Modulation of Heart Rate Variability following PAP Ion Magnetic Induction Intervention in Subjects with Chronic Musculoskeletal Pain: A Pilot Randomized Controlled Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Procedure
2.3. Interventions
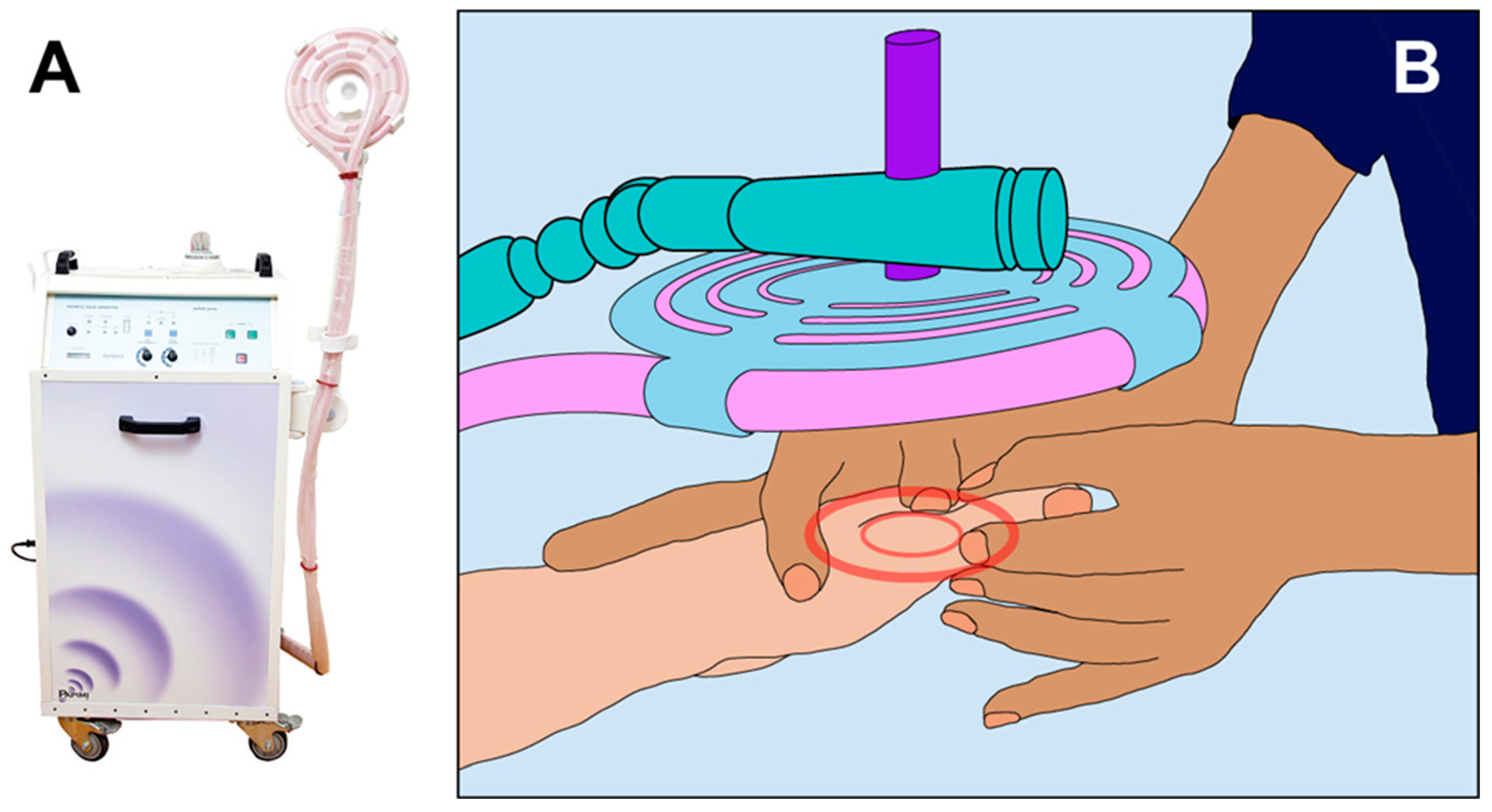
2.3.1. PAPIMI Intervention
2.3.2. Sham (Control) Papimi Intervention
2.4. Data Collection Measurements
2.5. Outcome Measures
2.6. Statistical Analysis
3. Results
3.1. Sample Characteristics and HRV Pre-Intervention Scores
3.2. HRV Post-Intervention Scores
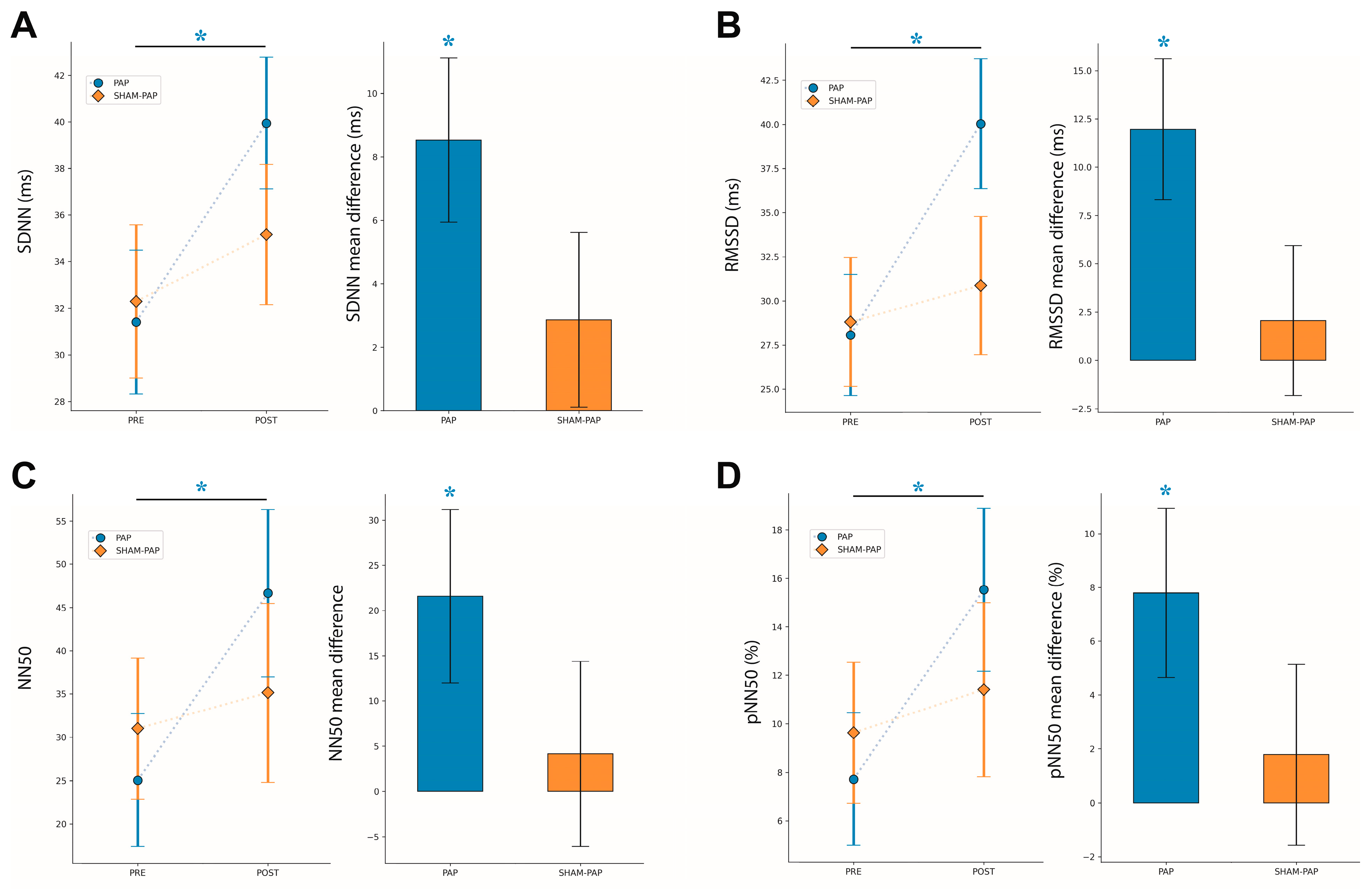
3.2.1. SDNN
3.2.2. RMSSD
3.2.3. NN50
3.2.4. pNN50
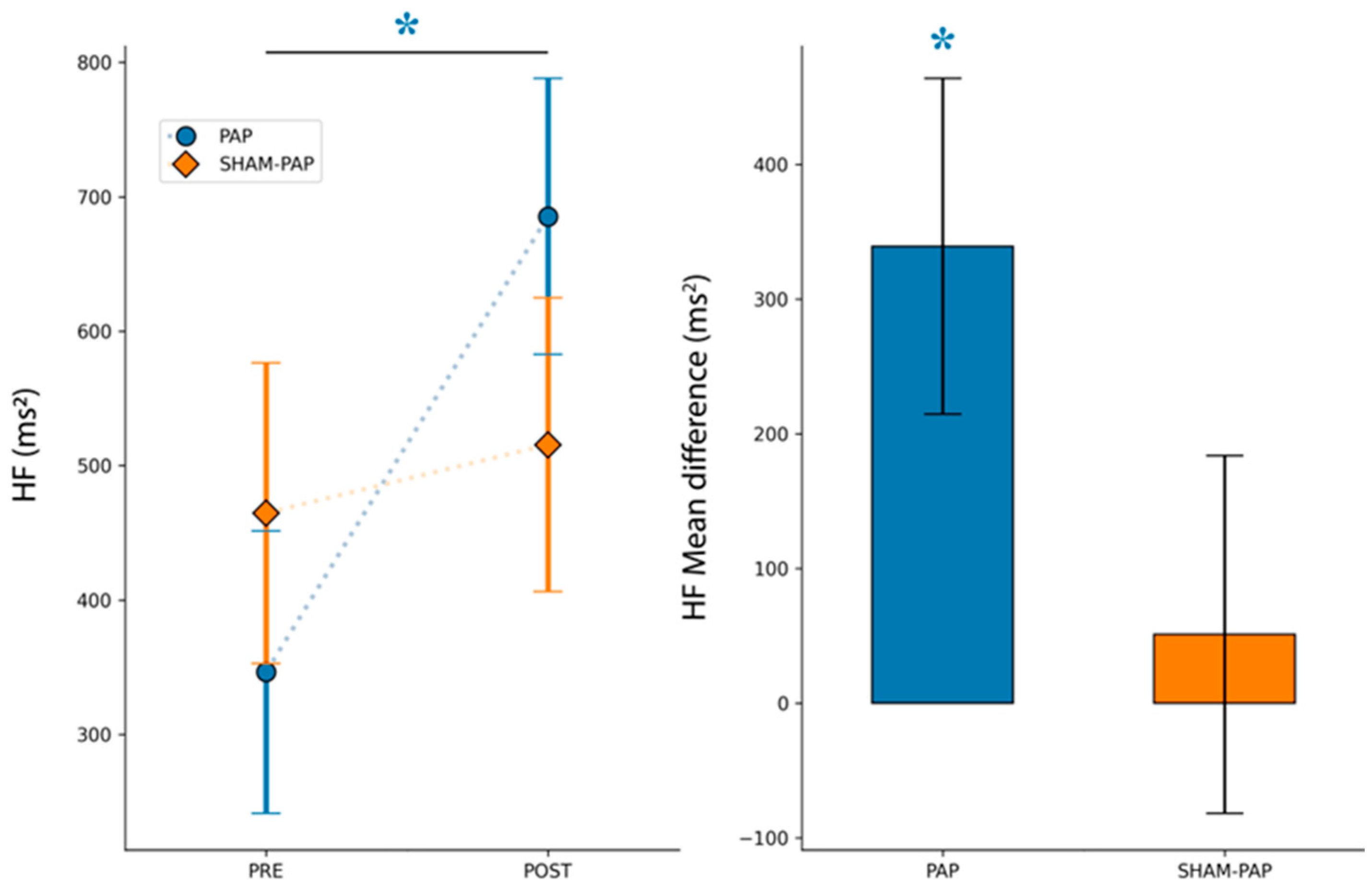
3.2.5. HF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thayer, J.F.; Lane, R.D. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 2000, 61, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Yamamoto, S.S.; Brosschot, J.F. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 2010, 141, 122–131. [Google Scholar] [CrossRef] [PubMed]
- McDougall, S.J.; Münzberg, H.; Derbenev, A.V.; Zsombok, A. Central control of autonomic functions in health and disease. Front. Neurosci. 2015, 8, 440. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Thayer, J.F.; Friedman, B.H. The Heart of Anxiety: A Dynamical Systems Approach. In The (Non) Expression of Emotions in Health and Disease; Vingerhoets, A., Ed.; Springer: Amsterdam, The Netherlands, 1997; pp. 39–49. [Google Scholar]
- Friedman, B.H.; Thayer, J.F. Anxiety and autonomic flexibility: A cardiovascular approach. Biol. Psychol. 1998, 47, 303–323. [Google Scholar]
- Friedman, B.H.; Thayer, J.F. Autonomic balance revisited: Panic anxiety and heart rate variability. J. Psychosom. Res. 1998, 44, 133–151. [Google Scholar] [CrossRef]
- Malik, M. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Pumprla, J.; Howorka, K.; Groves, D.; Chester, M.; Nolan, J. Functional assessment of heart rate variability: Physiological basis and practical applications. Int. J. Cardiol. 2002, 84, 1–14. [Google Scholar] [CrossRef]
- Freeman, J.V.; Dewey, F.E.; Hadley, D.M.; Myers, J.; Froelicher, V.F. Autonomic nervous system interaction with the cardiovascular system during exercise. Prog. Cardiovasc. Dis. 2006, 48, 342–362. [Google Scholar] [CrossRef]
- Kemp, A.H.; Quintana, D.S.; Felmingham, K.L.; Matthews, S.; Jelinek, H.F. Depression, comorbid anxiety disorders, and heart rate variability in physically healthy, unmedicated patients: Implications for cardiovascular risk. PLoS ONE 2012, 7, e30777. [Google Scholar] [CrossRef]
- Porta, A.; D’Addio, G.; Guzzetti, S.; Lucini, D.; Pagani, M. Testing the presence of non stationarities in short heart rate variability. Comput. Cardiol. 2004, 31, 645–648. [Google Scholar]
- Malliani, A.; Montano, N. Heart rate variability as a clinical tool. Ital. Heart. J. 2002, 3, 439–445. [Google Scholar]
- Malliani, A.; Pagani, M.; Lombardi, F.; Cerutti, S. Cardiovascular neural regulation explored in the frequency domain. Circulation 1991, 84, 482–492. [Google Scholar] [CrossRef]
- Malliani, A.; Pagani, M.; Lombardi, F. Methods for Assessment of Sympatho-Vagal Balance: Power Spectral Analysis; Futura: New York, NY, USA, 1994. [Google Scholar]
- Vanderlei, L.C.; Pastre, C.M.; Hoshi, R.A.; Carvalho, T.D.; Godoy, M.F. Basic notions of heart rate variability and its clinical applicability. Rev. Bras. Cir. Cardiovasc. 2009, 24, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Hoy, D.G.; Cross, M.; Vos, T.; Naghavi, M.; Buchbinder, R.; Woolf, A.; March, L. The global burden of other musculoskeletal disorders: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- El-Tallawy, S.N.; Nalamasu, R.; Salem, G.I.; LeQuang, J.A.K.; Pergolizzi, J.V.; Christo, P.J. Management of Musculoskeletal Pain: An Update with Emphasis on Chronic Musculoskeletal Pain. Pain Ther. 2021, 10, 181–209. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Pain-autonomic interactions: A selective review. Clin. Auton. Res. 2001, 11, 343–349. [Google Scholar] [CrossRef]
- Benarroch, E.E. Pain-autonomic interactions. Neurol. Sci. 2006, 27 (Suppl. 2), S130–S133. [Google Scholar] [CrossRef]
- Bruehl, S.; Chung, O.Y. Interactions between the cardiovascular and pain regulatory systems: An updated review of mechanisms and possible alterations in chronic pain. Neurosci. Biobehav. Rev. 2004, 28, 395–414. [Google Scholar] [CrossRef]
- Tracy, L.M.; Ioannou, L.; Baker, K.S.; Gibson, S.J.; Georgiou-Karistianis, N.; Giummarra, M.J. Meta-analytic evidence for decreased heart rate variability in chronic pain implicating parasympathetic nervous system dysregulation. Pain 2016, 157, 7–29. [Google Scholar] [CrossRef]
- Hallman, D.M.; Ekman, A.H.; Lyskov, E. Changes in physical activity and heart rate variability in chronic neck–shoulder pain: Monitoring during work and leisure time. Int. Arch. Occup. Environ. Health 2014, 87, 735–744. [Google Scholar] [CrossRef]
- Hautala, A.J.; Karppinen, J.; Seppanen, T. Short-Term Assessment of Autonomic Nervous System as a Potential Tool to Quantify Pain Experience. In Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016. [Google Scholar]
- Santos-de-Araújo, A.D.; Dibai-Filho, A.V.; Dos Santos, S.N.; de Alcântara, E.V.; Souza, C.D.S.; Gomes, C.A.F.P.; de Souza, J.N.; Pinheiro, J.S.; Bassi, D. Correlation Between Chronic Neck Pain and Heart Rate Variability Indices at Rest: A Cross-sectional Study. J. Manip. Physiol. Ther. 2019, 42, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Nowakowski, K.; Didier-Laurent, B.; Gombert, M.; Cottin, F. Effect of spinal manipulative treatment on cardiovascular autonomic control in patients with acute low back pain. Chiropr. Man. Ther. 2017, 25, 33. [Google Scholar] [CrossRef] [PubMed]
- Urits, I.; Schwartz, R.H.; Orhurhu, V.; Maganty, N.V.; Reilly, B.T.; Patel, P.M.; Wie, C.; Kaye, A.D.; Mancuso, K.F.; Kaye, A.J.; et al. A Comprehensive Review of Alternative Therapies for the Management of Chronic Pain Patients: Acupuncture, Tai Chi, Osteopathic Manipulative Medicine, and Chiropractic Care. Adv. Ther. 2020, 38, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Elshiwi, A.M.; Hamada, H.A.; Mosaad, D.; Ragab, I.M.A.; Koura, G.M.; Alrawaili, S. Effect of pulsed electromagnetic field on nonspecific low back pain patients: A randomized controlled trial. Braz. J. Phys. Ther. 2019, 23, 244–249. [Google Scholar] [CrossRef]
- Lisi, A.J.; Scheinowitz, M.; Saporito, R.; Onorato, A. A Pulsed Electromagnetic Field Therapy Device for Non-Specific Low Back Pain: A Pilot Randomized Controlled Trial. Pain Ther. 2019, 8, 133–140. [Google Scholar] [CrossRef]
- Auger, K.; Shedlock, G.; Coutinho, K.; Myers, N.E.; Lorenzo, S. Effects of osteopathic manipulative treatment and bio-electromagnetic energy regulation therapy on lower back pain. J. Am. Osteopat. Assoc. 2021, 121, 561–569. [Google Scholar] [CrossRef]
- Servodio Iammarrone, C.; Cadossi, M.; Sambri, A.; Grosso, E.; Corrado, B.; Iammarrone, F.S. Is there a role of pulsed electromagnetic fields in management of patellofemoral pain syndrome? Randomized controlled study at one year follow-up. Bioelectromagnetics 2016, 37, 81–88. [Google Scholar] [CrossRef]
- de Freitas, D.G.; Marcondes, F.B.; Monteiro, R.L.; Rosa, S.G.; Maria de Moraes Barros Fucs, P.; Fukuda, T.Y. Pulsed electromagnetic field and exercises in patients with shoulder impingement syndrome: A randomized, double-blind, placebo-controlled clinical trial. Arch. Phys. Med. Rehabil. 2014, 95, 345–352. [Google Scholar] [CrossRef]
- Uzunca, K.; Birtane, M.; Taştekin, N. Effectiveness of pulsed electromagnetic field therapy in lateral epicondylitis. Clin. Rheumatol. 2007, 26, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.L.; Zhou, Y.; McCall, C.E.; Soker, S.; Criswell, T.L. The Use of Pulsed Electromagnetic Field to Modulate Inflammation and Improve Tissue Regeneration: A Review. Bioelectricity 2019, 1, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Sait, M.L.; Wood, A.W.; Sadafi, H.A. A study of heart rate and heart rate variability in human subjects exposed to occupational levels of 50 Hz circularly polarised magnetic fields. Med. Eng. Phys. 1999, 21, 361–369. [Google Scholar] [CrossRef]
- Tabor, Z.; Michalski, J.; Rokita, E. Influence of 50 Hz magnetic field on human heart rate variability: Linear and nonlinear analysis. Bioelectromagnetics 2004, 25, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Misek, J.; Belyaev, I.; Jakusova, V.; Tonhajzerova, I.; Barabas, J.; Jakus, J. Heart rate variability affected by radiofrequency electromagnetic field in adolescent students. Bioelectromagnetics 2018, 39, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Binboğa, E.; Tok, S.; Munzuroğlu, M. The Short-Term Effect of Occupational Levels of 50 Hz Electromagnetic Field on Human Heart Rate Variability. Bioelectromagnetics 2021, 42, 60–75. [Google Scholar] [CrossRef]
- Mihajlovic-Madzarevic, V.; Pappas, P. Treatment of refractory seizures due to a benign mass present in the corpus callosum with an ion magnetic inductor: Case report. Brain Tumor Pathol. 2005, 22, 93–95. [Google Scholar] [CrossRef]
- Athanasiou, A.; Karkambounas, S.; Batistatou, A.; Lykoudis, E.; Katsaraki, A.; Kartsiouni, T.; Papalois, A.; Evangelou, A. The effect of pulsed electromagnetic fields on secondary skin wound healing: An experimental study. Bioelectromagnetics 2007, 28, 362–368. [Google Scholar] [CrossRef]
- Wagner, B.; Steiner, M.; Markovic, L.; Crevenna, R. Successful application of pulsed electromagnetic fields in a patient with post-COVID-19 fatigue: A case report. Wien. Med. Wochenschr. 2022, 172, 227–232. [Google Scholar] [CrossRef]
- Cimmino, M.A.; Ferrone, C.; Cutolo, M. Epidemiology of chronic musculoskeletal pain. Best Pract. Res. Clin. Rheumatol. 2011, 25, 173–183. [Google Scholar] [CrossRef]
- Ruffini, N.; D’Alessandro, G.; Mariani, N.; Pollastrelli, A.; Cardinali, L.; Cerritelli, F. Variations of high frequency parameter of heart rate variability following osteopathic manipulative treatment in healthy subjects compared to control group and sham therapy: Randomized controlled trial. Front. Neurosci. 2015, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Cerritelli, F.; Cardone, D.; Pirino, A.; Merla, A.; Scoppa, F. Does Osteopathic Manipulative Treatment Induce Autonomic Changes in Healthy Participants? A Thermal Imaging Study. Front. Neurosci. 2020, 14, 887. [Google Scholar] [CrossRef] [PubMed]
- Minami, J.; Ishimitsu, T.; Matsuoka, H. Effects of smoking cessation on blood pressure and heart rate variability in habitual smokers. Hypertension 1999, 33, 586–590. [Google Scholar] [CrossRef]
- Cardone, D.; Merla, A. New frontiers for applications of thermal infrared imaging devices: Computational psychopshysiology in the neurosciences. Sensors 2017, 17, 1042. [Google Scholar] [CrossRef] [PubMed]
- Papimi. Papimi® Manual. Experiences of Application; Version 05; Papimi: Vienna, Austria, 2019. [Google Scholar]
- Papimi® Official Homepage. Available online: https://papimi-therapie.eu/ (accessed on 23 December 2022).
- Aubert, A.E.; Seps, B.; Beckers, F. Heart rate variability in athletes. J. Sport. Med. 2003, 33, 889–919. [Google Scholar] [CrossRef]
- Umetani, K.; Singer, D.H.; McCraty, R.; Atkinson, M. Twenty-four hour time domain heart rate variability and heart rate: Relations to age and gender over nine decades. J. Am. Coll. Cardiol. 1998, 31, 593–601. [Google Scholar] [CrossRef]
- Roura, S.; Álvarez, G.; Solà, I.; Cerritelli, F. Do manual therapies have a specific autonomic effect? An overview of systematic reviews. PLoS ONE 2021, 16, e0260642. [Google Scholar] [CrossRef]
- Berntson, G.G.; Bigger, J.T., Jr.; Eckberg, D.L.; Grossman, P.; Kaufmann, P.G.; Malik, M.; Nagaraja, H.N.; Porges, S.W.; Saul, J.P.; Stone, P.H.; et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology 1997, 34, 623–648. [Google Scholar] [CrossRef]
- Kleiger, R.E.; Stein, P.K.; Bigger, J.T., Jr. Heart Rate Variability: Measurement and Clinical Utility. Ann. Noninvasive Electrocardiol. 2005, 10, 88–101. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Bentho, O.; Park, M.-Y.; Sharabi, Y. Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp. Physiol. 2011, 96, 1255–1261. [Google Scholar] [CrossRef]
- Heathers, J.A. Everything Hertz: Methodological issues in short-term frequency-domain HRV. Front. Physiol. 2014, 5, 177. [Google Scholar] [CrossRef]
- Quintana, D.S.; Alvares, G.A.; Heathers, J.A. Guidelines for Reporting Articles on Psychiatry and Heart rate variability (GRAPH): Recommendations to advance research communication. Transl. Psychiatry 2016, 6, e803. [Google Scholar] [CrossRef] [PubMed]
- Magagnin, V.; Bassani, T.; Bari, V.; Turiel, M.; Maestri, R.; Pinna, G.D.; Porta, A. Non-stationarities significantly distort short-term spectral, symbolic and entropy heart rate variability indices. Physiol. Meas. 2011, 32, 1775. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Yang, W.; Zeng, Q.; Chen, W.; Zhu, Y.; Liu, W.; Wang, S.; Wang, B.; Shao, Z.; Zhang, Y. Promising application of Pulsed Electromagnetic Fields (PEMFs) in musculoskeletal disorders. Biomed. Pharmacother. 2020, 131, 110767. [Google Scholar] [CrossRef] [PubMed]
- Myers, K.A.; Sivathamboo, S.; Perucca, P. Heart rate variability measurement in epilepsy: How can we move from research to clinical practice? Epilepsia 2018, 59, 2169–2178. [Google Scholar] [CrossRef] [PubMed]
- Marques, K.C.; Silva, C.C.; Trindade, S.D.S.; Santos, M.C.D.S.; Rocha, R.S.B.; Vasconcelos, P.F.D.C.; Quaresma, J.A.S.; Falcão, L.F.M. Reduction of Cardiac Autonomic Modulation and Increased Sympathetic Activity by Heart Rate Variability in Patients with Long COVID. Front. Cardiovasc. Med. 2022, 9, 862001. [Google Scholar] [CrossRef]
- Bachl, N.; Ruoff, G.; Wessner, B.; Tschan, H. Electromagnetic interventions in musculoskeletal disorders. Clin. Sport. Med. 2008, 27, 87–105. [Google Scholar] [CrossRef]
- Chiaramello, E.; Fiocchi, S.; Bonato, M.; Gallucci, S.; Benini, M.; Parazzini, M. Cell transmembrane potential in contactless permeabilization by time-varying magnetic fields. Comput. Biol. Med. 2021, 135, 104587. [Google Scholar] [CrossRef]
- Bau, J.-G.; Chia, T.; Wei, S.-H.; Li, Y.-H.; Kuo, F.-C. Correlations of Neck/Shoulder Perfusion Characteristics and Pain Symptoms of the Female Office Workers with Sedentary Lifestyle. PLoS ONE 2017, 12, e0169318. [Google Scholar] [CrossRef]
- Bau, J.-G.; Wu, S.-K.; Huang, B.-W.; Lin, T.T.-L.; Huang, S.-C. Myofascial Treatment for Microcirculation in Patients with Postural Neck and Shoulder Pain. Diagnostics 2021, 11, 2226. [Google Scholar] [CrossRef]
- Cagnie, B.; Dhooge, F.; Van Akeleyen, J.; Cools, A.; Cambier, D.; Danneels, L. Changes in microcirculation of the trapezius muscle during a prolonged computer task. Eur. J. Appl. Physiol. 2012, 112, 3305–3312. [Google Scholar] [CrossRef]
- Chrysafides, S.M.; Bordes, S.J.; Sharma, S. Physiology, Resting Potential. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Thayer, J.F.; Hansen, A.L.; Saus-Rose, E.; Johnsen, B.H. Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Ann. Behav. Med. 2009, 37, 141–153. [Google Scholar] [CrossRef]
- Williams, D.P.; Cash, C.; Rankin, C.; Bernardi, A.; Koenig, J.; Thayer, J.F. Resting heart rate variability predicts self-reported difficulties in emotion regulation: A focus on different facets of emotion regulation. Front. Psychol. 2015, 6, 261. [Google Scholar] [CrossRef] [PubMed]
- Carnevali, L.; Koenig, J.; Sgoifo, A.; Ottaviani, C. Autonomic and Brain Morphological Predictors of Stress Resilience. Front. Neurosci. 2018, 12, 228. [Google Scholar] [CrossRef] [PubMed]
- Billman, G.E.; Dujardin, J.P. Dynamic changes in cardiac vagal tone as measured by time-series analysis. Heart Circ. Physiol. 1990, 258, H896–H902. [Google Scholar] [CrossRef] [PubMed]
- Billman, G.E. Cardiac autonomic neural “remodeling” and susceptibility to sudden cardiac death: Effect of endurance exercise training. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1171–H1193. [Google Scholar] [CrossRef] [PubMed]
- Billman, G.E. Heart rate variability—A historical perspective. Front. Physiol. 2011, 2, 86. [Google Scholar] [CrossRef]
- Billman, G.E. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front. Physiol. 2013, 4, 26. [Google Scholar] [CrossRef]
- Billman, G.E.; Huikuri, H.V.; Sacha, J.; Trimmel, K. An introduction to heart rate variability: Methodological considerations and clinical applications. Front. Physiol. 2015, 6, 55. [Google Scholar] [CrossRef]
- Taylor, J.A.; Myers, C.W.; Halliwill, J.R.; Seidel, H.; Eckberg, D.L. Sympathetic restraint of respiratory sinus arrhythmia: Implications for vagal-cardiac tone assessment in humans. Am. J. Physiol. Hear. Circ. Physiol. 2001, 280, H2804–H2814. [Google Scholar] [CrossRef]
- Cohen, M.; Taylor, J.A. Short-term cardiovascular oscillations in man: Measuring and modeling the physiologies. J. Physiol. 2002, 542, 669–683. [Google Scholar] [CrossRef]
- Malik, M.; Xia, R.; Odemuyiwa, O.; Staunton, A.; Poloniecki, J.; Camm, A.J. Influence of the recognition artefact in the automatic analysis of long-term electrocardiograms on time domain measurements of heart rate variability. Med. Biol. Eng. Comput. 1993, 31, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Flynn, D.M. Chronic Musculoskeletal Pain: Nonpharmacologic, Noninvasive Treatments. Am. Fam. Physician 2020, 102, 465–477. [Google Scholar] [PubMed]
- Dersh, J.; Polatin, P.B.M.; Gatchel, R.J. Chronic Pain and Psychopathology: Research Findings and Theoretical Considerations. Psychosom. Med. 2002, 64, 773–786. [Google Scholar] [PubMed]
- Means-Christensen, A.J.; Roy-Byrne, P.P.; Sherbourne, C.D.; Craske, M.G.; Stein, M.B. Relationships among pain, anxiety, and depression in primary care. Depress. Anxiety 2008, 25, 593–600. [Google Scholar] [CrossRef]
- Woo, A.K. Depression and Anxiety in Pain. Rev. Pain 2010, 4, 8–12. [Google Scholar] [CrossRef]
- Gerrits, M.M.J.G.; Vogelzangs, N.; van Oppen, P.; van Marwijk, H.W.J.; van der Horst, H.; Penninx, B.W.J.H. Impact of pain on the course of depressive and anxiety disorders. Pain 2012, 153, 429–436. [Google Scholar] [CrossRef]
- Giovannini, S.; Macchi, C.; Liperoti, R.; Laudisio, A.; Coraci, D.; Loreti, C.; Vannetti, F.; Onder, G.; Padua, L.; Mugello Study Working Group. Association of Body Fat with Health-Related Quality of Life and Depression in Nonagenarians: The Mugello Study. J. Am. Med. Dir. Assoc. 2019, 20, 564–568. [Google Scholar] [CrossRef]
- Kanbara, K.; Fukunaga, M. Links among emotional awareness, somatic awareness and autonomic homeostatic processing. Biopsychosoc. Med. 2016, 10, 16. [Google Scholar] [CrossRef]
- Kremer, M.; Becker, L.J.; Barrot, M.; Yalcin, I. How to study anxiety and depression in rodent models of chronic pain? Eur. J. Neurosci. 2021, 53, 236–270. [Google Scholar] [CrossRef]
- Laudisio, A.; Incalzi, R.A.; Gemma, A.; Giovannini, S.; Monaco, M.R.L.; Vetrano, D.L.; Padua, L.; Bernabei, R.; Zuccalà, G. Use of proton-pump inhibitors is associated with depression: A population-based study. Int. Psychogeriatr. 2017, 30, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Levenson, R.W. The Autonomic Nervous System and Emotion. Emot. Rev. 2014, 6, 100–112. [Google Scholar] [CrossRef]
- Meda, R.T.; Nuguru, S.P.; Rachakonda, S.; Sripathi, S.; Khan, M.I.; Patel, N. Chronic Pain-Induced Depression: A Review of Prevalence and Management. Cureus 2022, 14, e28416. [Google Scholar] [CrossRef]
- Ru, Q.; Lu, Y.; Saifullah, A.B.; Blanco, F.A.; Yao, C.; Cata, J.P.; Li, D.P.; Tolias, K.F.; Li, L. TIAM1-mediated synaptic plasticity underlies comorbid depression-like and ketamine antidepressant-like actions in chronic pain. J. Clin. Investig. 2022, 132, e158545. [Google Scholar] [CrossRef] [PubMed]

| PAP | SHAM-PAP | p Value | |
|---|---|---|---|
| Age (years) a,§ | 49.18 ± 13.02 | 51.07 ± 9.42 | 0.63 |
| BMI (kg/m2) a,§ | 24.52 ± 3.62 | 25.19 ± 3.60 | 0.58 |
| Male b,$ | 10 (58.82) | 7 (46.67) | 0.47 |
| HR (bpm) a,§ | 72.18 ± 11.00 | 69.17 ± 6.83 | 0.37 |
| SDNN (ms) a,§ | 31.41 ± 13.74 | 32.30 ± 11.44 | 0.85 |
| RMSSD (ms) a,§ | 28.07 ± 16.67 | 28.80 ± 10.50 | 0.88 |
| NN50 a,§ | 25.06 ± 32.92 | 31.01 ± 29.86 | 0.60 |
| pNN50 (%) a,§ | 7.72 ± 11.75 | 9.63 ± 10.60 | 0.64 |
| HF (ms2) a,§ | 346.35 ± 458.01 | 464.67 ± 402.16 | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viti, A.; Panconi, G.; Guarducci, S.; Garfagnini, S.; Mondonico, M.; Bravi, R.; Minciacchi, D. Modulation of Heart Rate Variability following PAP Ion Magnetic Induction Intervention in Subjects with Chronic Musculoskeletal Pain: A Pilot Randomized Controlled Study. Int. J. Environ. Res. Public Health 2023, 20, 3934. https://doi.org/10.3390/ijerph20053934
Viti A, Panconi G, Guarducci S, Garfagnini S, Mondonico M, Bravi R, Minciacchi D. Modulation of Heart Rate Variability following PAP Ion Magnetic Induction Intervention in Subjects with Chronic Musculoskeletal Pain: A Pilot Randomized Controlled Study. International Journal of Environmental Research and Public Health. 2023; 20(5):3934. https://doi.org/10.3390/ijerph20053934
Chicago/Turabian StyleViti, Antonio, Giulia Panconi, Sara Guarducci, Susanna Garfagnini, Mosè Mondonico, Riccardo Bravi, and Diego Minciacchi. 2023. "Modulation of Heart Rate Variability following PAP Ion Magnetic Induction Intervention in Subjects with Chronic Musculoskeletal Pain: A Pilot Randomized Controlled Study" International Journal of Environmental Research and Public Health 20, no. 5: 3934. https://doi.org/10.3390/ijerph20053934
APA StyleViti, A., Panconi, G., Guarducci, S., Garfagnini, S., Mondonico, M., Bravi, R., & Minciacchi, D. (2023). Modulation of Heart Rate Variability following PAP Ion Magnetic Induction Intervention in Subjects with Chronic Musculoskeletal Pain: A Pilot Randomized Controlled Study. International Journal of Environmental Research and Public Health, 20(5), 3934. https://doi.org/10.3390/ijerph20053934








