 | 1.089 |
| XIII Diseases of the musculoskeletal system and connective tissue | 1.029 | 0.974 | 1.088 |
| XIV Diseases of the genitourinary system | 1.245 | 1.182 | 1.311 |
| XV Pregnancy, childbirth and the puerperium | 1.065 | 0.772 | 1.468 |
| XVI Certain conditions originating in the perinatal period | 1.177 | 0.689 | 2.008 |
| XVII Congenital malformations, deformations and chromosomal abnormalities | 0.950 | 0.887 | 1.019 |
| XVIII Symptoms, signs and abnormal clinical and laboratory findings, not elsewhere classified | 1.162 | 1.097 | 1.230 |
| Household size | 1.058 | 0.984 | 1.135 |
| Deprivation 2nd quintile (Ref. 1st quintile) | 1.110 | 1.018 | 1.208 |
| Deprivation 3rd quintile (Ref. 1st quintile) | 1.080 | 0.989 | 1.178 |
| Deprivation 4th quintile (Ref. 1st quintile) | 1.026 | 0.932 | 1.130 |
| Deprivation 5th quintile (Ref. 1st quintile) | 1.058 | 0.984 | 1.135 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kauhl, B.; König, J.; Wolf, S. Spatial Distribution of COVID-19 Hospitalizations and Associated Risk Factors in Health Insurance Data Using Bayesian Spatial Modelling. Int. J. Environ. Res. Public Health 2023, 20, 4375. https://doi.org/10.3390/ijerph20054375
Kauhl B, König J, Wolf S. Spatial Distribution of COVID-19 Hospitalizations and Associated Risk Factors in Health Insurance Data Using Bayesian Spatial Modelling. International Journal of Environmental Research and Public Health. 2023; 20(5):4375. https://doi.org/10.3390/ijerph20054375
Chicago/Turabian StyleKauhl, Boris, Jörg König, and Sandra Wolf. 2023. "Spatial Distribution of COVID-19 Hospitalizations and Associated Risk Factors in Health Insurance Data Using Bayesian Spatial Modelling" International Journal of Environmental Research and Public Health 20, no. 5: 4375. https://doi.org/10.3390/ijerph20054375
APA StyleKauhl, B., König, J., & Wolf, S. (2023). Spatial Distribution of COVID-19 Hospitalizations and Associated Risk Factors in Health Insurance Data Using Bayesian Spatial Modelling. International Journal of Environmental Research and Public Health, 20(5), 4375. https://doi.org/10.3390/ijerph20054375






