Risk of Subsequent Preeclampsia by Maternal Country of Birth: A Norwegian Population-Based Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
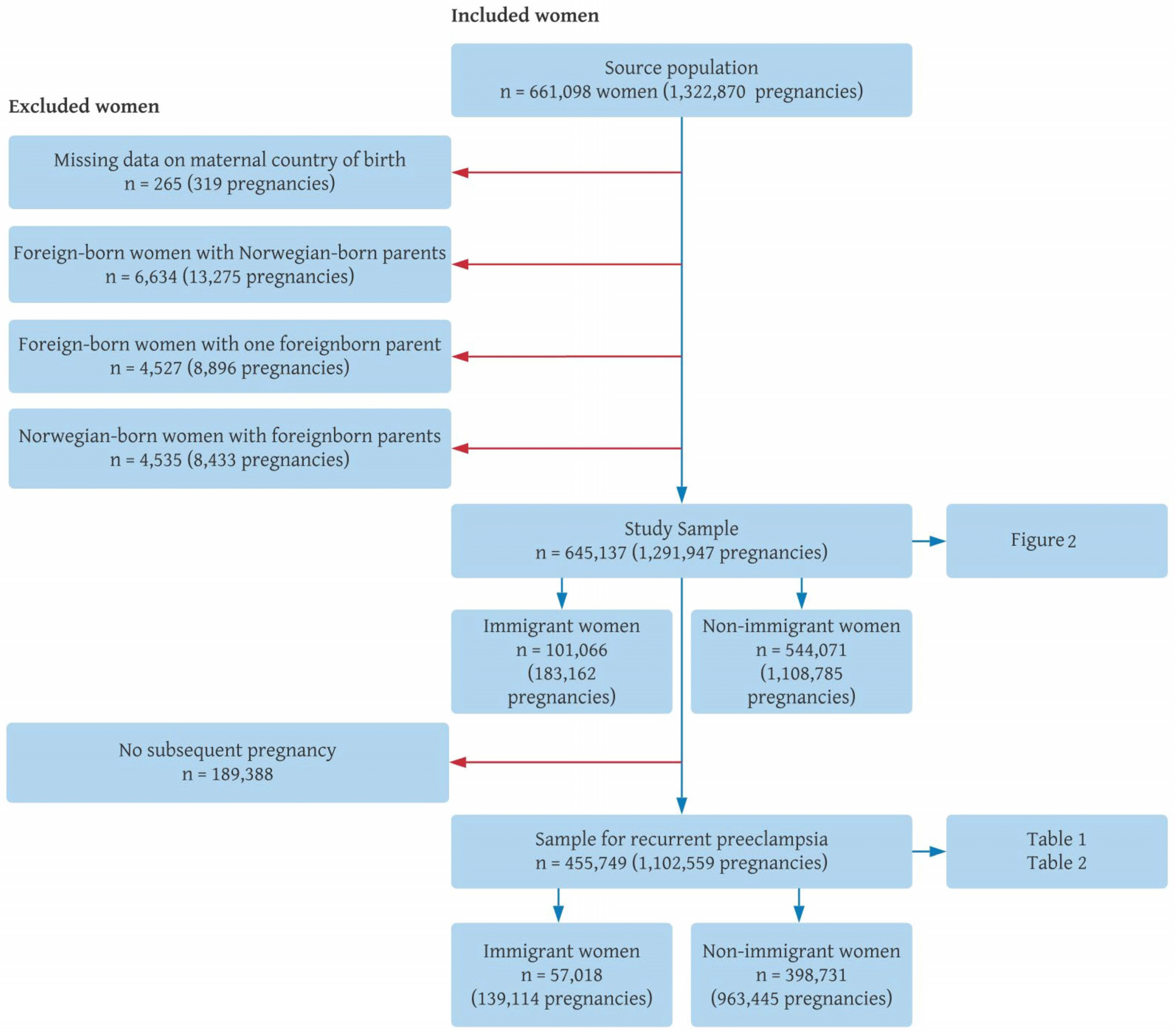
2.2. Study Sample
2.3. Preeclampsia
2.4. Region of Birth
2.5. Other Variables
2.6. Statistical Analyses
2.7. Ethics and Public Involvement
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walker, J.J. Pre-eclampsia. Lancet 2000, 356, 1260–1265. [Google Scholar] [CrossRef]
- Khan, K.S.; Wojdyla, D.; Say, L.; Gülmezoglu, A.M.; Van Look, P.F. WHO analysis of causes of maternal death: A systematic review. Lancet 2006, 367, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.C.; Best, K.E.; Pearce, M.S.; Waugh, J.; Robson, S.C.; Bell, R. Cardiovascular disease risk in women with pre-eclampsia: Systematic review and meta-analysis. Eur. J. Epidemiol. 2013, 28, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, L.; Casas, J.-P.; Hingorani, A.D.; Williams, D.J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. BMJ 2007, 335, 974. [Google Scholar] [CrossRef] [PubMed]
- Villamor, E.; Cnattingius, S. Interpregnancy weight change and risk of adverse pregnancy outcomes: A population-based study. Lancet 2006, 368, 1164–1170. [Google Scholar] [CrossRef]
- Weissgerber, T.L.; Mudd, L.M. Preeclampsia and diabetes. Curr. Diab. Rep. 2015, 15, 9. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Delli Muti, N.; Balercia, G.; Ciavattini, A.; Giannubilo, S.R.; Marzioni, D. Preeclampsia and severe acute respiratory syndrome coronavirus 2 infection: A systematic review. J. Hypertens. 2022, 40, 1629–1638. [Google Scholar] [CrossRef]
- Hernández-Díaz, S.; Toh, S.; Cnattingius, S. Risk of pre-eclampsia in first and subsequent pregnancies: Prospective cohort study. BMJ 2009, 338, b2255. [Google Scholar] [CrossRef]
- Bartsch, E.; Medcalf, K.E.; Park, A.L.; Ray, J.G.; High Risk of Pre-eclampsia Identification Group. Clinical risk factors for pre-eclampsia determined in early pregnancy: Systematic review and meta-analysis of large cohort studies. BMJ 2016, 353, i1753. [Google Scholar] [CrossRef]
- Mogos, M.F.; Salinas-Miranda, A.A.; Salemi, J.L.; Medina, I.M.; Salihu, H.M. Pregnancy-Related Hypertensive Disorders and Immigrant Status: A Systematic Review and Meta-analysis of Epidemiological Studies. J. Immigr. Minor. Health 2017, 19, 1488–1497. [Google Scholar] [CrossRef]
- Urquia, M.; Glazier, R.; Gagnon, A.; Mortensen, L.; Nybo Andersen, A.-M.; Janevic, T.; Guendelman, S.; Thornton, D.; Bolumar, F.; Río Sánchez, I.; et al. Disparities in pre-eclampsia and eclampsia among immigrant women giving birth in six industrialised countries. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.G.; Vermeulen, M.J.; Schull, M.J.; Singh, G.; Shah, R.; Redelmeier, D.A. Results of the Recent Immigrant Pregnancy and Perinatal Long-term Evaluation Study (RIPPLES). CMAJ Can. Med. Assoc. J. 2007, 176, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, R.M.; Vik, E.S.; Rasmussen, S.A.; Small, R.; Moster, D.; Schytt, E.; Aasheim, V. Preeclampsia by maternal reasons for immigration: A population-based study. BMC Pregnancy Childbirth 2018, 18, 423. [Google Scholar] [CrossRef]
- Gushulak, B. Healthier on arrival? Further insight into the “healthy immigrant effect”. CMAJ Can. Med. Assoc. J. 2007, 176, 1439–1440. [Google Scholar] [CrossRef] [PubMed]
- Naimy, Z.; Grytten, J.; Monkerud, L.; Eskild, A. The prevalence of pre-eclampsia in migrant relative to native Norwegian women: A population-based study. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 859–865. [Google Scholar] [CrossRef]
- Ministry of Health and Welfare. Lov om Pasient- og Brukerrettigheter [The Law on Patient and User Rights Law]; LOV-1999-07-02-63; Lovdata: Oslo, Norway, 2001.
- The Norwegian Directorate of Health. Healthcare for Persons without Legal Residence in Norway. 2019. Available online: https://www.helsenorge.no/en/foreigners-in-norway/healthcare-for-persons-without-legal-residence/ (accessed on 1 December 2022).
- Bains, S.; Skråning, S.; Sundby, J.; Vangen, S.; Sorbye, I.; Lindskog, B. Challenges and barriers to optimal maternity care for recently migrated women—A mixed-method study in Norway. BMC Pregnancy Childbirth 2021, 21, 686. [Google Scholar] [CrossRef]
- Small, R.; Roth, C.; Raval, M.; Shafiei, T.; Korfker, D.; Heaman, M.; McCourt, C.; Gagnon, A. Immigrant and non-immigrant women’s experiences of maternity care: A systematic and comparative review of studies in five countries. BMC Pregnancy Childbirth 2014, 14, 152. [Google Scholar] [CrossRef]
- Staff, A.; Kvie, A.; Langesæter, E.; Michelsen, T.M.; Moe, K.; Strand, K.M.; Værnesbranden, M.; Øian, P. [Hypertensive Pregnancy Complications and Eclampsia] Hypertensive Svangerskapskomplikasjoner og Eklampsi. The Norwegian Medical Association: The Norwegian Society of Obstetrics and Gynecology. 2020. Available online: https://www.legeforeningen.no/foreningsledd/fagmed/norsk-gynekologisk-forening/veiledere/veileder-i-fodselshjelp/hypertensive-svangerskapskomplikasjoner-og-eklampsi/ (accessed on 5 September 2022).
- Irgens, L.M. The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet. Gynecol. Scand. 2000, 79, 435–439. [Google Scholar]
- Statistics Norway. About Statistics Norway. 2020. Available online: https://www.ssb.no/en/omssb/om-oss (accessed on 28 October 2022).
- Dzamarija, M. Statistics on Reasons for Immigration 1990–2011, What Do We Know and How Can We Best Use This Information; Statistics Norway: Oslo-Kongsvinger, Norway, 2013.
- Thomsen, L.C.V.; Klungsøyr, K.; Roten, L.T.; Tappert, C.; Araya, E.; Bærheim, G.; Tollaksen, K.; Fenstad, M.H.; Macsali, F.; Austgulen, R.; et al. Validity of the diagnosis of pre-eclampsia in the Medical Birth Registry of Norway. Acta Obstet. Gynecol. Scand. 2013, 92, 943–950. [Google Scholar] [CrossRef]
- Vestrheim, L.C.; Austgulen, R.; Melve, K.K.; Roten, L.T.; Tappert, C.; Araya, E.; Tollaksen, K.; Bærheim, G.; Leuchsner, H.; Fenstad, M.H.; et al. P54 Classification of pre-eclamptic pregnancies in health registries. Pregnancy Hypertens. Int. J. Womens Cardiovasc. Health 2010, 1, S56–S57. [Google Scholar] [CrossRef]
- Klungsøyr, K.; Harmon, Q.E.; Skard, L.B.; Simonsen, I.; Austvoll, E.T.; Alsaker, E.R.; Starling, A.; Trogstad, L.; Magnus, P.; Engel, S.M. Validity of pre-eclampsia registration in the medical birth registry of Norway for women participating in the Norwegian mother and child cohort study, 1999-2010. Paediatr. Perinat. Epidemiol. 2014, 28, 362–371. [Google Scholar] [CrossRef]
- Institute for Health Metrics and Evaluation. About GBD. The Global Burden of Disease: A Critical Resource for Informed Policymaking. Available online: https://www.healthdata.org/gbd/about (accessed on 4 June 2022).
- Institute for Health Metrics and Evaluation. What Countries Are in Each Region? Available online: http://www.healthdata.org/gbd/faq (accessed on 4 June 2022).
- Nilsen, R.M.; Vollset, S.E.; Rasmussen, S.A.; Ueland, P.M.; Daltveit, A.K. Folic acid and multivitamin supplement use and risk of placental abruption: A population-based registry study. Am. J. Epidemiol. 2008, 167, 867–874. [Google Scholar] [CrossRef]
- Quaresima, P.; Visconti, F.; Interlandi, F.; Puccio, L.; Caroleo, P.; Amendola, G.; Morelli, M.; Venturella, R.; Di Carlo, C. Awareness of gestational diabetes mellitus foetal-maternal risks: An Italian cohort study on pregnant women. BMC Pregnancy Childbirth 2021, 21, 692. [Google Scholar] [CrossRef] [PubMed]
- Mahande, M.J.; Daltveit, A.K.; Mmbaga, B.T.; Masenga, G.; Obure, J.; Manongi, R.; Lie, R.T. Recurrence of preeclampsia in northern Tanzania: A registry-based cohort study. PLoS ONE 2013, 8, e79116. [Google Scholar] [CrossRef] [PubMed]
- Diaz, E.; Kumar, B.N.; Gimeno-Feliu, L.-A.; Calderón-Larrañaga, A.; Poblador-Pou, B.; Prados-Torres, A. Multimorbidity among registered immigrants in Norway: The role of reason for migration and length of stay. Trop. Med. Int. Health 2015, 20, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Gushulak, B.D.; MacPherson, D.W. Health aspects of the pre-departure phase of migration. PLoS Med. 2011, 8, e1001035. [Google Scholar] [CrossRef]
- Kennedy, S.; Kidd, M.P.; McDonald, J.T.; Biddle, N. The Healthy Immigrant Effect: Patterns and Evidence from Four Countries. J. Int. Migr. Integr. 2015, 16, 317–332. [Google Scholar] [CrossRef]
| Maternal Characteristic | Non-Immigrants | Central Europe, Eastern Europe, Central Asia | High Income Countries | Latin America, Caribbean | North Africa, Middle East | South Asia | Southeast Asia, East Asia, Oceania | Sub-Saharan Africa |
|---|---|---|---|---|---|---|---|---|
| No. of women (%) | 398,731 (87.5) | 12,151 (2.7) | 13,508 (3.0) | 1445 (0.3) | 9340 (2.1) | 4641 (1.0) | 9721 (2.1) | 6212 (1.4) |
| Maternal age a (mean ± SD) | 26.2 ± 4.5 | 26.6 ± 4.4 | 29.9 ± 4.4 | 28.3 ± 4.9 | 25.2 ± 4.6 | 24.9 ± 3.9 | 26.9 ± 4.4 | 25.8 ± 4.6 |
| Year of childbirth a (mean ± SD) | 2001 ± 7.0 | 2007 ± 5.8 | 2003 ± 7.0 | 2005 ± 6.0 | 2004 ± 6.3 | 2002 ± 6.9 | 2002 ± 6.8 | 2006 ± 6.3 |
| Parity b (mean ± SD) | 2.4 ± 0.7 | 2.3 ± 0.6 | 2.3 ± 0.6 | 2.2 ± 0.5 | 2.6 ± 0.8 | 2.8 ± 1.0 | 2.4 ± 0.6 | 2.8 ± 1.1 |
| Interpregnancy interval a,c | ||||||||
| months (mean ± SD) | 33.3 ± 26.6 | 31.0 ± 24.4 | 27.2 ± 20.8 | 35.1 ± 29.4 | 31.2 ± 26.3 | 27.7 ± 23.4 | 33.1 ± 26.1 | 24.3 ± 22.6 |
| Length of residence a,d | ||||||||
| years (mean ± SD) | - | 4.1 ± 5.1 | 5.5 ± 5.6 | 3.8 ± 4.6 | 4.2 ± 5.7 | 6.1 ± 7.8 | 5.0 ± 6.2 | 3.6 ± 4.5 |
| Maternal education a,e (%) | ||||||||
| No education | 0.0 | 0.3 | 0.2 | 0.9 | 3.0 | 2.0 | 1.3 | 8.1 |
| Primary education | 18.9 | 18.8 | 9.9 | 22.0 | 43.7 | 39.9 | 34.0 | 50.1 |
| Secondary education | 37.9 | 29.5 | 25.3 | 24.4 | 27.8 | 27.3 | 29.7 | 24.6 |
| University/college | 43.2 | 51.5 | 64.6 | 52.7 | 25.6 | 30.8 | 35.0 | 17.3 |
| Missing b | 0.2 | 22.9 | 15.3 | 25.3 | 36.5 | 31.5 | 24.9 | 33.5 |
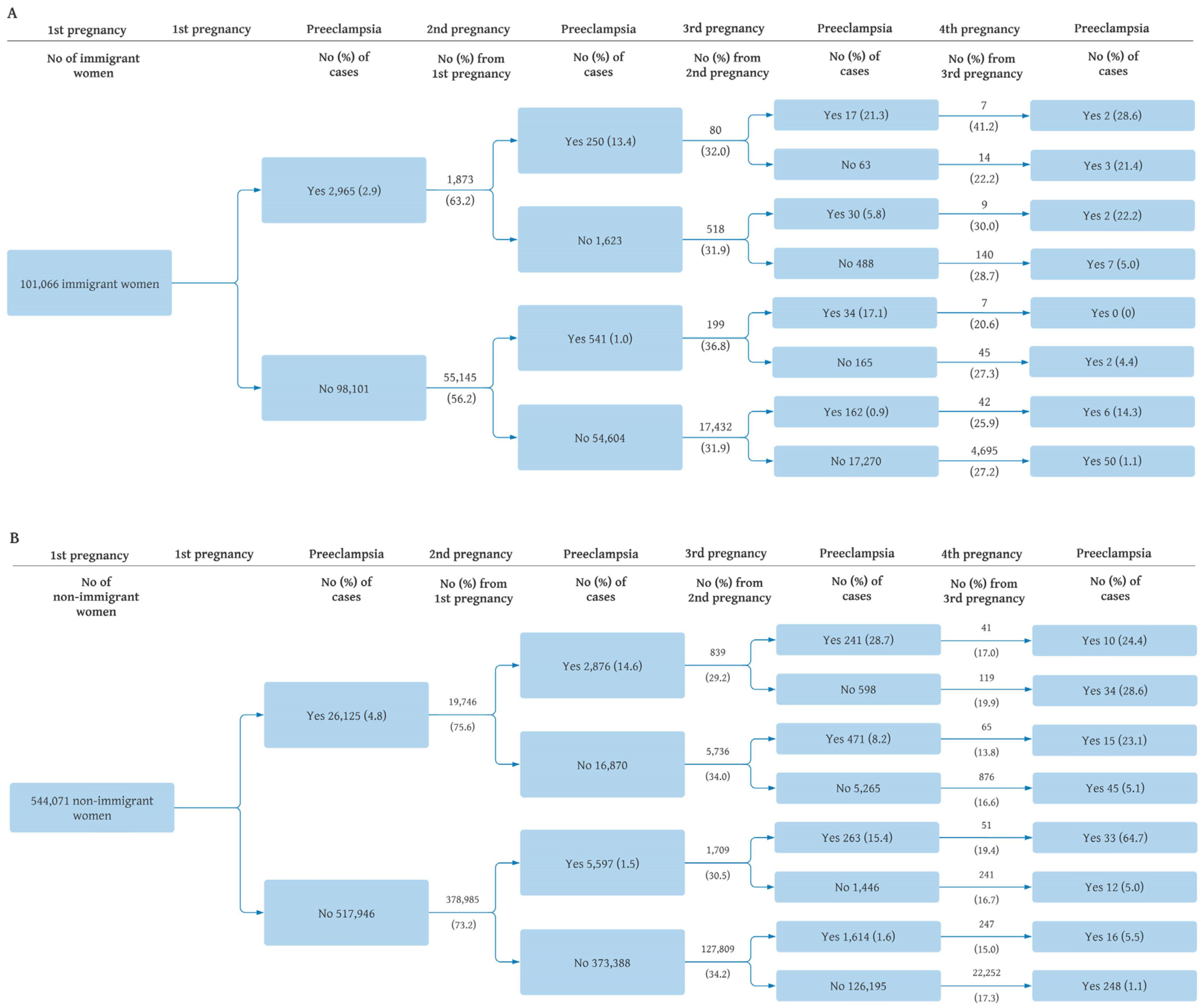
| Maternal Region of Birth | No. of Women a | Preeclampsia in Second | Crude RR (95% CI) | Adjusted RR (95% CI) b |
|---|---|---|---|---|
| No. | No. (%) | |||
| Total Sample | ||||
| No preeclampsia in first | 434,130 | 6138 (1.4) | 1.00 (Reference) | 1.00 (Reference) |
| Preeclampsia in first | 21,619 | 3126 (14.5) | 10.2 (9.82, 10.7) | 9.8 (9.4, 10.2) |
| Immigrant | ||||
| No preeclampsia in first | 55,145 | 541 (1.0) | 1.00 (Reference) | 1.00 (Reference) |
| Preeclampsia in first | 1873 | 250 (13.4) | 13.6 (11.8, 15.7) | 12.9 (11.2, 14.9) |
| Non-Immigrant | ||||
| No preeclampsia in first | 378,985 | 5597 (1.5) | 1.00 (Reference) | 1.00 (Reference) |
| Preeclampsia in first | 19,746 | 2876 (14.6) | 9.86 (9.45, 10.3) | 9.5 (9.1, 10.0) |
| Central Europe, Eastern Europe, and Central Asia | ||||
| No preeclampsia in first | 11,831 | 82 (0.7) | 1.00 (Reference) | 1.00 (Reference) |
| Preeclampsia in first | 320 | 34 (10.6) | 15.3 (10.4, 22.5) | 14.1 (9.7, 20.7) |
| High Income Countries | ||||
| No preeclampsia in first | 12, 993 | 124 (1.0) | 1.00 (Reference) | 1.00 (Reference) |
| Preeclampsia in first | 515 | 73 (14.2) | 14.9 (11.3, 19.6) | 14.5 (11.0, 19.1) |
| Latin America and Caribbean | ||||
| No preeclampsia in first | 1396 | 14 (1.0) | 1.00 (Reference) | 1.00 (Reference) |
| Preeclampsia in first | 49 | 9 (18.4) | 18.3 (8.33, 40.3) | 17.4 (8.1, 37.4) |
| North Africa and Middle East | ||||
| No preeclampsia in first | 9092 | 87 (1.0) | 1.00 (Reference) | 1.00 (Reference) |
| Preeclampsia in first | 248 | 38 (15.3) | 16.0 (11.2, 22.9) | 14.9 (10.5, 21.3) |
| South Asia | ||||
| No preeclampsia in first | 4493 | 61 (1.4) | 1.00 (Reference) | 1.00 (Reference) |
| Preeclampsia in first | 148 | 23 (15.5) | 11.5 (7.29, 18.0) | 10.6 (6.8, 16.6) |
| Southeast Asia, East Asia, and Oceania | ||||
| No preeclampsia in first | 9447 | 103 (1.1) | 1.00 (Reference) | 1.00 (Reference) |
| Preeclampsia in first | 274 | 32 (11.7) | 10.7 (7.34, 15.6) | 10.4 (7.2, 15.2) |
| Sub-Saharan Africa | ||||
| No preeclampsia in first | 5893 | 70 (1.2) | 1.00 (Reference) | 1.00 (Reference) |
| Preeclampsia in first | 319 | 41 (12.9) | 10.8 (7.48, 15.6) | 10.4 (7.2, 15.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mæland, K.S.; Morken, N.-H.; Schytt, E.; Aasheim, V.; Nilsen, R.M. Risk of Subsequent Preeclampsia by Maternal Country of Birth: A Norwegian Population-Based Study. Int. J. Environ. Res. Public Health 2023, 20, 4109. https://doi.org/10.3390/ijerph20054109
Mæland KS, Morken N-H, Schytt E, Aasheim V, Nilsen RM. Risk of Subsequent Preeclampsia by Maternal Country of Birth: A Norwegian Population-Based Study. International Journal of Environmental Research and Public Health. 2023; 20(5):4109. https://doi.org/10.3390/ijerph20054109
Chicago/Turabian StyleMæland, Karolina S., Nils-Halvdan Morken, Erica Schytt, Vigdis Aasheim, and Roy M. Nilsen. 2023. "Risk of Subsequent Preeclampsia by Maternal Country of Birth: A Norwegian Population-Based Study" International Journal of Environmental Research and Public Health 20, no. 5: 4109. https://doi.org/10.3390/ijerph20054109
APA StyleMæland, K. S., Morken, N.-H., Schytt, E., Aasheim, V., & Nilsen, R. M. (2023). Risk of Subsequent Preeclampsia by Maternal Country of Birth: A Norwegian Population-Based Study. International Journal of Environmental Research and Public Health, 20(5), 4109. https://doi.org/10.3390/ijerph20054109









