Bacterial Colonization of Microplastics at the Beaches of an Oceanic Island, Tenerife, Canary Islands
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Field Work
2.2. Laboratory Analysis of MPs for Microorganism Detection
2.3. MPs Color Classification
2.4. MPs Composition
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plastics Europe. Plastics-the Facts; Plastics Europe: Brussels, Belgium, 2021. [Google Scholar]
- De-la-Torre, G.E.; Dioses-Salinas, D.C.; Pizarro-Ortega, C.I.; Santillán, L. New Plastic Formations in the Anthropocene. Sci. Total Environ. 2021, 754, 142216. [Google Scholar] [CrossRef]
- Domínguez-Hernández, C.; Villanova-Solano, C.; Sevillano-González, M.; Hernández-Sánchez, C.; González-Sálamo, J.; Ortega-Zamora, C.; Díaz-Peña, F.J.; Hernández-Borges, J. Plastitar: A New Threat for Coastal Environments. Sci. Total Environ. 2022, 839, 156261. [Google Scholar] [CrossRef]
- Ehlers, S.M.; Ellrich, J.A. First Record of ‘Plasticrusts’ and ‘Pyroplastic’ from the Mediterranean Sea. Mar. Pollut. Bull. 2020, 151, 110845. [Google Scholar] [CrossRef]
- Turner, A.; Wallerstein, C.; Arnold, R.; Webb, D. Marine Pollution from Pyroplastics. Sci. Total Environ. 2019, 694, 133610. [Google Scholar] [CrossRef]
- Ryan, P.G.; Moore, C.J.; Van Franeker, J.A.; Moloney, C.L. Monitoring the Abundance of Plastic Debris in the Marine Environment. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1999–2012. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olson, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The Physical Impacts of Microplastics on Marine Organisms: A Review the Physical Impacts of Microplastics on Marine Organisms: A Review; Elsevier: Amsterdam, The Netherlands, 2013; Volume 178, pp. 483–492. [Google Scholar]
- Zarfl, C.; Fleet, D.; Fries, E.; Galgani, F.; Gerdts, G.; Hanke, G.; Matthies, M. Microplastics in Oceans. Mar. Pollut. Bull. 2011, 62, 1589–1591. [Google Scholar] [CrossRef]
- Chambault, P.; Vandeperre, F.; Machete, M.; Lagoa, J.C.; Pham, C.K. Distribution and Composition of Floating Macro Litter off the Azores Archipelago and Madeira (NE Atlantic) Using Opportunistic Surveys. Mar. Environ. Res. 2018, 141, 225–232. [Google Scholar] [CrossRef]
- Pieper, C.; Ventura, M.A.; Martins, A.; Cunha, R.T. Beach Debris in the Azores (NE Atlantic): Faial Island as a First Case Study. Mar. Pollut. Bull. 2015, 101, 575–582. [Google Scholar] [CrossRef]
- Álvarez, S.; Gestoso, I.; Herrera, A.; Riera, L.; Canning-Clode, J. A Comprehensive First Baseline for Marine Litter Characterization in the Madeira Archipelago (NE Atlantic). Water Air Soil Pollut. 2020, 231, 182. [Google Scholar] [CrossRef]
- Monteiro, R.C.P.; Ivar do Sul, J.A.; Costa, M.F. Plastic Pollution in Islands of the Atlantic Ocean. Environ. Pollut. 2018, 238, 103–110. [Google Scholar] [CrossRef]
- Silvestrova, K.; Stepanova, N. The Distribution of Microplastics in the Surface Layer of the Atlantic Ocean from the Subtropics to the Equator According to Visual Analysis. Mar. Pollut. Bull. 2021, 162, 111836. [Google Scholar] [CrossRef]
- Álvarez-Hernández, C.; Cairós, C.; López-Darias, J.; Mazzetti, E.; Hernández-Sánchez, C.; González-Sálamo, J.; Hernández-Borges, J. Microplastic Debris in Beaches of Tenerife (Canary Islands, Spain). Mar. Pollut. Bull. 2019, 146, 26–32. [Google Scholar] [CrossRef]
- Baztan, J.; Carrasco, A.; Chouinard, O.; Cleaud, M.; Gabaldon, J.E.; Huck, T.; Jaffrès, L.; Jorgensen, B.; Miguelez, A.; Paillard, C.; et al. Protected Areas in the Atlantic Facing the Hazards of Micro-Plastic Pollution: First Diagnosis of Three Islands in the Canary Current. Mar. Pollut. Bull. 2014, 80, 302–311. [Google Scholar] [CrossRef]
- Edo, C.; Tamayo-Belda, M.; Martínez-Campos, S.; Martín-Betancor, K.; González-Pleiter, M.; Pulido-Reyes, G.; García-Ruiz, C.; Zapata, F.; Leganés, F.; Fernández-Piñas, F.; et al. Occurrence and Identification of Microplastics along a Beach in the Biosphere Reserve of Lanzarote. Mar. Pollut. Bull. 2019, 143, 220–227. [Google Scholar] [CrossRef]
- Sevillano-González, M.; González-Sálamo, J.; Díaz-Peña, F.J.; Hernández-Sánchez, C.; Catalán Torralbo, S.; Ródenas Seguí, A.; Hernández-Borges, J. Assessment of Microplastic Content in Diadema Africanum Sea Urchin from Tenerife (Canary Islands, Spain). Mar. Pollut. Bull. 2022, 175, 113174. [Google Scholar] [CrossRef]
- Herrera, A.; Asensio, M.; Martínez, I.; Santana, A.; Packard, T.; Gómez, M. Microplastic and Tar Pollution on Three Canary Islands Beaches: An Annual Study. Mar. Pollut. Bull. 2018, 129, 494–502. [Google Scholar] [CrossRef]
- Rapp, J.; Herrera, A.; Martinez, I.; Raymond, E.; Santana, Á.; Gómez, M. Study of Plastic Pollution and Its Potential Sources on Gran Canaria Island Beaches (Canary Islands, Spain). Mar. Pollut. Bull. 2020, 153, 110967. [Google Scholar] [CrossRef]
- Reinold, S.; Herrera, A.; Hernández-González, C.; Gómez, M. Plastic Pollution on Eight Beaches of Tenerife (Canary Islands, Spain): An Annual Study. Mar. Pollut. Bull. 2020, 151, 110847. [Google Scholar] [CrossRef]
- 2006/7/EC Document. Directive 2006/7/ec of the European Parliament and of the Council Concerning the Management of Bathing Water Quality and Repealing Directive 76/160/EEC. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX:32006L0007 (accessed on 27 July 2022).
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “Plastisphere”: Microbial Communities on Plastic Marine Debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Carpenter, E.J.; Anderson, S.J.; Harvey, G.R.; Miklas, H.P.; Peck, B.B. Polystyrene Spherules in Coastal Waters. Science 1972, 178, 749–750. [Google Scholar] [CrossRef] [PubMed]
- Amaral-Zettler, L.A.; Zettler, E.R.; Mincer, T.J. Ecology of the Plastisphere. Nat. Rev. Microbiol. 2020, 18, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Bowley, J.; Baker-Austin, C.; Porter, A.; Hartnell, R.; Lewis, C. Oceanic Hitchhikers—Assessing Pathogen Risks from Marine Microplastic. Trends Microbiol. 2021, 29, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, A. A Roadmap for a Plastisphere. Mar. Pollut. Bull. 2021, 167, 112322. [Google Scholar] [CrossRef]
- Kirstein, I.V.; Kirmizi, S.; Wichels, A.; Garin-Fernandez, A.; Erler, R.; Löder, M.; Gerdts, G. Dangerous Hitchhikers? Evidence for Potentially Pathogenic Vibrio Spp. on Microplastic Particles. Mar. Environ. Res. 2016, 120, 1–8. [Google Scholar] [CrossRef]
- De Tender, C.; Devriese, L.I.; Haegeman, A.; Maes, S.; Vangeyte, J.; Cattrijsse, A.; Dawyndt, P.; Ruttink, T. Temporal Dynamics of Bacterial and Fungal Colonization on Plastic Debris in the North Sea. Environ. Sci. Technol. 2017, 51, 7350–7360. [Google Scholar] [CrossRef]
- McCormick, A.; Hoellein, T.J.; Mason, S.A.; Schluep, J.; Kelly, J.J. Microplastic Is an Abundant and Distinct Microbial Habitat in an Urban River. Environ. Sci. Technol. 2014, 48, 11863–11871. [Google Scholar] [CrossRef]
- Miao, L.; Wang, P.; Hou, J.; Yao, Y.; Liu, Z.; Liu, S.; Li, T. Distinct Community Structure and Microbial Functions of Biofilms Colonizing Microplastics. Sci. Total Environ. 2019, 650, 2395–2402. [Google Scholar] [CrossRef]
- Rodrigues, A.; Oliver, D.M.; McCarron, A.; Quilliam, R.S. Colonisation of Plastic Pellets (Nurdles) by E. Coli at Public Bathing Beaches. Mar. Pollut. Bull. 2019, 139, 376–380. [Google Scholar] [CrossRef]
- Silva, M.M.; Maldonado, G.C.; Castro, R.O.; de Sá Felizardo, J.; Cardoso, R.P.; dos Anjos, R.M.; Araújo, F.V. de Dispersal of Potentially Pathogenic Bacteria by Plastic Debris in Guanabara Bay, RJ, Brazil. Mar. Pollut. Bull. 2019, 141, 561–568. [Google Scholar] [CrossRef]
- Liang, H.; de Haan, W.P.; Cerdà-Domènech, M.; Méndez, J.; Lucena, F.; García-Aljaro, C.; Sanchez-Vidal, A.; Ballesté, E. Detection of Faecal Bacteria and Antibiotic Resistance Genes in Biofilms Attached to Plastics from Human-Impacted Coastal Areas. Environ. Pollut. 2023, 319, 120983. [Google Scholar] [CrossRef] [PubMed]
- Martí, E.; Martin, C.; Galli, M.; Echevarría, F.; Duarte, C.M.; Andreés, A.; Coózar, C.; Cózar, A. The Colors of the Ocean Plastics. Environ. Sci. Technol. 2020, 54, 6594–6601. [Google Scholar] [CrossRef] [PubMed]
- The European Comission Law—EU Coastal and Marine Policy—Environment—European Commission. Available online: https://ec.europa.eu/environment/marine/eu-coast-and-marine-policy/marine-strategy-framework-directive/index_en.htm (accessed on 17 September 2019).
- González-Hernández, M.; Hernández-Sánchez, C.; González-Sálamo, J.; López-Darias, J.; Hernández-Borges, J. Monitoring of Meso and Microplastic Debris in Playa Grande Beach (Tenerife, Canary Islands, Spain) during a Moon Cycle. Mar. Pollut. Bull. 2020, 150, 110757. [Google Scholar] [CrossRef]
- Hernández-Sánchez, C.; González-Sálamo, J.; Díaz-Peña, F.J.; Fraile-Nuez, E.; Hernández-Borges, J. Arenas Blancas (El Hierro Island), a New Hotspot of Plastic Debris in the Canary Islands (Spain). Mar. Pollut. Bull. 2021, 169, 112548. [Google Scholar] [CrossRef] [PubMed]
- Reinold, S.; Herrera, A.; Saliu, F.; Hernández-González, C.; Martinez, I.; Lasagni, M.; Gómez, M. Evidence of Microplastic Ingestion by Cultured European Sea Bass (Dicentrarchus labrax). Mar. Pollut. Bull. 2021, 168, 112450. [Google Scholar] [CrossRef]
- Abaroa-Pérez, B.; Ortiz-Montosa, S.; Hernández-Brito, J.J.; Vega-Moreno, D. Yellowing, Weathering and Degradation of Marine Pellets and Their Influence on the Adsorption of Chemical Pollutants. Polymers 2022, 14, 1305. [Google Scholar] [CrossRef]
- Guo, X.; Wang, J. The Chemical Behaviors of Microplastics in Marine Environment: A Review. Mar. Pollut. Bull. 2019, 142, 1–14. [Google Scholar] [CrossRef]
- Frère, L.; Maignien, L.; Chalopin, M.; Huvet, A.; Rinnert, E.; Morrison, H.; Kerninon, S.; Cassone, A.L.; Lambert, C.; Reveillaud, J.; et al. Microplastic Bacterial Communities in the Bay of Brest: Influence of Polymer Type and Size. Environ. Pollut. 2018, 242, 614–625. [Google Scholar] [CrossRef]
- Viršek, M.K.; Lovšin, M.N.; Koren, Š.; Kržan, A.; Peterlin, M. Microplastics as a Vector for the Transport of the Bacterial Fish Pathogen Species Aeromonas Salmonicida. Mar. Pollut. Bull. 2017, 125, 301–309. [Google Scholar] [CrossRef]
- Jacobs Slifka, K.M.; Newton, A.E.; Mahon, B.E. Vibrio Alginolyticus Infections in the USA, 1988–2012. Epidemiol. Infect. 2017, 145, 1491–1499. [Google Scholar] [CrossRef]
- Pezzlo, M.; Valter, P.J.; Burns, M.J. Wound Infection Associated with Vibrio Alginolyticus. Am. J. Clin. Pathol. 1979, 71, 476–478. [Google Scholar] [CrossRef] [PubMed]
- Pedrotti, M.L.; de Figueiredo Lacerda, A.L.; Petit, S.; Ghiglione, J.F.; Gorsky, G. Vibrio Spp and Other Potential Pathogenic Bacteria Associated to Microfibers in the North-Western Mediterranean Sea. PLoS ONE 2022, 17, e0275284. [Google Scholar] [CrossRef] [PubMed]
- Elmir, S.M.; Wright, M.E.; Abdelzaher, A.; Solo-Gabriele, H.M.; Fleming, L.E.; Miller, G.; Rybolowik, M.; Peter Shih, M.T.; Pillai, S.P.; Cooper, J.A.; et al. Quantitative Evaluation of Bacteria Released by Bathers in a Marine Water. Water Res. 2007, 41, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Topić, N.; Cenov, A.; Jozić, S.; Glad, M.; Mance, D.; Lušić, D.; Kapetanović, D.; Mance, D.; Lušić, D.V. Staphylococcus Aureus—An Additional Parameter of Bathing Water Quality for Crowded Urban Beaches. Int. J. Environ. Res. Public Health 2021, 18, 5234. [Google Scholar] [CrossRef]
- Beloe, C.J.; Browne, M.A.; Johnston, E.L. Plastic Debris As a Vector for Bacterial Disease: An Interdisciplinary Systematic Review. Environ. Sci. Technol. 2022, 56, 2950–2958. [Google Scholar] [CrossRef]
- Ministerio de Sanidad. y B.S. Sistema de Información Nacional de Aguas de Consumo SINAC. Available online: http://nayadeciudadano.sanidad.gob.es/ (accessed on 16 February 2023).
- Grimes, D.J. The Vibrios: Scavengers, Symbionts, and Pathogens from the Sea. Microb. Ecol. 2020, 80, 501–506. [Google Scholar] [CrossRef]
- Bhagwat, G.; Zhu, Q.; O’Connor, W.; Subashchandrabose, S.; Grainge, I.; Knight, R.; Palanisami, T. Exploring the Composition and Functions of Plastic Microbiome Using Whole-Genome Sequencing. Environ. Sci. Technol. 2021, 55, 4899–4913. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, M.; Su, H.; Xiao, H.; Wu, S.; Qin, X.; Li, S.; Mi, H.; Lu, Z.; Shi, D.; et al. Selection and Characterization of SsDNA Aptamers Specifically Recognizing Pathogenic Vibrio Alginolyticus. J. Fish Dis. 2019, 42, 851–858. [Google Scholar] [CrossRef]
- Krüger, L.; Casado-Coy, N.; Valle, C.; Ramos, M.; Sánchez-Jerez, P.; Gago, J.; Carretero, O.; Beltran-Sanahuja, A.; Sanz-Lazaro, C. Plastic Debris Accumulation in the Seabed Derived from Coastal Fish Farming. Environ. Pollut. 2020, 257, 113336. [Google Scholar] [CrossRef]
- Alomar, C.; Sanz-Martín, M.; Compa, M.; Rios-Fuster, B.; Álvarez, E.; Ripolles, V.; Valencia, J.M.; Deudero, S. Microplastic Ingestion in Reared Aquaculture Fish: Biological Responses to Low-Density Polyethylene Controlled Diets in Sparus Aurata. Environ. Pollut. 2021, 280, 116960. [Google Scholar] [CrossRef]
- Jovanović, B. Ingestion of Microplastics by Fish and Its Potential Consequences from a Physical Perspective. Integr. Environ. Assess. Manag. 2017, 13, 510–515. [Google Scholar] [CrossRef] [PubMed]
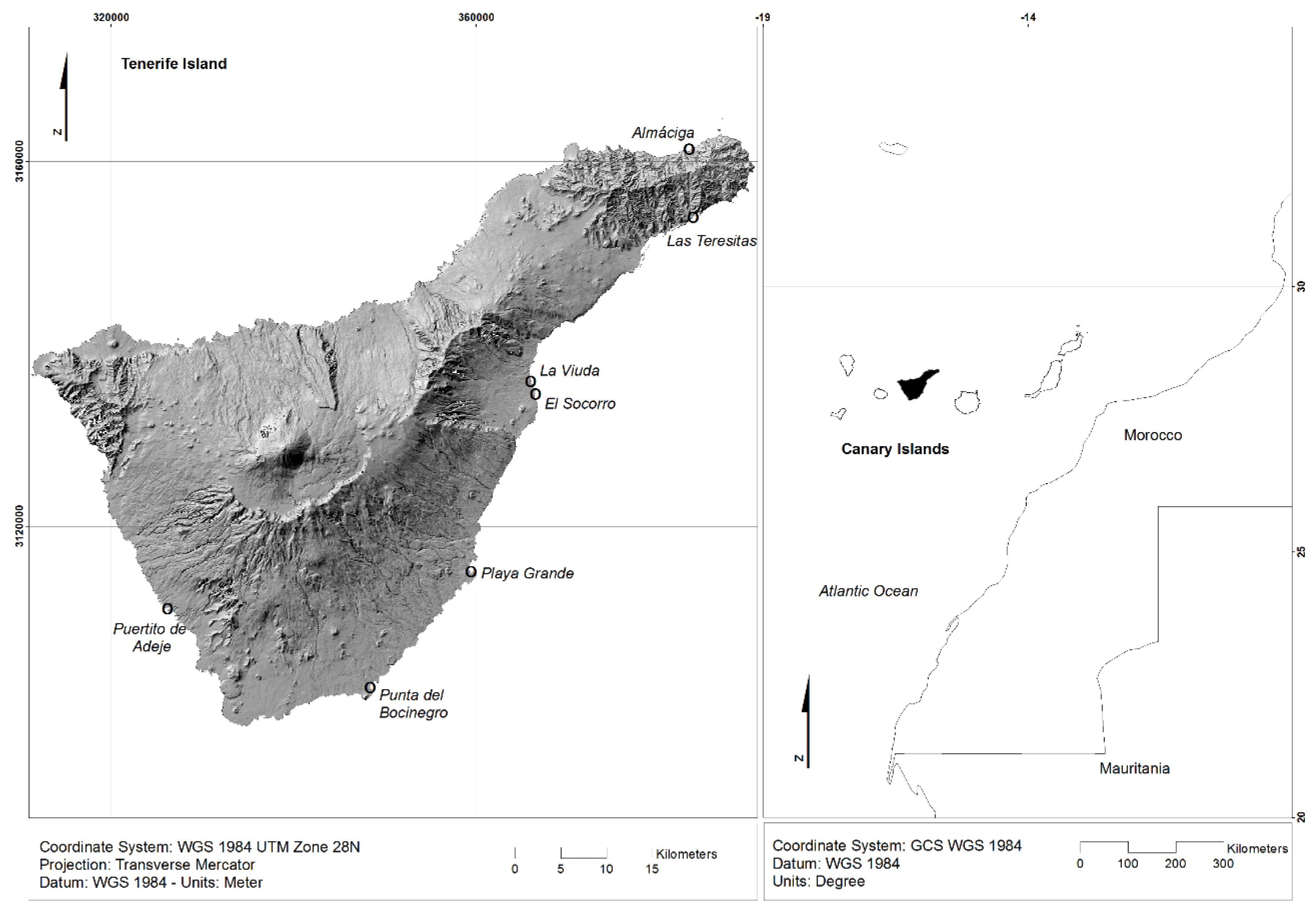
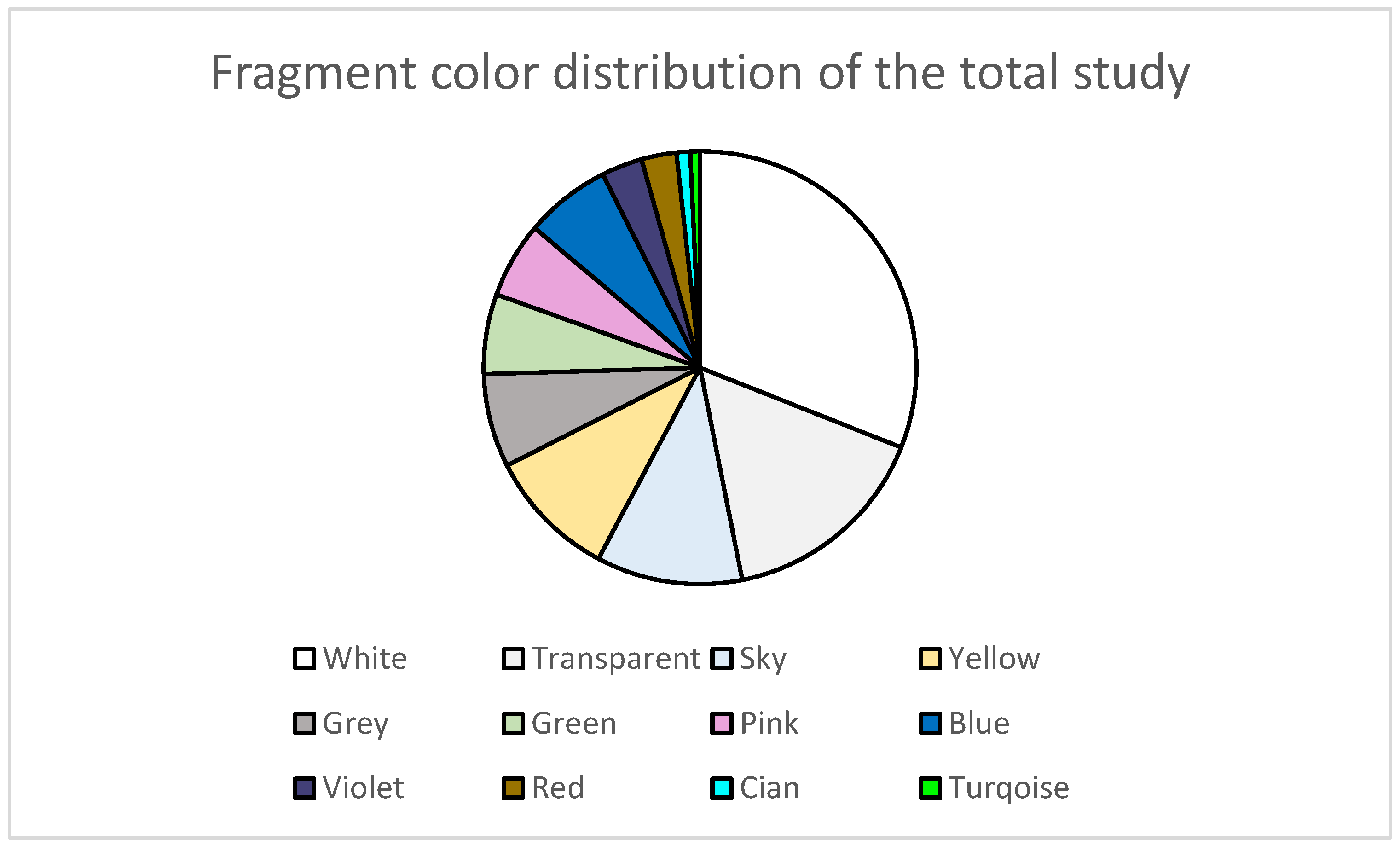
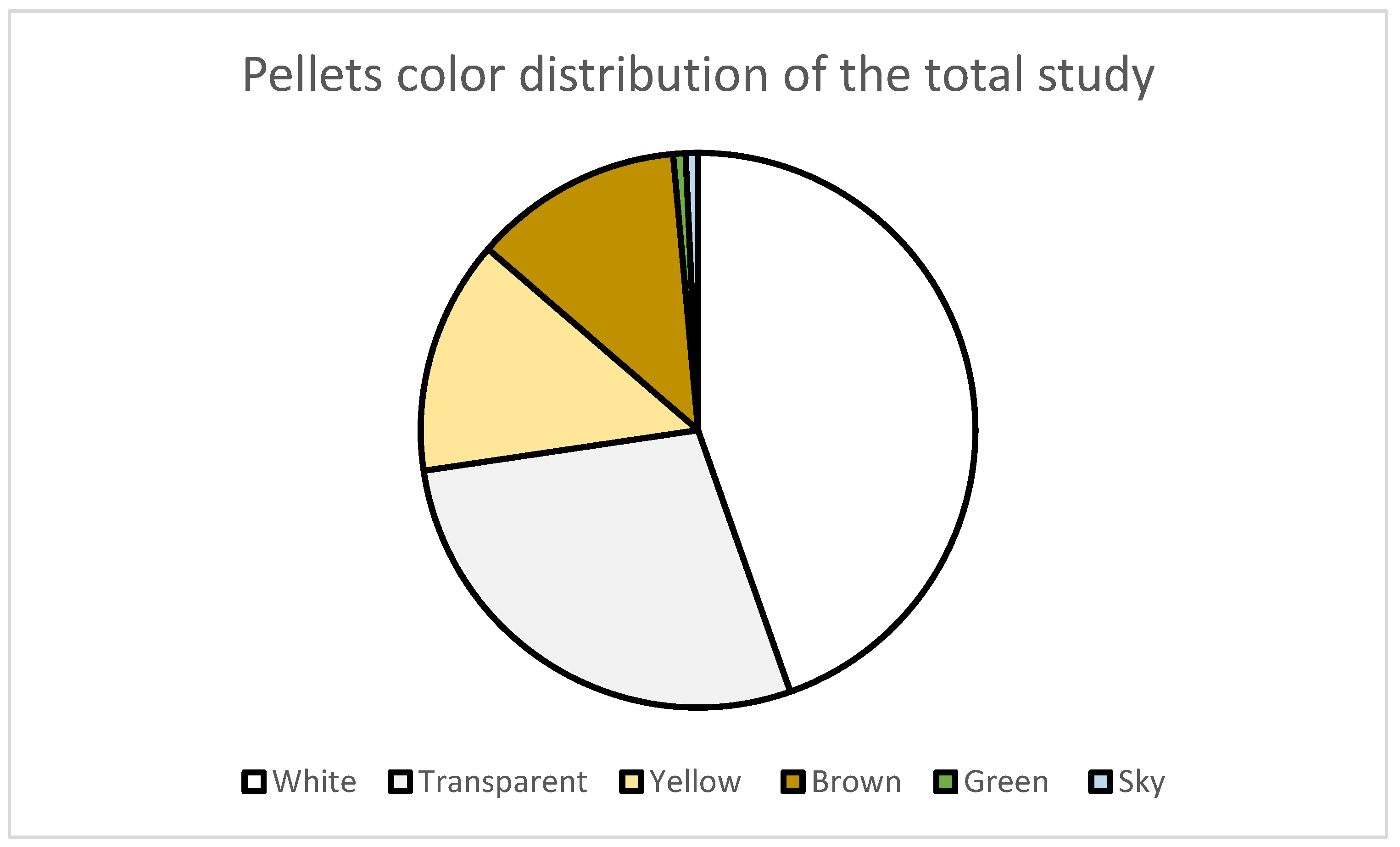
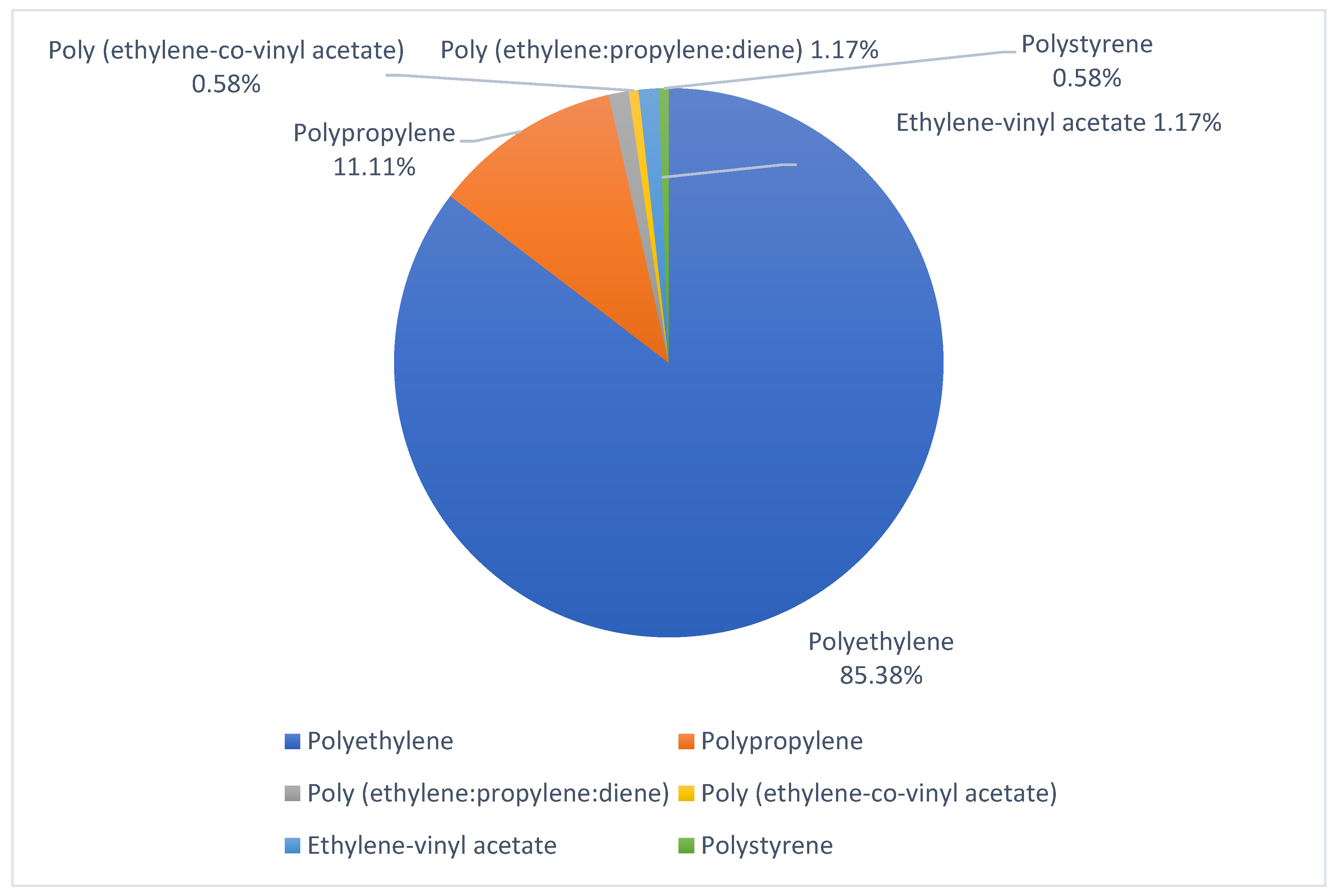

| Beach | UTM Coordinates | Sampling Date | Extension | Orientation | Characteristics |
|---|---|---|---|---|---|
| Almáciga | X = 383.337,38 Y = 3.161.160,50 | 20 April 2021 | 307 m | N | Black sand and pebbles |
| Las Teresitas | X = 383.720,42 Y = 3.153.954,60 | 12 May 2021 | 1.229 m | SE | White sand |
| La Viuda | X = 365.963,43 Y = 3.135.761,21 | 9 May 2021 | 126 m | E | Black sand and pebbles |
| El Socorro | X = 366.359,82 Y = 3.134.403,47 | 11 May 2021 | 58 m | SE | Black sand |
| Playa Grande | X = 359.414,00 Y = 3.114.927,65 | 21 April 2021 | 180 m | NE | Black sand |
| Punta del Bocinegro | X = 348.334,87 Y = 3.102.181,66 | 27 April 2021 | 286 m | E | Black sand and pebbles |
| Puertito Adeje | X = 326.313,94 Y = 3.111.047,50 | 9 May 2021 | 92 m | SW | Black sand |
| Beach | MP | E. coli | Faecal Coliforms | Intestinal Eterococci | S. aureus | Vibrio spp. |
|---|---|---|---|---|---|---|
| Almáciga | Fragments | - | + | + | - | + |
| Pellets | - | - | - | + | - | |
| El Socorro | Fragments | - | - | + | + | + |
| Pellets | - | + | + | + | - | |
| Las Teresitas | Fragments | + | + | + | - | + |
| Pellets | + | + | + | + | + | |
| Playa Grande | Fragments | + | + | + | + | + |
| Pellets | - | - | - | - | - | |
| Puertito de Adeje | Fragments | + | + | + | + | + |
| Pellets | - | + | + | - | + | |
| La Viuda | Fragments | - | + | - | + | + |
| Pellets | - | - | - | + | - | |
| Punta del Bocinegro | Fragments | + | + | + | + | + |
| Pellets | + | + | - | + | + | |
| Percentage of positive | Fragments * | 57.1% | 85.7% | 85.7% | 71.4% | 100% |
| Pellets ** | 28.5% | 57.1% | 42.8% | 57.1% | 42.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Sánchez, C.; Pestana-Ríos, Á.A.; Villanova-Solano, C.; Domínguez-Hernández, C.; Díaz-Peña, F.J.; Rodríguez-Álvarez, C.; Lecuona, M.; Arias, Á. Bacterial Colonization of Microplastics at the Beaches of an Oceanic Island, Tenerife, Canary Islands. Int. J. Environ. Res. Public Health 2023, 20, 3951. https://doi.org/10.3390/ijerph20053951
Hernández-Sánchez C, Pestana-Ríos ÁA, Villanova-Solano C, Domínguez-Hernández C, Díaz-Peña FJ, Rodríguez-Álvarez C, Lecuona M, Arias Á. Bacterial Colonization of Microplastics at the Beaches of an Oceanic Island, Tenerife, Canary Islands. International Journal of Environmental Research and Public Health. 2023; 20(5):3951. https://doi.org/10.3390/ijerph20053951
Chicago/Turabian StyleHernández-Sánchez, Cintia, Ángel Antonio Pestana-Ríos, Cristina Villanova-Solano, Cristopher Domínguez-Hernández, Francisco Javier Díaz-Peña, Cristobalina Rodríguez-Álvarez, María Lecuona, and Ángeles Arias. 2023. "Bacterial Colonization of Microplastics at the Beaches of an Oceanic Island, Tenerife, Canary Islands" International Journal of Environmental Research and Public Health 20, no. 5: 3951. https://doi.org/10.3390/ijerph20053951
APA StyleHernández-Sánchez, C., Pestana-Ríos, Á. A., Villanova-Solano, C., Domínguez-Hernández, C., Díaz-Peña, F. J., Rodríguez-Álvarez, C., Lecuona, M., & Arias, Á. (2023). Bacterial Colonization of Microplastics at the Beaches of an Oceanic Island, Tenerife, Canary Islands. International Journal of Environmental Research and Public Health, 20(5), 3951. https://doi.org/10.3390/ijerph20053951






