Effectiveness of Individual Oral Health Care Training in Hospitalized Inpatients in Geriatric Wards
Abstract
1. Introduction
2. Materials and Methods
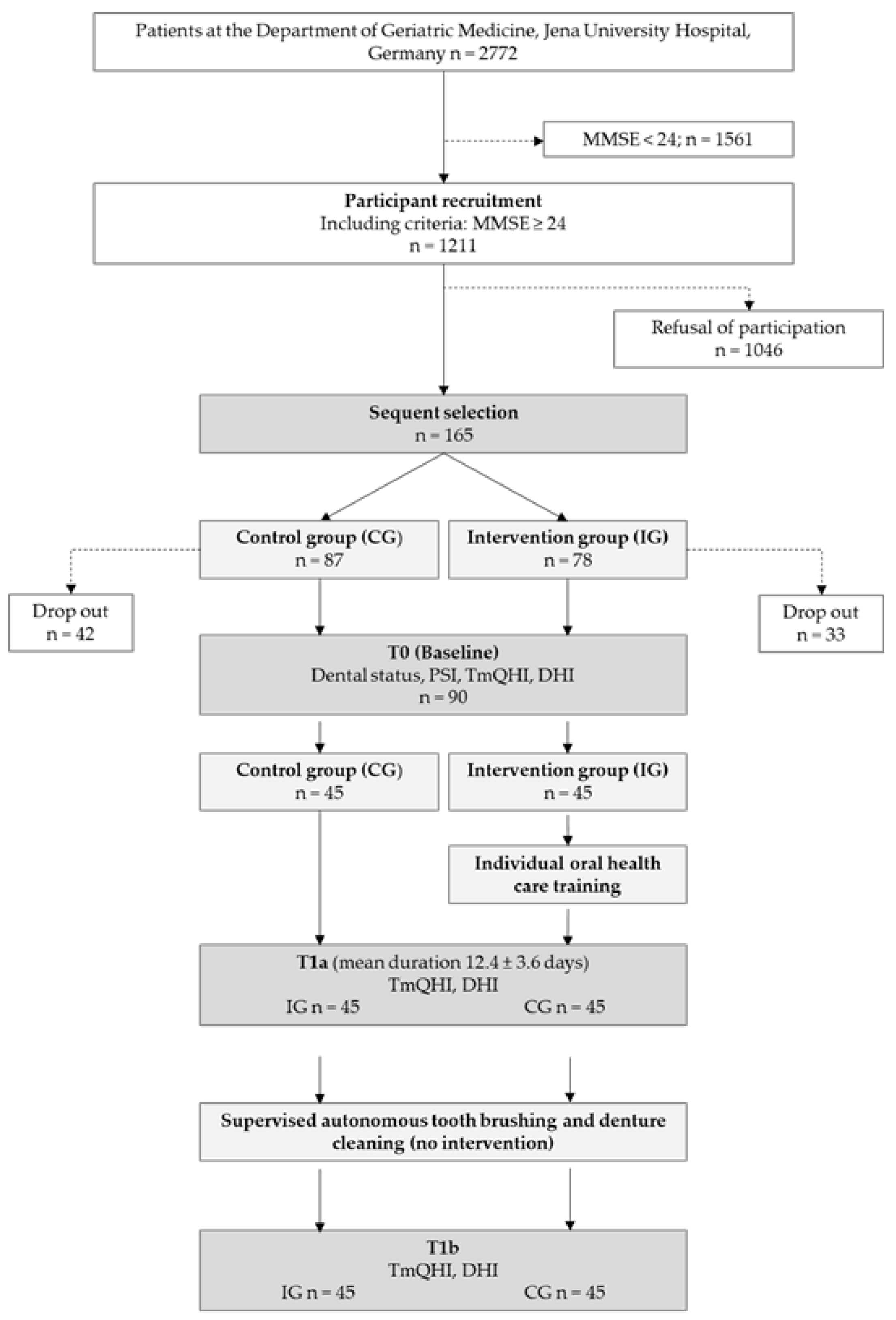
2.1. Study Sample
2.2. Oral Examinations
2.3. Intervention
2.4. Collection of Geriatric Assessment Data
2.5. Statistical Data Analysis
3. Results
3.1. Study Sample at Baseline
3.2. Evaluation of oral Hygiene Status between the Beginning (T0) and End (T1a) of Hospitalization
3.3. Evaluation of Oral Hygiene Status before and after Autonomous Tooth Brushing and Denture Cleaning (T1a-T1b)
4. Discussion
4.1. Evaluation of Oral Hygiene Status between the Beginning (T0) and end (T1a) of Hospitalization
4.2. Evaluation of Oral Hygiene Status before and after Autonomous Tooth Brushing and Denture Cleaning (T1a-T1b)
4.3. Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Branca, S.; Bennati, E.; Ferlito, L.; Spallina, G.; Cardillo, E.; Malaguarnera, M.; Motta, M. Imusce The health-care in the extreme longevity. Arch. Gerontol. Geriatr. 2009, 49, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Marengoni, A.; Angleman, S.; Melis, R.; Mangialasche, F.; Karp, A.; Garmen, A.; Meinow, B.; Fratiglioni, L. Aging with multimorbidity: A systematic review of the literature. Ageing Res. Rev. 2011, 10, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Miegel, K.; Wachtel, T. Improving the oral health of older people in long-term residential care: A review of the literature. Int. J. Older People Nurs. 2009, 4, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, I.; Müller, F.; Hopfenmüller, W. The uptake of dental services by elderly Germans. Gerodontology 2001, 18, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Jordan, R.A.; Bodechtel, C.; Hertrampf, K.; Hoffmann, T.; Kocher, T.; Nitschke, I.; Schiffner, U.; Stark, H.; Zimmer, S.; Micheelis, W. Group DVSI The Fifth German Oral Health Study (Fünfte Deutsche Mundgesundheitsstudie, DMS V)-rationale, design, and methods. BMC Oral Health 2014, 14, 161. [Google Scholar] [CrossRef] [PubMed]
- Gaszynska, E.; Szatko, F.; Godala, M.; Gaszynski, T. Oral health status, dental treatment needs, and barriers to dental care of elderly care home residents in Lodz, Poland. Clin. Interv. Aging 2014, 9, 1637. [Google Scholar] [CrossRef] [PubMed]
- Ceena, D.E.; Navya, K.; Nayak, S.U.; Shenoy, R.; Binnal, A.; Bastian, T.S. Oral health status among the geriatric population-A cross sectional study. J. Gerontol. Geriatr. 2022, 70, 164–168. [Google Scholar] [CrossRef]
- Hung, M.; Moffat, R.; Gill, G.; Lauren, E.; Ruiz-Negrón, B.; Rosales, M.N.; Richey, J.; Licari, F.W. Oral health as a gateway to overall health and well-being: Surveillance of the geriatric population in the United States. Spec. Care Dent. 2019, 39, 354–361. [Google Scholar] [CrossRef]
- Ní Chróinín, D.; Montalto, A.; Jahromi, S.; Ingham, N.; Beveridge, A.; Foltyn, P. Oral health status is associated with common medical comorbidities in older hospital inpatients. J. Am. Geriatr. Soc. 2016, 64, 1696–1700. [Google Scholar] [CrossRef]
- Lalla, E.; Papapanou, P.N. Diabetes mellitus and periodontitis: A tale of two common interrelated diseases. Nat. Rev. Endocrinol. 2011, 7, 738–748. [Google Scholar] [CrossRef]
- Gaetti-Jardim, E.; Marcelino, S.L.; Feitosa, A.C.R.; Romito, G.A.; Avila-Campos, M.J. Quantitative detection of periodontopathic bacteria in atherosclerotic plaques from coronary arteries. J. Med. Microbiol. 2009, 58 Pt 12, 1568–1575. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, L.L.; Fu, R.; Buckley, D.I.; Freeman, M.; Helfand, M. Periodontal disease and coronary heart disease incidence: A systematic review and meta-analysis. J. Gen. Intern. Med. 2008, 23, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Azarpazhooh, A.; Leake, J.L. Systematic review of the association between respiratory diseases and oral health. J. Periodontol. 2006, 77, 1465–1482. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, P.; Nilsson, E.; Forsell, M.; Johansson, O.; Hoogstraate, J. A systematic review of the preventive effect of oral hygiene on pneumonia and respiratory tract infection in elderly people in hospitals and nursing homes: Effect estimates and methodological quality of randomized controlled trials. J. Am. Geriatr. Soc. 2008, 56, 2124–2130. [Google Scholar] [CrossRef]
- Tôrres, L.H.; Tellez, M.; Hilgert, J.B.; Hugo, F.N.; de Sousa, M.D.; Ismail, A.I. Frailty, frailty components, and oral health: A systematic review. J. Am. Geriatr. Soc. 2015, 63, 2555–2562. [Google Scholar] [CrossRef]
- Hakeem, F.F.; Bernabé, E.; Sabbah, W. Association between oral health and frailty: A systematic review of longitudinal studies. Gerodontology 2019, 36, 205–215. [Google Scholar] [CrossRef]
- Ramsay, S.E.; Papachristou, E.; Watt, R.G.; Tsakos, G.; Lennon, L.T.; Papacosta, A.O.; Moynihan, P.; Sayer, A.A.; Whincup, P.H.; Wannamethee, S.G. Influence of poor oral health on physical frailty: A population-based cohort study of older British men. J. Am. Geriatr. Soc. 2018, 66, 473–479. [Google Scholar] [CrossRef]
- Maeda, K.; Mori, N. Poor oral health and mortality in geriatric patients admitted to an acute hospital: An observational study. BMC Geriatr. 2020, 20, 26. [Google Scholar] [CrossRef]
- Kossioni, A.E. The association of poor oral health parameters with malnutrition in older adults: A review considering the potential implications for cognitive impairment. Nutrients 2018, 10, 1709. [Google Scholar] [CrossRef]
- Ozkan, Y.; Ozcan, M.; Kulak, Y.; Kazazoglu, E.; Arikan, A. General health, dental status and perceived dental treatment needs of an elderly population in Istanbul. Gerodontology 2011, 28, 28–36. [Google Scholar] [CrossRef]
- Nakre, P.D.; Harikiran, A.G. Effectiveness of oral health education programs: A systematic review. J. Int. Soc. Prev. Community Dent. 2013, 3, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Schwindling, F.S.; Krisam, J.; Hassel, A.J.; Rammelsberg, P.; Zenthöfer, A. Long-term success of oral health intervention among care-dependent institutionalized seniors: Findings from a controlled clinical trial. Community Dent. Oral Epidemiol. 2018, 46, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Skorupka, W.; Zurek, K.; Kokot, T.; Nowakowska-Zajdel, E.; Fatyga, E.; Niedworok, E.; Muc-Wierzgon, M. Assessment of oral hygiene in adults. Cent. Eur. J. Public Health 2012, 20, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Hoogendam, Y.Y.; van der Lijn, F.; Vernooij, M.W.; Hofman, A.; Niessen, W.J.; van der Lugt, A.; Ikram, M.A.; van der Geest, J.N. Older age relates to worsening of fine motor skills: A population-based study of middle-aged and elderly persons. Front. Aging Neurosci. 2014, 6, 259. [Google Scholar] [CrossRef]
- Lewis, A.; Wallace, J.; Deutsch, A.; King, P. Improving the oral health of frail and functionally dependent elderly. Aust. Dent. J. 2015, 60 (Suppl. S1), 95–105. [Google Scholar] [CrossRef]
- Gati, D.; Vieira, A.R. Elderly at greater risk for root caries: A look at the multifactorial risks with emphasis on genetics susceptibility. Int. J. Dent. 2011, 2011, 647168. [Google Scholar] [CrossRef]
- Zenthöfer, A.; Rammelsberg, P.; Cabrera, T.; Schröder, J.; Hassel, A.J. Determinants of oral health-related quality of life of the institutionalized elderly. Psychogeriatrics 2014, 14, 247–254. [Google Scholar] [CrossRef]
- Porter, J.; Ntouva, A.; Read, A.; Murdoch, M.; Ola, D.; Tsakos, G. The impact of oral health on the quality of life of nursing home residents. Health Qual. Life Outcomes 2015, 13, 102. [Google Scholar] [CrossRef]
- Saliba, T.A.; Ortega, M.M.; Goya, K.K.; Moimaz, S.A.S.; Garbin, C.A.S. Influence of oral health on the quality of life of institutionalized and noninstitutionalized elderly people. Dent. Res. J. 2018, 15, 256–263. [Google Scholar]
- Sheng, X.; Xiao, X.; Song, X.; Qiao, L.; Zhang, X.; Zhong, H. Correlation between oral health and quality of life among the elderly in Southwest China from 2013 to 2015. Medicine 2018, 97, e10777. [Google Scholar] [CrossRef]
- Bilder, L.; Yavnai, N.; Zini, A. Oral health status among long-term hospitalized adults: A cross sectional study. PeerJ 2014, 2, e423. [Google Scholar] [CrossRef] [PubMed]
- Solemdal, K.; Sandvik, L.; Willumsen, T.; Mowe, M.; Hummel, T. The impact of oral health on taste ability in acutely hospitalized elderly. PLoS ONE 2012, 7, e36557. [Google Scholar] [CrossRef] [PubMed]
- Schnieder, R. Einflussfaktoren Auf Die Compliance von Patienten in Einem Geriatrischen Zentrum unter Besonderer Berücksichtigung der Mundgesundheit. Influencing Factors on the Compliance of Patients in a Geriatric Centre with Special Attention to Oral Health. Ph.D. Thesis, Charité University Hospital, Berlin, Germany, 2006. [Google Scholar]
- Pajukoski, H.; Meurman, J.H.; Snellman-Gröhn, S.; Sulkava, R. Oral health in hospitalized and nonhospitalized community-dwelling elderly patients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1999, 88, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Peltola, P.; Vehkalahti, M.M.; Wuolijoki-Saaristo, K. Oral health and treatment needs of the long-term hospitalised elderly. Gerodontology 2004, 21, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Peltola, P.; Vehkalahti, M.M.; Simoila, R. Effects of 11-month interventions on oral cleanliness among the long-term hospitalised elderly. Gerodontology 2007, 24, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Petelin, M.; Cotič, J.; Perkič, K.; Pavlič, A. Oral health of the elderly living in residential homes in Slovenia. Gerodontology 2012, 29, e447–e457. [Google Scholar] [CrossRef]
- World Health Organization. Oral Health Surveys: Basic Methods; WHO: Geneva, Swizerland, 1997.
- Meyle, J.; Jepsen, S. Der parodontale screening-index (PSI). Parodontologie 2000, 11, 17–21. [Google Scholar]
- Turesky, S.; Gilmore, N.D.; Glickman, I. Reduced plaque formation by the chloromethyl analogue of victamine C. J. Periodontol. 1970, 41, 41–43. [Google Scholar] [CrossRef]
- Wefers, K. Der “Denture Hygiene Index” (DHI). Dental. Forum. 1999, 1, 13–15. [Google Scholar]
- Srinivasan, M.; Delavy, J.; Schimmel, M.; Duong, S.; Zekry, D.; Trombert, V.; Gold, G.; Müller, F. Prevalence of oral hygiene tools amongst hospitalised elders: A cross-sectional survey. Gerodontology 2019, 36, 125–133. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Brink, T.L.; Yesavage, J.A.; Lum, O.; Heersema, P.H.; Adey, M.; Rose, T.L. Screening tests for geriatric depression. Clin. Gerontol. 1982, 1, 37–43. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Tsakos, G.; Allen, P.F.; Steele, J.G.; Locker, D. Interpreting oral health-related quality of life data. Community Dent. Oral Epidemiol. 2012, 40, 193–200. [Google Scholar] [CrossRef]
- Adachi, M.; Ishihara, K.; Abe, S.; Okuda, K.; Ishikawa, T. Effect of professional oral health care on the elderly living in nursing homes. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 94, 191–195. [Google Scholar] [CrossRef]
- Reed, R.; Broder, H.L.; Jenkins, G.; Spivack, E.; Janal, M.N. Oral health promotion among older persons and their care providers in a nursing home facility. Gerodontology 2006, 23, 73–78. [Google Scholar] [CrossRef]
- Sweeney, M.P.; Williams, C.; Kennedy, C.; Macpherson, L.M.; Turner, S.; Bagg, J. Oral health care and status of elderly care home residents in Glasgow. Community Dent. Health 2007, 24, 37–42. [Google Scholar]
- Hanne, K.; Ingelise, T.; Linda, C.; Ulrich, P.P. Oral status and the need for oral health care among patients hospitalised with acute medical conditions. J. Clin. Nurs. 2012, 21, 2851–2859. [Google Scholar] [CrossRef]
- Ghaffari, M.; Rakhshanderou, S.; Ramezankhani, A.; Buunk-Werkhoven, Y.; Noroozi, M.; Armoon, B. Are educating and promoting interventions effective in oral health?: A systematic review. Int. J. Dent. Hyg. 2018, 16, 48–58. [Google Scholar] [CrossRef]
- Gibney, J.M.; Wright, F.A.; D’Souza, M.; Naganathan, V. Improving the oral health of older people in hospital. Australas J. Ageing 2019, 38, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Supervía, A.; Aranda, D.; Márquez, M.A.; Aguirre, A.; Skaf, E.; Gutiérrez, J. Predicting length of hospitalisation of elderly patients, using the Barthel Index. Age Ageing 2008, 37, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Grönbeck Lindén, I.; Hägglin, C.; Gahnberg, L.; Andersson, P. Factors affecting older persons’ ability to manage oral hygiene: A qualitative study. JDR Clin. Trans. Res. 2017, 2, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Curreri, C.; Trevisan, C.; Carrer, P.; Facchini, S.; Giantin, V.; Maggi, S.; Noale, M.; De Rui, M.; Perissinotto, E.; Zambon, S.; et al. Difficulties with fine motor skills and cognitive impairment in an elderly population: The Progetto Veneto Anziani. J. Am. Geriatr. Soc. 2018, 66, 350–356. [Google Scholar] [CrossRef]
- Ruiz-Medina, P.; Bravo, M.; Gil-Montoya, J.A.; Montero, J. Discrimination of functional capacity for oral hygiene in elderly Spanish people by the Barthel General Index. Community Dent. Oral Epidemiol. 2005, 33, 363–369. [Google Scholar] [CrossRef]
- Almas, K.; Al-Lazzam, S.; Al-Quadairi, A. The effect of oral hygiene instructions on diabetic type 2 male patients with periodontal diseases. J. Contemp. Dent. Pract. 2003, 4, 24–35. [Google Scholar] [CrossRef]
- Stewart, R.; Weyant, R.J.; Garcia, M.E.; Harris, T.; Launer, L.J.; Satterfield, S.; Simonsick, E.M.; Yaffe, K.; Newman, A.B. Adverse oral health and cognitive decline: The health, aging and body composition study. J. Am. Geriatr. Soc. 2013, 61, 177–184. [Google Scholar] [CrossRef]
- Åstrøm, A.N.; Ekback, G.; Ordell, S.; Nasir, E. Long-term routine dental attendance: Influence on tooth loss and oral health-related quality of life in Swedish older adults. Community Dent. Oral Epidemiol. 2014, 42, 460–469. [Google Scholar] [CrossRef]
- Blinkhorn, F.; Weingarten, L.; Boivin, L.; Plain, J.; Kay, M. An intervention to improve the oral health of residents in an aged care facility led by nurses. Health Educ. J. 2012, 71, 527–535. [Google Scholar] [CrossRef]
- Frenkel, H.; Harvey, I.; Newcombe, R.G. Improving oral health in institutionalised elderly people by educating caregivers: A randomised controlled trial. Community Dent. Oral Epidemiol. 2001, 29, 289–297. [Google Scholar] [CrossRef]
- Chávez, E.M.; Kossioni, A.; Fukai, K. Policies Supporting Oral Health in Ageing Populations Are Needed Worldwide. Int. Dent. J. 2022, 72, S27–S38. [Google Scholar] [CrossRef] [PubMed]
- Jordan, R.A.; Sirsch, E.; Gesch, D.; Zimmer, S.; Bartholomeyczik, S. Verbesserung der zahnmedizinischen Betreuung in der Altenpflege durch Schulungen von Pflegekräften [Improvement of oral health care in geriatric care by training of nurses and nursing assistants for the elderly]. Pflege 2012, 25, 97–105. [Google Scholar] [CrossRef]
- Sigurdardottir, A.S.; Geirsdottir, O.G.; Ramel, A.; Arnadottir, I.B. Cross-sectional study of oral health care service, oral health beliefs and oral health care education of caregivers in nursing homes. Geriatr. Nurs. 2022, 43, 138–145. [Google Scholar] [CrossRef]
- Thavarajah, R.; Amico, K.R.; Fisher, W.A.; Harman, J.J. Drilling Deeper into Toothbrushing Skills: Is Proactive Interference an Under-Recognized Factor in Oral Hygiene Behavior Change? Curr. Oral Health Rep. 2015, 2, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Riddle, M.; Clark, D. Behavioral and social intervention research at the National Institute of Dental and Craniofacial Research (NIDCR). J. Public Health Dent. 2011, 71, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Agostini, B.A.; Cericato, G.O.; Silveira, E.R.D.; Nascimento, G.G.; Costa, F.D.S.; Thomson, W.M.; Demarco, F.F. How common is dry mouth? Systematic review and meta-regression analysis of prevalence estimates. Braz. Dent. J. 2018, 29, 606–618. [Google Scholar] [CrossRef]
- Zenthöfer, A.; Dieke, R.; Dieke, A.; Wege, K.C.; Rammelsberg, P.; Hassel, A.J. Improving oral hygiene in the long-term care of the elderly—A RCT. Community Dent. Oral Epidemiol. 2013, 41, 261–268. [Google Scholar] [CrossRef]
| Total | Control Group | Intervention Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | Age < 75 Years | Age ≥ 75 Years | All | Age < 75 Years | Age ≥ 75 Years | All | Age < 75 Years | Age ≥ 75 Years | ||
| Patients, n (%) | 90 (100) | 16 (17.8) | 74 (82.2) | 45 (50) | 9 (10) | 36 (40) | 45 (50) | 7 (7.8) | 38 (42.2) | |
| Sex, n (%) | Male ♂ | 18 (20) | 6 (6.7) | 12 (13.3) | 9 (20) | 2 (2.2) | 7 (7.8) | 9 (10) | 4 (4.4) | 5 (5.6) |
| Female ♀ | 72 (80) | 10 (11.1) | 62 (68.9) | 36 (40) | 7 (7.8) | 29 (32.2) | 36 (40) | 3 (3.3) | 33 (36.7) | |
| Age, mean ± SD | 81.1 ± 7.1 | 69.2 ± 3.8 | 83.6 ± 4.7 | 79.9 ± 6.8 | 69.3 ± 3.5 | 82.5 ± 4.5 | 82.2 ± 7.3 | 69 ± 4.4 | 84.6 ± 4.4 | |
| Edentulism, n (%) | 26 (28.9) | 4 (4.4) | 22 (24.4) | 13 (14.4) | 3 (3.3) | 10 (11.1) | 13 (14.4) | 1 (1.1) | 12 (13.3) | |
| Number of teeth, mean ± SD | 8.4 ± 8.4 | 9.6 ± 8.0 | 8.1 ± 8.5 | 9.5 ± 8.6 | 9.0 ± 8.3 | 9.6 ± 8.8 | 7.2 ± 8.0 | 10.4 ± 8.2 | 6.6 ± 8.0 | |
| DMFT, mean ± SD | 25.4 ± 3.7 | 24.5 ± 3.4 | 25.5 ± 3.8 | 25.1 ± 3.6 | 25.0 ± 3.1 | 25.1 ± 3.7 | 25.6 ± 3.9 | 23.9 ± 4.0 | 25.9 ± 3.8 | |
| DT, mean ± SD | 2.0 ± 2.8 | 3.2 ± 4.0 | 1.7 ± 2.5 | 2.1 ± 2.9 | 3.8 ± 4.8 | 1.7 ± 2.2 | 1.9 ± 2.8 | 2.5 ± 3.3 | 1.7 ± 2.7 | |
| PSI max, mean ± SD | 2.5 ± 1.1 | 2.75 ± 1.0 | 2.5 ± 1.1 | 2.5 ± 0.9 | 2.7 ± 0.8 | 2.5 ± 1.0 | 2.5 ± 1.2 | 2.8 ± 1.2 | 2.5 ± 1.3 | |
| Dentures, n (%) | 74 (82.2) | 13 (13.3) | 61 (67.8) | 37 (41.1) | 8 (8.9) | 29 (32.2) | 37 (41.1) | 5 (5.6) | 32 (35.6) | |
| TmQHI, mean ± SD | 3.0 ± 0.9 | 3.0 ± 0.8 | 3.0 ± 1.0 | 2.9 ± 1.0 | 2.94 ± 1.1 | 2.9 ± 1.0 | 3.1 ± 0.8 | 3.1 ± 0.3 | 3.1 ± 0.9 | |
| DHI, mean ± SD | 10.1 ± 5.5 | 9.8 ± 6.7 | 10.2 ± 5.3 | 11.1 ± 5.2 | 12.6 ± 6.6 | 10.6 ± 4.9 | 9.2 ± 5.7 | 5.2 ± 4.3 | 9.8 ± 5.7 | |
| Geriatric assessment, mean ± SD | GDS | 3.6 ± 2.5 | 5.1 ± 3.7 | 3.3 ± 2.1 | 3.6 ± 2.5 | 5.9 ± 3.6 | 3.1 ± 1.8 | 3.6 ± 2.6 | 4.1 ± 3.8 | 3.5 ± 2.4 |
| MMS | 26.5 ± 1.8 | 26.9 ± 1.9 | 26.5 ± 1.8 | 26.9 ± 1.7 | 27.4 ± 1.8 | 26.7 ± 1.6 | 26.2 ± +1.9 | 26.1 ± 1.9 | 26.2 ± 1.9 | |
| Barthel | 62.3 ± 17.7 | 61.6 ± 17.5 | 62.5 ± 17.8 | 62.7 ± 16.9 | 59.4 ± 19.1 | 63.5 ± 16.5 | 62.0 ± 18.6 | 64.3 ± 16.2 | 61.6 ± 19.1 | |
| Variables | TmQHI At Baseline | DHI at Baseline | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N Patients CG/IG | All | CG | IG | p-Value | N Patients CG/IG | All | CG | IG | p-Value | |||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||||||
| All | 32/32 | 3.0 ± 0.9 | 2.9 ± 1.0 | 3.1 ± 0.8 | 0.275 | 37/37 | 10.1 ± 5.5 | 11.1 ± 5.2 | 9.2 ± 5.7 | 0.146 | ||
| Age | <75 | 6/6 | 3.0 ± 0.8 | 2.9 ± 1.1 | 3.1 ± 0.3 | 0.693 | 8/5 | 9.8 ± 6.7 | 12.6 ± 6.6 | 5.2 ± 4.3 | 0.047 | |
| ≥75 | 29/32 | 3.0 ± 1.0 | 3.0 ± 1.0 | 3.1 ± 0.9 | 0.318 | 26/26 | 10.2 ± 5.3 | 10.6 ± 4.9 | 9.8 ± 5.7 | 0.554 | ||
| Sex | Male ♂ | 6/7 | 3.2 ± 0.8 | 3.2 ± 0.9 | 3.2 ± 0.8 | 0.999 | 8/6 | 10.5 ± 3.4 | 11.1 ± 3.2 | 9.7 ± 3.8 | 0.451 | |
| Female ♀ | 26/25 | 2.9 ± 0.9 | 2.8 ± 1.0 | 3.1 ± 0.8 | 0.255 | 29/31 | 10.0 ± 5.9 | 11.0 ± 5.7 | 9.1 ± 6.0 | 0.207 | ||
| Number of teeth | 0 | 0 | - | - | - | - | 13/13 | 11.9 ± 5.7 | 12.1 ± 4.8 | 11.7 ± 6.7 | 0.868 | |
| 1–9 | 11/18 | 3.2 ± 0.9 | 3.3 ± 0.9 | 3.2 ± 0.9 | 0.768 | 11/16 | 10.0 ± 4.9 | 11.4 ± 5.8 | 9.1 ± 4.0 | 0.246 | ||
| 10–19 | 12/8 | 3.0 ± 0.7 | 2.9 ± 0.9 | 3.1 ± 0.4 | 0.662 | 13/8 | 8.0 ± 5.5 | 9.8 ± 5.4 | 5.3 ± 4.9 | 0.068 | ||
| ≥20 | 9/6 | 2.6 ± 1.1 | 2.3 ± 1.1 | 3.1 ± 1.0 | 0.210 | 0/0 | - | - | - | - | ||
| Type of prostheses | upper jaw | partial | 17/18 | 3.0 ± 1.2 | 3.1 ± 1.1 | 2.8 ± 1.3 | 0.495 | 17/18 | 4.9 ± 2.9 | 6.3 ± 3.0 | 3.7 ± 2.2 | 0.007 |
| total | - | - | - | - | - | 19/19 | 6.2 ± 2.1 | 6.8 ± 2.3 | 5.6 ± 3.4 | 0.224 | ||
| lower jaw | partial | 19/18 | 3.1 ± 1.0 | 2.8 ± 1.0 | 3.5 ± 0.9 | 0.031 | 19/18 | 5.0 ± 3.6 | 5.8 ± 4.1 | 4.3 ± 2.7 | 0.275 | |
| total | - | - | - | - | - | 12/16 | 6.5 ± 3.1 | 6.4 ± 3.0 | 6.5 ± 3.2 | 0.893 | ||
| PSI | 0 | 1/4 | 2.8 ± 0.4 | 2.7 ± 0.0 | 2.8 ± 0.5 | 0.812 | 1/3 | 10.8 ± 4.0 | 15.0 ± 0.0 | 9.3 ± 3.5 | 0.297 | |
| 1–2 | 13/9 | 2.9 ± 1.0 | 2.85 ± 1.0 | 3.0 ± 1.1 | 0.711 | 10/7 | 7.8 ± 5.1 | 9.4 ± 5.4 | 5.6 ± 4.0 | 0.133 | ||
| 3–4 | 18/19 | 3.1 ± 0.9 | 2.9 ± 1.0 | 3.2 ± 0.7 | 0.252 | 13/14 | 9.8 ± 5.4 | 11.0 ± 5.8 | 8.6 ± 4.9 | 0.264 | ||
| Plaque Reduction between T0 and T1a (Mean Duration, 12.4 Days) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | TmQHI-Difference | DHI-Difference | |||||||||
| N CG/IG | All Mean ± SD | CG Mean ± SD | IG Mean ± SD | CG vs. IG Effect Size (p-Value) | N CG/IG | All Mean ± SD | CG Mean ± SD | IG Mean ± SD | CG vs. IG Effect Size (p-Value) | ||
| All | 32/32 | 0.1 ± 0.6 | 0.0 ± 0.5 | 0.1 ± 0.6 | 0.2 (0.333) | 37/37 | −0.3 ± 4.3 | 0.0 ± 4.1 | −0.7 ± 4.6 | −0.2 (0.473) | |
| Age | <75 | 6/6 | −0.1 ± 0.8 | −0.2 ± 0.7 | 0.0 ± 0.8 | 0.3 (0.642) | 8/5 | 1.3 ± 5.1 | 2.4 ± 5.4 | −0.4 ± 4.6 | −0.6 (0.363) |
| ≥75 | 26/26 | 0.1 ± 0.5 | 0.0 ± 0.5 | 0.2 ± 0.6 | 0.2 (0.409) | 29/32 | −0.7 ± 4.1 | −0.6 ± 3.4 | −0.8 ± 4.7 | 0 (0.904) | |
| ES (p-value) (<75 vs. ≥75) | - | 0.3 (0.386) | 0.3 (0.409) | 0.3 (0.680) | - | - | 1.3 * (0.133) | −0.7 (0.064) | −0.1 (0.878) | - | |
| Sex | Male ♂ | 6/7 | 0.0 ± 0.4 | 0.2 ± 0.4 | −0.1 ± 0.4 | −0.7 (0.219) | 8/6 | −0.4 ± 2.5 | 0.0 ± 1.2 | −1.0 ± 3.7 | −0.4 (0.487) |
| Female ♀ | 26/25 | 0.1 ± 0.6 | −0.1 ± 0.6 | 0.2 ± 0.6 | 0.4 (0.139) | 29/31 | −0.3 ± 4.7 | 0.0 ± 4.6 | −0.7 ± 4.8 | −0.1 (0.578) | |
| ES (p-value) (♂ vs. ♀) | - | 0.2 (0.691) | −0.6 (0.396) | 0.6 (0.198) | - | - | <0.1 (0.931) | <0.01 (0.983) | 0.3 (0.866) | - | |
| Number of teeth | 0 | 0/0 | - | - | - | - | 13/13 | −0.6 ± 3.3 | −0.3 ± 3.5 | −0.9 ± 3.3 | −0.2 (0.645) |
| 1–9 | 11/18 | 0.2 ± 0.6 | 0.1 ± 0.6 | 0.3 ± 0.6 | 0.3 (0.533) | 11/16 | 0.4 ± 4.7 | 0.1 ± 4.5 | 0.6 ± 4.9 | 0.1 (0.777) | |
| 10–19 | 13/9 | −0.1 ± 0.6 | 0.0 ± 0.5 | −0.2 ± 0.7 | −0.3 (0.511) | 13/8 | −1.0 ± 5.0 | 0.3 ± 4.6 | −3.0 ± 5.4 | −0.7 (0.149) | |
| ≥20 | 8/5 | 0.0 ± 0.5 | −0.2 ± 0.5 | 0.2 ± 0.2 | 1.1 * (0.159) | 0/0 | - | - | - | - | |
| ES (p-value) (0–9 vs. 10–28) | - | 0.5 (0.069) | 0.3 (0.366) | 0.5 (0.175) | - | - | 0.2 (0.447) | −0.1 (0.762) | 0.6 (0.114) | - | |
| PSI | 0 | 1/4 | −0.3 ± 0.6 | 0.0 ± 0.0 | −0.3 ± 0.7 | (0.688) | 1/3 | 0.5 ± 1.9 | 0.0 ± 0.0 | 0.7 ± 2.3 | (0.826) |
| 1–2 | 13/9 | 0.1 ± 0.6 | 0.1 ± 0.6 | 0.2 ± 0.6 | 0.2 (0.771) | 10/7 | −0.3 ± 4.7 | −0.6 ± 2.0 | 0.1 ± 7.2 | 0.1 (0.759) | |
| 3–4 | 18/19 | 0.1 ± 0.6 | −0.1 ± 0.5 | 0.2 ± 0.6 | 0.5 (0.112) | 13/14 | −0.2 ± 5.3 | 0.9 ± 5.8 | −1.2 ± 4.8 | −0.4 (0.323) | |
| ES (p-value) (0–2 vs. 3–4) | - | <0.1 (0.961) | 0.4 (0.311) | −0.3 (0.422) | - | - | 0.1 (0.956) | −0.3 (0.454) | 0.3 (0.501) | - | |
| MMS | <MAV | 10/19 | 0.2 ± 0.6 | 0.2 ± 0.7 | 0.1 ± 0.6 | −0.2 (0.802) | 14/24 | −0.6 ± 4.7 | 0.1 ± 3.2 | −1.0 ± 5.5 | −0.3 (0.493) |
| ≥MAV | 22/13 | 0.0 ± 0.6 | −0.1 ± 0.4 | 0.1 ± 0.6 | 0.4 (0.218) | 23/13 | 0.0 ± 3.9 | 0.0 ± 4.6 | −0.1 ± 2.5 | 0 (0.956) | |
| ES (p-value) (<MAV vs. ≥MAV) | - | −0.3 (0.227) | −0.5 (0.146) | 0 (0.964) | - | 0.1 (0.553) | 0 (0.959) | 0.2 (0.553) | - | ||
| GDS | <MAV | 18/17 | 0.1 ± 0.6 | 0.0 ± 0.5 | 0.2 ± 0.7 | 0.3 (0.450) | 22/20 | −0.3 ± 3.9 | −0.3 ± 4.0 | −0.4 ± 4.0 | 0 (0.950) |
| ≥MAV | 14/15 | 0.0 ± 0.6 | −0.1 ± 0.6 | 0.1 ± 0.5 | 0.4 (0.534) | 15/17 | −0.4 ± 4.9 | 0.5 ± 4.2 | −1.1 ± 5.4 | −0.3 (0.367) | |
| ES (p-value) (<MAV vs. ≥MAV) | - | −0.2 (0.443) | −0.2 (0.599) | −0.2 (0.557) | - | - | 0.0 (0.949) | 0.2 (0.594) | −0.1 (0.622) | - | |
| Reduced BI | ≤20 | 14/12 | 0.1 ± 0.5 | 0.0 ± 0.6 | 0.3 ± 0.4 | 0.6 (0.149) | 16/12 | 1.1 ± 4.5 | 0.6 ± 4.1 | 1.7 ± 5.1 | 0.2 (0.557) |
| >20 | 18/20 | 0.0 ± 0.6 | 0.0 ± 0.6 | 0.0 ± 0.7 | 0 (0.809) | 21/25 | −1.2 ± 4.0 | −0.4 ± 4.0 | −1.8 ± 4.0 | −0.4 (0.241) | |
| ES (p-value) (≤20 vs. >20) | - | 0.2 (0.382) | 0 (0.936) | 0.5 (0.245) | - | - | 0.5 (0.028) | 0.2 (0.442) | 0.8 (0.029) | - | |
| Effect of Intervention T1a-T1b | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TmQHI-Difference | DHI-Difference | ||||||||||
| N CG/IG | All Mean ± SD | CG Mean ± SD | IG Mean ± SD | CG vs. IG Effect Size (p-Value) | N CG/IG | All Mean ± SD | CG Mean ± SD | IG Mean ± SD | CG vs. IG Effect Size (p-Value) | ||
| All | 32/32 | 0.9 ± 0.5 | 0.7 ± 0.3 | 1.1 ± 0.5 | 1.0 (<0.001) | 37/37 | 4.6 ± 4.0 | 3.8 ± 3.5 | 5.4 ± 4.4 | 0.4 (0.093) | |
| Age | <75 | 6/6 | 0.9 ± 0.4 | 0.7 ± 0.2 | 1.2 ± 0.5 | 1.3 * (0.046) | 8/5 | 2.5 ± 2.6 | 2.1 ± 1.7 | 3.2 ± 3.8 | 0.4 (0.498) |
| ≥75 | 26/26 | 0.9 ± 0.5 | 0.7 ± 0.3 | 1.1 ± 0.5 | 1.0 * (0.001) | 29/32 | 5.0 ± 4.1 | 4.2 ± 3.8 | 5.7 ± 4.4 | 0.4 (0.175) | |
| ES (p-value) (<75 vs. ≥75) | - | 0.0 (0.952) | 0.0 (0.908) | −0.2 (0.878) | - | - | 0.7 (0.044) | 0.7 (0.135) | 0.6 (0.241) | - | |
| Sex | Male ♂ | 6/7 | 1.0 ± 0.5 | 0.8 ± 0.3 | 1.1 ± 0.7 | 0.6 (0.218) | 8/6 | 4.4 ± 3.6 | 4.0 ± 4.3 | 5.0 ± 2.6 | 0.3 (0.626) |
| Female ♀ | 26/25 | 0.9 ± 0.5 | 0.7 ± 0.3 | 1.1 ± 0.5 | 1.0 * (<0.001) | 29/31 | 4.6 ± 4.1 | 3.7 ± 3.4 | 5.4 ± 4.7 | 0.4 (0.114) | |
| ES (p-value) (♂ vs. ♀) | - | −0.2 (0.703) | −0.3 (0.619) | 0.0 (0.997) | - | - | 0.1 (0.887) | −0.1 (0.848) | 0.1 (0.833) | - | |
| Number of teeth | 0 | 0/0 | - | - | - | - | 13/13 | 5.2 ± 4.0 | 3.7 ± 2.6 | 6.8 ± 4.8 | 0.8 * (0.051) |
| 1–9 | 11/18 | 1.1 ± 0.5 | 0.7 ± 0.3 | 1.3 ± 0.5 | 1.5 * (<0.001) | 11/16 | 4.6 ± 4.0 | 4.3 ± 3.7 | 4.9 ± 4.3 | 0.2 (0.707) | |
| 10–19 | 13/9 | 1.0 ± 0.4 | 0.9 ± 0.3 | 1.1 ± 0.4 | 0.6 (0.094) | 13/8 | 3.7 ± 4.0 | 3.5 ± 4.4 | 4.0 ± 3.6 | 0.1 (0.775) | |
| ≥ 20 | 8/5 | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.6 ± 0.2 | 0.5 (0.617) | 0/0 | - | - | - | - | |
| ES (p-value) (0–9 vs. 10–28) | - | 0.6 (0.021) | −0.1 (0.72) | 0.8 * (0.026) | - | - | 0.3 (0.227) | 0.1 (0.689) | 0.4 (0.329) | - | |
| PSI | 0 | 1/4 | 1.2 ± 0.8 | 0.7 ± 0.0 | 1.3 ± 0.8 | 1.1 * (0.532) | 1/3 | 4.3 ± 4.8 | 5.7 ± 4.7 | 0.0 ± 0.0 | (0.408) |
| 1–2 | 13/9 | 1.0 ± 0.6 | 0.7 ± 0.4 | 1.4 ± 0.5 | 1.5 * (0.001) | 10/7 | 3.5 ± 3.3 | 3.9 ± 3.7 | 2.9 ± 1.1 | −0.4 (0.543) | |
| 3–4 | 18/19 | 0.8 ± 0.4 | 0.7 ± 0.3 | 1.0 ± 0.4 | 0.8 * (0.016) | 13/14 | 4.7 ± 4.3 | 4.1 ± 4.4 | 5.2 ± 4.3 | 0.3 (0.505) | |
| ES (p-value) (0–2 vs. 3–4) | - | 0.4 (0.12) | <0.01 (0.978) | 0.9 * (0.02) | - | - | 0.3 (0.372) | −0.1 (0.754) | −0.4 (0.375) | - | |
| MMS | <MAV | 10/19 | 1.0 ± 0.5 | 0.8 ± 0.3 | 1.2 ± 0.5 | 1.0 * (0.037) | 14/24 | 5.6 ± 3.7 | 4.4 ± 2.8 | 6.3 ± 4.1 | 0.5 (0.139) |
| ≥MAV | 22/13 | 0.8 ± 0.4 | 0.7 ± 0.3 | 1.1 ± 0.5 | 1.0 * (0.003) | 23/13 | 3.5 ± 4.1 | 3.4 ± 3.9 | 3.6 ± 4.5 | 0.0 (0.877) | |
| ES (p-value) (<MAV vs. ≥MAV) | - | −0.4 (0.096) | −0.3 (0.457) | −0.2 (0.725) | - | - | −0.5 (0.021) | −0.3 (0.393) | −0.6 (0.074) | - | |
| GDS | <MAV | 18/17 | 0.9 ± 0.4 | 0.8 ± 0.3 | 1.0 ± 0.5 | 0.5 (0.145) | 22/20 | 4.4 ± 3.9 | 4.1 ± 3.9 | 4.7 ± 4.0 | 0.2 (0.620) |
| ≥MAV | 14/15 | 1.0 ± 0.5 | 0.6 ± 0.3 | 1.3 ± 0.5 | 1.7 * (<0.001) | 15/17 | 4.8 ± 4.2 | 3.3 ± 3.1 | 6.1 ± 4.7 | 0.7 (0.061) | |
| ES (p-value) (<MAV vs. ≥MAV) | - | 0.2 (0.607) | −0.7 (0.07) | 0.6 (0.091) | - | - | 0.1 (0.65) | −0.2 (0.529) | 0.3 (0.331) | - | |
| Reduced BI | ≤20 | 14/12 | 0.9 ± 0.5 | 0.6 ± 0.3 | 1.1 ± 0.5 | 1.2 (0.010) | 16/12 | 4.1 ± 2.9 | 3.9 ± 3.0 | 4.3 ± 2.9 | 0.1 (0.785) |
| >20 | 18/20 | 1.0 ± 0.5 | 0.7 ± 0.3 | 1.2 ± 0.5 | 1.2 (0.004) | 21/25 | 4.9 ± 4.6 | 3.7 ± 4.0 | 5.9 ± 4.9 | 0.5 (0.102) | |
| ES (p-value) (≤20 vs. >20) | - | −0.2 (0.394) | −0.3 (0.377) | −0.2 (0.784) | - | - | −0.2 (0.411) | 0.1 (0.821) | −0.4 (0.293) | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viebranz, S.; Dederichs, M.; Kwetkat, A.; Schüler, I.M. Effectiveness of Individual Oral Health Care Training in Hospitalized Inpatients in Geriatric Wards. Int. J. Environ. Res. Public Health 2023, 20, 4275. https://doi.org/10.3390/ijerph20054275
Viebranz S, Dederichs M, Kwetkat A, Schüler IM. Effectiveness of Individual Oral Health Care Training in Hospitalized Inpatients in Geriatric Wards. International Journal of Environmental Research and Public Health. 2023; 20(5):4275. https://doi.org/10.3390/ijerph20054275
Chicago/Turabian StyleViebranz, Stephanie, Marco Dederichs, Anja Kwetkat, and Ina Manuela Schüler. 2023. "Effectiveness of Individual Oral Health Care Training in Hospitalized Inpatients in Geriatric Wards" International Journal of Environmental Research and Public Health 20, no. 5: 4275. https://doi.org/10.3390/ijerph20054275
APA StyleViebranz, S., Dederichs, M., Kwetkat, A., & Schüler, I. M. (2023). Effectiveness of Individual Oral Health Care Training in Hospitalized Inpatients in Geriatric Wards. International Journal of Environmental Research and Public Health, 20(5), 4275. https://doi.org/10.3390/ijerph20054275





