Changes in the Histological Structure of Adrenal Glands and Corticosterone Level after Whey Protein or Bee Pollen Supplementation in Running and Non-Running Rats
Abstract
1. Introduction
2. Materials and Methods
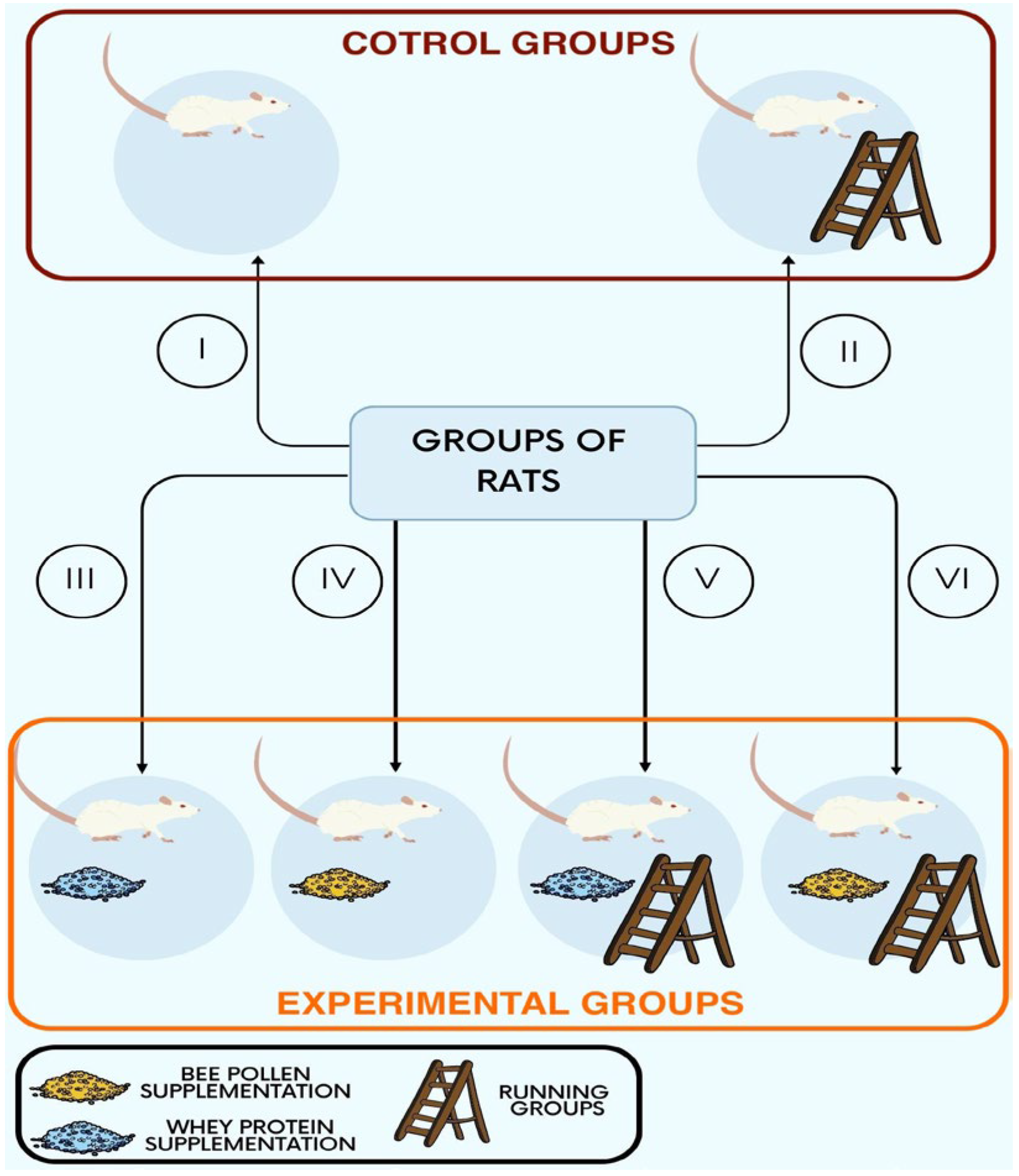
2.1. Study Protocol
2.2. Supplements
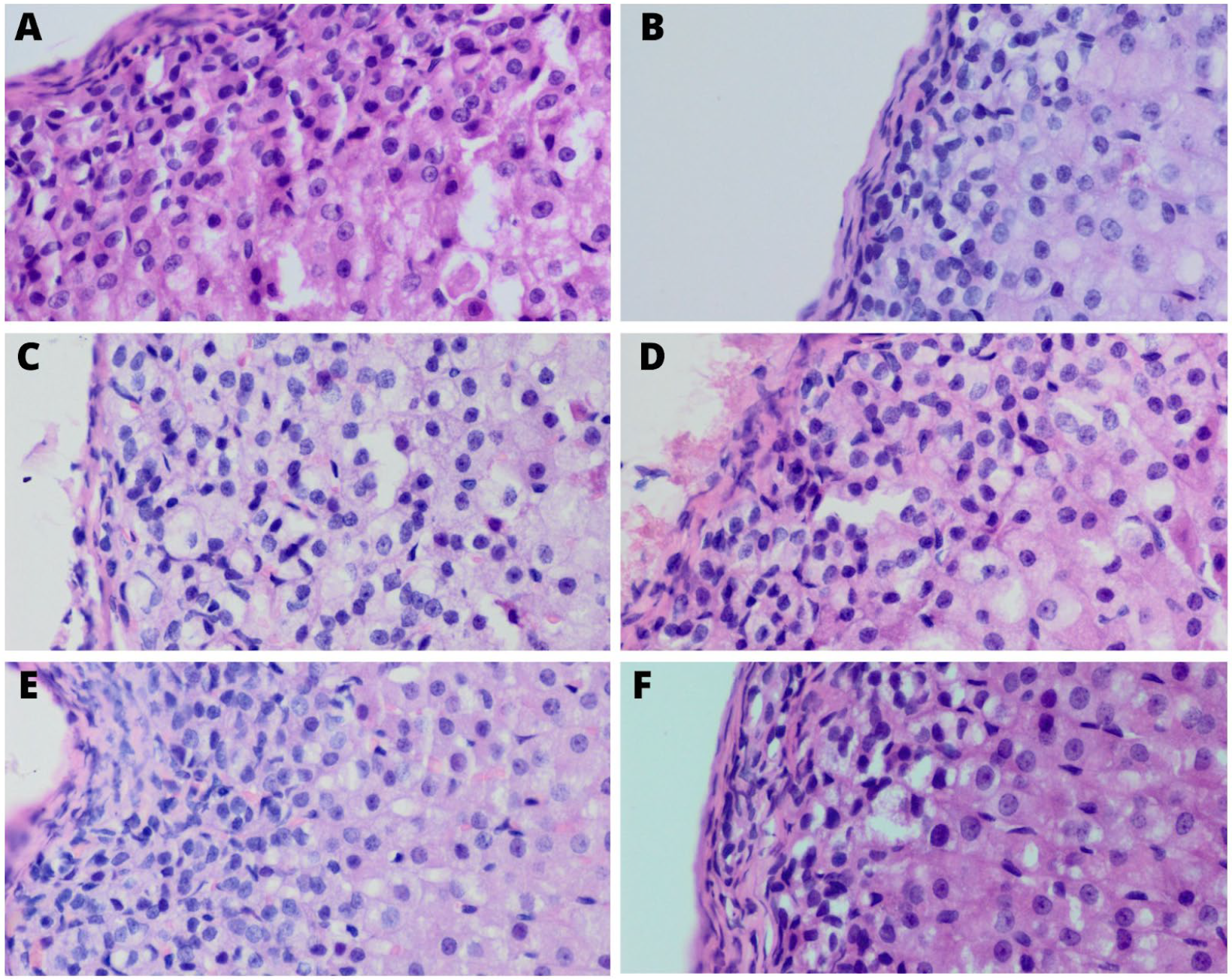
2.3. Histological Staining and Analysis
2.4. ELISA
2.5. Statistical Analysis
3. Results
3.1. Food Intake
3.2. Adrenal Gland Mass
3.3. Vacuoles and Structure
3.4. Fibrosis
3.5. Corticosterone Production
3.6. Pyknotic Nuclei
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicolaides, N.C.; Kyratzi, E.; Lamprokostopoulou, A.; Chrousos, G.P.; Charmandari, E. Stress, the Stress System and the Role of Glucocorticoids. Neuroimmunomodulation 2015, 22, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Gjerstad, J.K.; Lightman, S.L.; Spiga, F. Role of Glucocorticoid Negative Feedback in the Regulation of HPA Axis Pulsatility. Stress 2018, 21, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Vale, W.W. The Role of the Hypothalamic-Pituitary-Adrenal Axis in Neuroendocrine Responses to Stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Pacák, K.; Palkovits, M. Stressor Specificity of Central Neuroendocrine Responses: Implications for Stress-Related Disorders. Endocr. Rev. 2001, 22, 502–548. [Google Scholar] [CrossRef]
- Silva, A.A.; Perilhão, M.S.; Portes, L.A.; Serra, A.J.; Tucci, P.J.F.; Leopoldo, A.S.; dos Santos, L.; Bocalini, D.S. Physical Exercise Attenuates Stress-Induced Hypertension in Rats but Not the Impairments on the Myocardial Mechanics. J. Hypertens. 2021, 40, 528–535. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Panahi, Y.; Sahraei, H.; Johnston, T.P.; Sahebkar, A. The Impact of Stress on Body Function: A Review. EXCLI J. 2017, 16, 1057. [Google Scholar] [CrossRef]
- Barnthouse, M.; Jones, B.L. The Impact of Environmental Chronic and Toxic Stress on Asthma. Clin. Rev. Allergy Immunol. 2019, 57, 427–438. [Google Scholar] [CrossRef]
- Xu, W.; Tan, L.; Wang, H.-F.; Jiang, T.; Tan, M.-S.; Tan, L.; Zhao, Q.-F.; Li, J.-Q.; Wang, J.; Yu, J.-T. Meta-Analysis of Modifiable Risk Factors for Alzheimer’s Disease. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1299–1306. [Google Scholar] [CrossRef]
- Yang, T.; Qiao, Y.; Xiang, S.; Li, W.; Gan, Y.; Chen, Y. Work Stress and the Risk of Cancer: A Meta-analysis of Observational Studies. Int. J. Cancer 2019, 144, 2390–2400. [Google Scholar] [CrossRef]
- Torres, S.J.; Nowson, C.A. Relationship between Stress, Eating Behavior, and Obesity. Nutrition 2007, 23, 887–894. [Google Scholar] [CrossRef]
- Pecoraro, N.; Gomez, F.; Dallman, M.F. Glucocorticoids Dose-Dependently Remodel Energy Stores and Amplify Incentive Relativity Effects. Psychoneuroendocrinology 2005, 30, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Groesz, L.M.; McCoy, S.; Carl, J.; Saslow, L.; Stewart, J.; Adler, N.; Laraia, B.; Epel, E. What Is Eating You? Stress and the Drive to Eat. Appetite 2012, 58, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, N.; Reyes, F.; Gomez, F.; Bhargava, A.; Dallman, M.F. Chronic Stress Promotes Palatable Feeding, Which Reduces Signs of Stress: Feedforward and Feedback Effects of Chronic Stress. Endocrinology 2004, 145, 3754–3762. [Google Scholar] [CrossRef]
- Yeap, S.K.; Beh, B.K.; Ali, N.M.; Yusof, H.M.; Ho, W.Y.; Koh, S.P.; Alitheen, N.B.; Long, K. Antistress and Antioxidant Effects of Virgin Coconut Oil in Vivo. Exp. Ther. Med. 2015, 9, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Yeap, S.K.; Beh, B.K.; Ali, N.M.; Mohd Yusof, H.; Ho, W.Y.; Koh, S.P.; Alitheen, N.B.; Long, K. In Vivo Antistress and Antioxidant Effects of Fermented and Germinated Mung Bean. BioMed Res. Int. 2014, 2014, 694842. [Google Scholar] [CrossRef] [PubMed]
- Komosinska-Vassev, K.; Olczyk, P.; Kaźmierczak, J.; Mencner, L.; Olczyk, K. Bee Pollen: Chemical Composition and Therapeutic Application. Evid. Based Complement. Alternat. Med. 2015, 2015, 297425. [Google Scholar] [CrossRef]
- Denisow, B.; Denisow-Pietrzyk, M. Biological and Therapeutic Properties of Bee Pollen: A Review: Bee Pollen and Medicine. J. Sci. Food Agric. 2016, 96, 4303–4309. [Google Scholar] [CrossRef]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxid. Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elashal, M.H.; Yosri, N.; Du, M.; Musharraf, S.G.; Nahar, L.; Sarker, S.D.; Guo, Z.; Cao, W.; Zou, X.; et al. Bee Pollen: Current Status and Therapeutic Potential. Nutrients 2021, 13, 1876. [Google Scholar] [CrossRef]
- Habryka, C.; Socha, R.; Juszczak, L. Effect of Bee Pollen Addition on the Polyphenol Content, Antioxidant Activity, and Quality Parameters of Honey. Antioxidants 2021, 10, 810. [Google Scholar] [CrossRef]
- Lagrange, V.; Whitsett, D.; Burris, C. Global Market for Dairy Proteins: Global Market for Dairy Proteins. J. Food Sci. 2015, 80, A16–A22. [Google Scholar] [CrossRef] [PubMed]
- Krissansen, G.W. Emerging Health Properties of Whey Proteins and Their Clinical Implications. J. Am. Coll. Nutr. 2007, 26, 713S–723S. [Google Scholar] [CrossRef]
- Patel, S. Emerging Trends in Nutraceutical Applications of Whey Protein and Its Derivatives. J. Food Sci. Technol. 2015, 52, 6847–6858. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.H.A.; de Araújo, F.H.S.; Olimpio, M.Y.M.; Primo, R.B.D.B.; Pereira, T.T.; Lopes, L.A.F.; Trindade, E.B.S.D.M.; Fernandes, R.; Oesterreich, S.A. Comparative Meta-Analysis of the Effect of Concentrated, Hydrolyzed, and Isolated Whey Protein Supplementation on Body Composition of Physical Activity Practitioners. Nutrients 2019, 11, 2047. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Perez-Cueto, F.; Bredie, W. Sensory-Driven Development of Protein-Enriched Rye Bread and Cream Cheese for the Nutritional Demands of Older Adults. Nutrients 2018, 10, 1006. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, U.T.; Amen, O.A.; Applegate, T.J.; Cheng, H.W. Brazilian Propolis Effects on Growth, Productivity Performance, Gut Characteristics and Physiological Changes in Broiler Chickens. Int. J. Poult. Sci. 2017, 16, 169–179. [Google Scholar] [CrossRef]
- Mahmoud, U.T.; Abdel-Rahman, M.A.M.; Darwish, M.H.A. Effects of Propolis, Ascorbic Acid and Vitamin E on Thyroid and Corticosterone Hormones in Heat Stressed Broilers. J. Adv. Vet. Res. 2014, 4, 18–27. [Google Scholar]
- Chegini, S.; Kiani, A.; Rokni, H. Alleviation of Thermal and Overcrowding Stress in Finishing Broilers by Dietary Propolis Supplementation. Ital. J. Anim. Sci. 2018, 17, 377–385. [Google Scholar] [CrossRef]
- Caixeta, D.C.; Teixeira, R.R.; Peixoto, L.G.; Machado, H.L.; Baptista, N.B.; de Souza, A.V.; Vilela, D.D.; Franci, C.R.; Salmen Espindola, F. Adaptogenic Potential of Royal Jelly in Liver of Rats Exposed to Chronic Stress. PLoS ONE 2018, 13, e0191889. [Google Scholar] [CrossRef]
- Teixeira, R.R.; de Souza, A.V.; Peixoto, L.G.; Machado, H.L.; Caixeta, D.C.; Vilela, D.D.; Baptista, N.B.; Franci, C.R.; Espindola, F.S. Royal Jelly Decreases Corticosterone Levels and Improves the Brain Antioxidant System in Restraint and Cold Stressed Rats. Neurosci. Lett. 2017, 655, 179–185. [Google Scholar] [CrossRef]
- Ortolani, D.; Garcia, M.C.; Melo-Thomas, L.; Spadari-Bratfisch, R.C. Stress-Induced Endocrine Response and Anxiety: The Effects of Comfort Food in Rats. Stress 2014, 17, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Harvey, S.; Phillips, J.G.; Rees, A.; Hall, T.R. Stress and Adrenal Function. J. Exp. Zool. 1984, 232, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Zarobkiewicz, M.K.; Sławiński, M.A.; Wawryk-Gawda, E.; Woźniakowski, M.M.; Kulak-Janczy, E.; Korzeniowska, S.; Jodłowska-Jędrych, B. Changes in Histological Structure and Nitric Oxide Synthase Expression in Aorta of Rats Supplemented with Bee Pollen or Whey Protein. Appl. Physiol. Nutr. Metab. 2019, 44, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Zarobkiewicz, M.K.; Woźniakowski, M.M.; Wawryk-Gawda, E.; Sławiński, M.A.; Halczuk, P.; Korolczuk, A.; Jodłowska-Jędrych, B. Decrease in Lipid Droplets in Adrenal Cortex of Male Wistar Rats after Chronic Exposure to Energy Drinks. Medicina 2018, 54, 90. [Google Scholar] [CrossRef] [PubMed]
- Pihl, L.; Hau, J. Faecal Corticosterone and Immunoglobulin A in Young Adult Rats. Lab. Anim. 2003, 37, 166–171. [Google Scholar] [CrossRef]
- Maniam, J.; Morris, M.J. The Link between Stress and Feeding Behaviour. Neuropharmacology 2012, 63, 97–110. [Google Scholar] [CrossRef]
- Zellner, D.A.; Loaiza, S.; Gonzalez, Z.; Pita, J.; Morales, J.; Pecora, D.; Wolf, A. Food Selection Changes under Stress. Physiol. Behav. 2006, 87, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Adam, T.C.; Epel, E.S. Stress, Eating and the Reward System. Physiol. Behav. 2007, 91, 449–458. [Google Scholar] [CrossRef]
- Ulrich-Lai, Y.M.; Fulton, S.; Wilson, M.; Petrovich, G.; Rinaman, L. Stress Exposure, Food Intake and Emotional State. Stress Amst. Neth. 2015, 18, 381–399. [Google Scholar] [CrossRef]
- Berger, I.; Werdermann, M.; Bornstein, S.R.; Steenblock, C. The Adrenal Gland in Stress—Adaptation on a Cellular Level. J. Steroid Biochem. Mol. Biol. 2019, 190, 198–206. [Google Scholar] [CrossRef]
- Marin, M.T.; Cruz, F.C.; Planeta, C.S. Chronic Restraint or Variable Stresses Differently Affect the Behavior, Corticosterone Secretion and Body Weight in Rats. Physiol. Behav. 2007, 90, 29–35. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, M.Y.; Choi, C.S.; Sohn, Y.W.; Park, B.R.; Choi, M.-G.; Nah, Y.-H.; Choi, S.C. The Effect of Chronic Variable Stress on Bowel Habit and Adrenal Function in Rats. J. Gastroenterol. Hepatol. 2008, 23, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Aguila, Y.; Cuevas-Romero, E.; Castelán, F.; Martínez-Gómez, M.; Rodríguez-Antolín, J.; Nicolás-Toledo, L. Chronic Stress and High Sucrose Intake Cause Distinctive Morphometric Effects in the Adrenal Glands of Post-Weaned Rats. Biotech. Histochem. 2018, 93, 565–574. [Google Scholar] [CrossRef]
- Zeeni, N.; Bassil, M.; Fromentin, G.; Chaumontet, C.; Darcel, N.; Tome, D.; Daher, C.F. Environmental Enrichment and Cafeteria Diet Attenuate the Response to Chronic Variable Stress in Rats. Physiol. Behav. 2015, 139, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Martí, O.; Martí, J.; Armario, A. Effects of Chronic Stress on Food Intake in Rats: Influence of Stressor Intensity and Duration of Daily Exposure. Physiol. Behav. 1994, 55, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Macedo, I.C.; Medeiros, L.F.; Oliveira, C.; Oliveira, C.M.; Rozisky, J.R.; Scarabelot, V.L.; Souza, A.; Silva, F.R.; Santos, V.S.; Cioato, S.G.; et al. Cafeteria Diet-Induced Obesity plus Chronic Stress Alter Serum Leptin Levels. Peptides 2012, 38, 189–196. [Google Scholar] [CrossRef]
- Oliveira, M.J.; Monteiro, M.; Ribeiro, A.; Pignatelli, D.; Aguas, A. Chronic Exposure of Rats to Occupational Textile Noise Causes Cytological Changes in Adrenal Cortex. Noise Health 2009, 11, 118. [Google Scholar] [CrossRef]
- Zaki, S.M.; Abdelgawad, F.A.; El-Shaarawy, E.A.A.; Radwan, R.A.K.; Aboul-Hoda, B.E. Stress-Induced Changes in the Aged-Rat Adrenal Cortex. Histological and Histomorphometric Study. Folia Morphol. 2018, 77, 629–641. [Google Scholar] [CrossRef]
- Webster, M.; Witkin, K.L.; Cohen-Fix, O. Sizing up the Nucleus: Nuclear Shape, Size and Nuclear-Envelope Assembly. J. Cell Sci. 2009, 122, 1477–1486. [Google Scholar] [CrossRef]
- Saad Al-Dh, I. Possible Protective Role of Whey Protein on the Rat’s Liver Tissues Treated with Nandrolone Decanoate. Pak. J. Biol. Sci. 2018, 21, 262–274. [Google Scholar] [CrossRef]
- Mahmoud, O.M.; Salem, N.A.; Al Badawi, M.H. Protective Effect of Propolis on Manganese Chloride Neurotoxicity of Olfactory Bulb in Adult Male Albino Rat. Folia Morphol. 2020, 79, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Koko, V.; Djordjeviæ, J.; Cvijiæ, G.; Davidoviæ, V. Effect of Acute Heat Stress on Rat Adrenal Glands: A Morphological and Stereological Study. J. Exp. Biol. 2004, 207, 4225–4230. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.; Othman, Z.A.; Suleiman, J.B.; Che Jalil, N.A.; Ghazali, W.S.W.; Nna, V.U.; Mohamed, M. Hepatoprotective Effect of Bee Bread in Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) Rats: Impact on Oxidative Stress and Inflammation. Antioxidants 2021, 10, 2031. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, J.; Gould, K.; Borman, E.; deCatanzaro, D. Circulating and Urinary Adrenal Corticosterone, Progesterone, and Estradiol in Response to Acute Stress in Female Mice (Mus musculus). Horm. Metab. Res. 2014, 46, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, J.; Rajabi, N.; deCatanzaro, D. Circadian Rhythm and Response to an Acute Stressor of Urinary Corticosterone, Testosterone, and Creatinine in Adult Male Mice. Horm. Metab. Res. 2012, 44, 429–435. [Google Scholar] [CrossRef]
- Zylan, K.D.; Brown, S.D. Effect of Stress and Food Variety on Food Intake in Male and Female Rats. Physiol. Behav. 1996, 59, 165–169. [Google Scholar] [CrossRef]
- Sudo, A.; Miki, K. Dissociation of Catecholamine and Corticosterone Responses to Different Types of Stress in Rats. Ind. Health 1993, 31, 101–111. [Google Scholar] [CrossRef]
- Premack, D.; Premack, A.J. Increased Eating in Rats Deprived of Running. J. Exp. Anal. Behav. 1963, 6, 209–212. [Google Scholar] [CrossRef]
- Kashima, Y.; Kanematsu, S.; Asai, S.; Kusada, M.; Watanabe, S.; Kawashima, T.; Nakamura, T.; Shimada, M.; Goto, T.; Nagaoka, S. Identification of a Novel Hypocholesterolemic Protein, Major Royal Jelly Protein 1, Derived from Royal Jelly. PLoS ONE 2014, 9, e105073. [Google Scholar] [CrossRef]
- Ortolani, D.; Oyama, L.M.; Ferrari, E.M.; Melo, L.L.; Spadari-Bratfisch, R.C. Effects of Comfort Food on Food Intake, Anxiety-like Behavior and the Stress Response in Rats. Physiol. Behav. 2011, 103, 487–492. [Google Scholar] [CrossRef]
- Corona-Pérez, A.; Díaz-Muñoz, M.; Rodríguez, I.S.; Cuevas, E.; Martínez-Gómez, M.; Castelán, F.; Rodríguez-Antolín, J.; Nicolás-Toledo, L. High Sucrose Intake Ameliorates the Accumulation of Hepatic Triacylglycerol Promoted by Restraint Stress in Young Rats. Lipids 2015, 50, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Oi, Y.; Imafuku, M.; Shishido, C.; Kominato, Y.; Nishimura, S.; Iwai, K. Garlic Supplementation Increases Testicular Testosterone and Decreases Plasma Corticosterone in Rats Fed a High Protein Diet. J. Nutr. 2001, 131, 2150–2156. [Google Scholar] [CrossRef] [PubMed]
- Oi-Kano, Y.; Kawada, T.; Watanabe, T.; Koyama, F.; Watanabe, K.; Senbongi, R.; Iwai, K. Oleuropein Supplementation Increases Urinary Noradrenaline and Testicular Testosterone Levels and Decreases Plasma Corticosterone Level in Rats Fed High-Protein Diet. J. Nutr. Biochem. 2013, 24, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Makkar, S.K.; Rath, N.C.; Packialakshmi, B.; Zhou, Z.Y.; Huff, G.R.; Donoghue, A.M. Nutritional Supplement of Hatchery Eggshell Membrane Improves Poultry Performance and Provides Resistance against Endotoxin Stress. PLoS ONE 2016, 11, e0159433. [Google Scholar] [CrossRef]
- Greco, A.M.; Boschi, G.; Valentino, R. Evaluation of Aldosterone, Corticosterone, Glucose and Triglyceride Blood Levels in Pregnant Rats Fed a High Protein Diet. Boll. Soc. Ital. Biol. Sper. 1982, 58, 115–120. [Google Scholar]
- Maekawa, Y.; Sugiyama, A.; Takeuchi, T. Lactoferrin Ameliorates Corticosterone-Related Acute Stress and Hyperglycemia in Rats. J. Vet. Med. Sci. 2017, 79, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Matsunaga, K.; Sugiyama, A. Antidepressant-like Effect of Milk-Derived Lactoferrin in the Repeated Forced-Swim Stress Mouse Model. J. Vet. Med. Sci. 2017, 79, 1803–1806. [Google Scholar] [CrossRef]
| Group | I | II | III | IV | V | VI | p A |
|---|---|---|---|---|---|---|---|
| Zona glomerulosa | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.7612 |
| Zona fasciculata | 0.04 | 0.02 | 0.02 | 0.03 | 0.03 | 0.02 | 0.0516 |
| Zona reticularis | 0.03 | 0.03 | 0.03 | 0.03 | 0.02 | 0.03 | 0.1043 |
| Group | I | II | III | IV | V | VI | p A |
|---|---|---|---|---|---|---|---|
| Nucleus diameter [μm] | |||||||
| Zona glomerulosa | 3.88, 0.905 | 4.12, 1.25 | 4.14, 0.843 | 4.74, 1.15 | 4.57, 1.16 | 5.21, 1.22 | <0.0001 |
| Zona fasciculata | 5.23, 1.03 | 4.94, 1.50 | 5.53, 1.12 | 5.83, 1.38 | 6.13, 1.31 | 6.23, 1.11 | <0.0001 |
| Zona reticularis | 4.54, 0.84 | 4.70, 1.04 | 4.44, 0.57 | 4.20, 0.66 | 5.00, 0.61 | 5.22, 0.62 | <0.0001 |
| Medulla | 5.43, 0.75 | 6.38, 0.86 | 6.02, 0.75 | 6.63, 0.58 | 6.14, 0.81 | 6.70, 0.95 | <0.0001 |
| Other measurements [μm] | |||||||
| Sinusoid width | 2.97, 0.82 | 4.24, 1.20 | 2.98, 1.16 | 4.08, 1.18 | 4.36, 2.06 | 3.72, 1.67 | 0.0009 |
| Sinusoid epithelium thickness | 1.40, 0.37 | 1.22, 0.29 | 1.18, 0.28 | 1.09, 0.30 | 1.54, 0.38 | 1.28, 0.32 | 0.0057 |
| Sinusoid epithelial cell nucleus thickness | 2.40, 0.46 | 2.79, 0.53 | 2.36, 0.48 | 2.74, 0.49 | 2.81, 0.59 | 2.71, 0.58 | 0.0025 |
| Capsule thickness | 14.03,1.90 | 11.58,2.80 | 14.24,2.74 | 16.01,4.60 | 15.17, 3.59 | 14.25, 4.04 | 0.0019 |
| Group | I | II | III | IV | V | VI | p A |
|---|---|---|---|---|---|---|---|
| Zona glomerulosa | 0.40 ± 0.10 | 0.37 ± 0.05 | 0.43±0.08 | 0.28 ± 0.05 | 0.34 ± 0.07 | 0.45 ± 0.13 | 0.0003 |
| Zona fasciculata | 0.45 ± 0.09 | 0.31 ± 0.03 | 0.38±0.04 | 0.30 ± 0.02 | 0.32 ± 0.04 | 0.43 ± 0.09 | <0.0001 |
| Zona reticularis | 0.45 ± 0.05 | 0.38 ± 0.08 | 0.38±0.03 | 0.45 ± 0.07 | 0.34 ± 0.07 | 0.52 ± 0.11 | <0.0001 |
| Group | I | II | III | IV | V | VI | p A |
|---|---|---|---|---|---|---|---|
| Corticosterone in urine [ng/mL] | 74.51 ± 16.12 | 64.23 ± 28.69 | 23.89 ± 7.80 | 36.60 ± 19.38 | 83.51 ± 37.24 | 88.99 ± 26.83 | 0.0037 |
| Corticosterone in feces [μg/mg] | 0.84 ± 0.33 | 1.68 ± 0.46 | 0.91 ± 0.11 | 1.98 ± 0.74 | 1.21 ± 0.72 | 1.24 ± 0.79 | 0.0612 |
| Total daily corticosterone excretion [μg] | 5.74 ± 2.16 | 8.51 ± 3.14 | 8.28 ± 5.13 | 11.10 ± 4.71 | 7.70 ± 3.40 | 12.58 ± 1149 | 0.4016 |
| Group | I | II | III | IV | V | VI | p A |
|---|---|---|---|---|---|---|---|
| Zona glomerulosa | 18.65 | 8.39 | 9.64 | 7.01 | 10.23 | 7.00 | <0.0001 |
| Zona fasciculata | 13.68 | 8.38 | 7.26 | 7.09 | 6.46 | 4.09 | 0.0003 |
| Zona reticularis | 14.97 | 7.40 | 6.96 | 7.71 | 4.01 | 2.88 | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frankowska, K.; Zarobkiewicz, M.; Sławiński, M.A.; Wawryk-Gawda, E.; Abramiuk, M.; Jodłowska-Jędrych, B. Changes in the Histological Structure of Adrenal Glands and Corticosterone Level after Whey Protein or Bee Pollen Supplementation in Running and Non-Running Rats. Int. J. Environ. Res. Public Health 2023, 20, 4105. https://doi.org/10.3390/ijerph20054105
Frankowska K, Zarobkiewicz M, Sławiński MA, Wawryk-Gawda E, Abramiuk M, Jodłowska-Jędrych B. Changes in the Histological Structure of Adrenal Glands and Corticosterone Level after Whey Protein or Bee Pollen Supplementation in Running and Non-Running Rats. International Journal of Environmental Research and Public Health. 2023; 20(5):4105. https://doi.org/10.3390/ijerph20054105
Chicago/Turabian StyleFrankowska, Karolina, Michał Zarobkiewicz, Mirosław A. Sławiński, Ewelina Wawryk-Gawda, Monika Abramiuk, and Barbara Jodłowska-Jędrych. 2023. "Changes in the Histological Structure of Adrenal Glands and Corticosterone Level after Whey Protein or Bee Pollen Supplementation in Running and Non-Running Rats" International Journal of Environmental Research and Public Health 20, no. 5: 4105. https://doi.org/10.3390/ijerph20054105
APA StyleFrankowska, K., Zarobkiewicz, M., Sławiński, M. A., Wawryk-Gawda, E., Abramiuk, M., & Jodłowska-Jędrych, B. (2023). Changes in the Histological Structure of Adrenal Glands and Corticosterone Level after Whey Protein or Bee Pollen Supplementation in Running and Non-Running Rats. International Journal of Environmental Research and Public Health, 20(5), 4105. https://doi.org/10.3390/ijerph20054105






