Molecular Mechanisms of Tebuconazole Affecting the Social Behavior and Reproduction of Zebrafish
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals Used in This Study
2.2. Zebrafish Maintenance and Experimental Design
2.3. Contents of TEB in Gonads and Exposure to Water
2.4. Measure of Sperm Motility
2.5. Histological Examination
2.6. Behavior Tests
2.7. Quantification of Sex Hormones
2.8. RNA Extraction and Real-Time Quantitative PCR (RT-qPCR) Analysis
2.9. Statistical Analyses
3. Results
3.1. Accumulation of TEB in Gonads and Exposure to Water
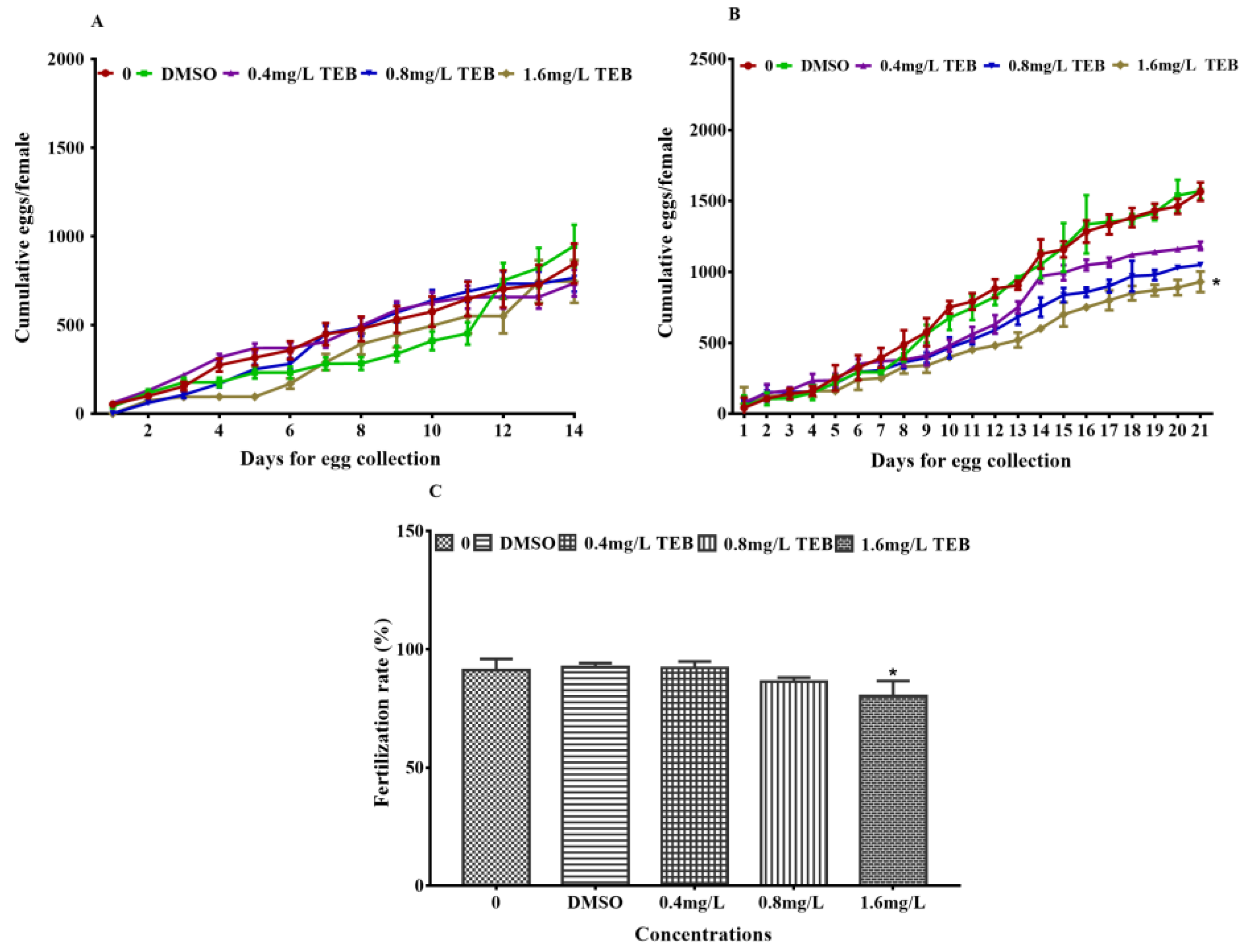
3.2. Egg Production, Fertilization Rate, and Somatic Indexes
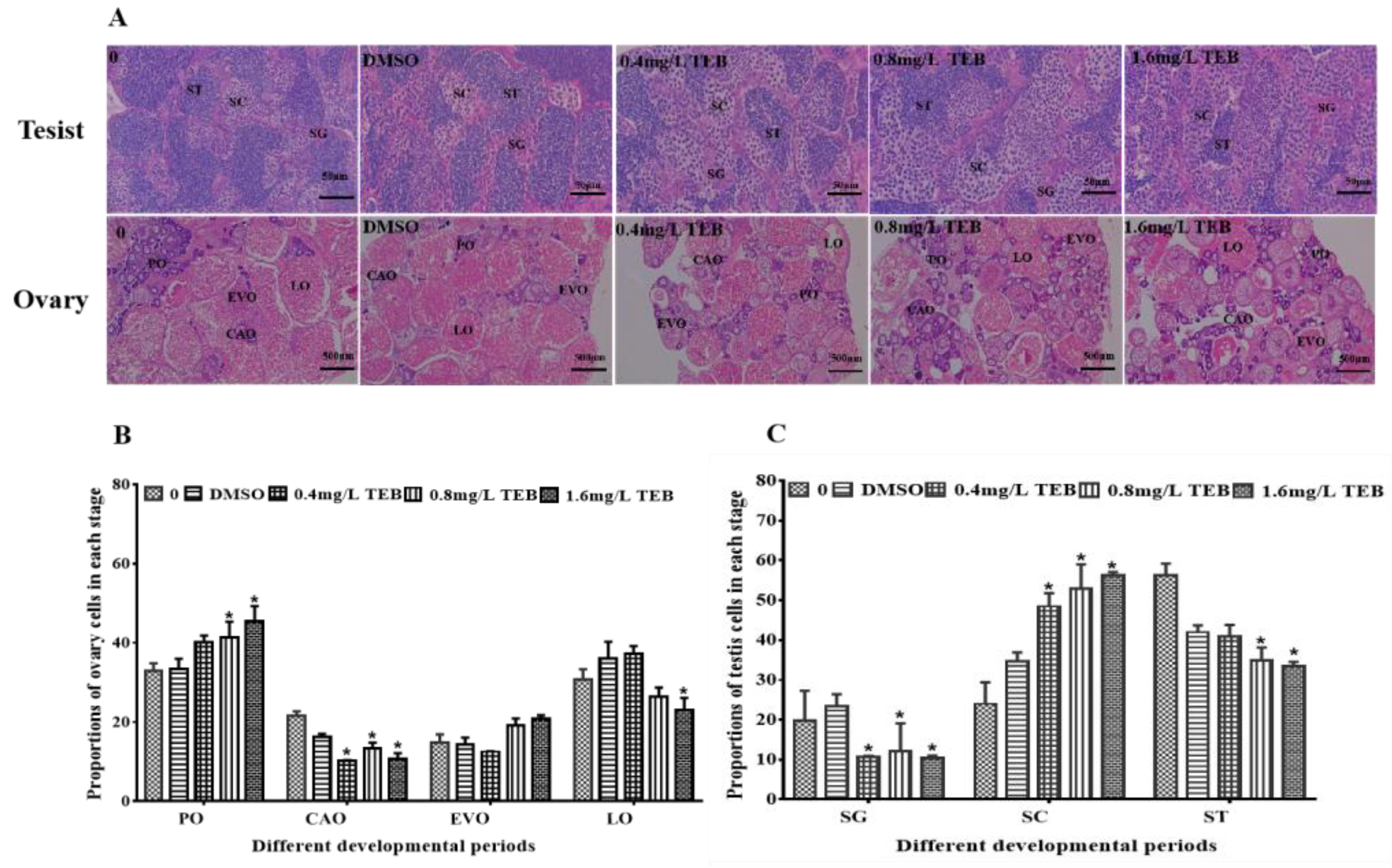
3.3. Histological Analysis of Gonads
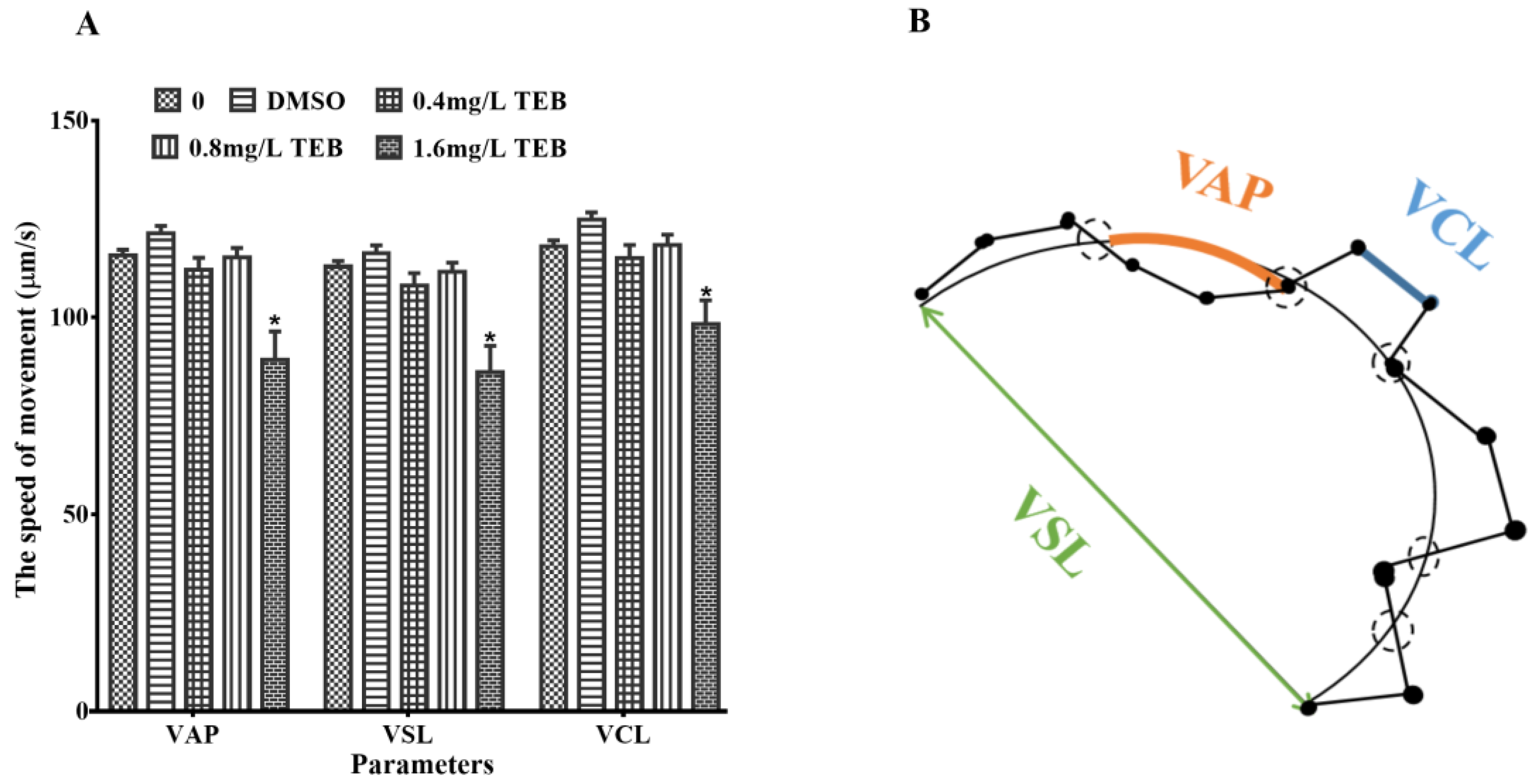
3.4. Sperm Motility of Male Zebrafish
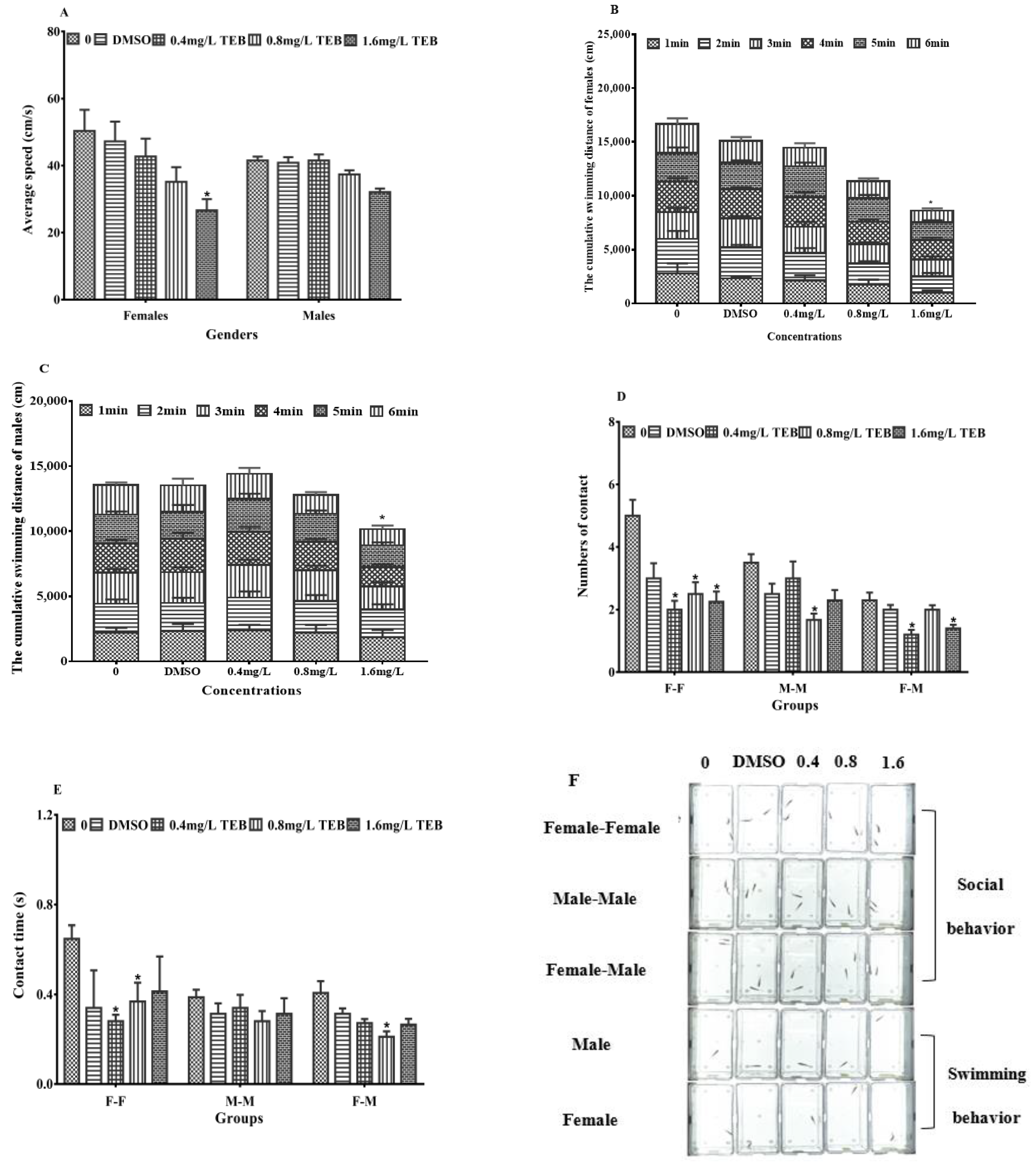
3.5. Behavior of Zebrafish
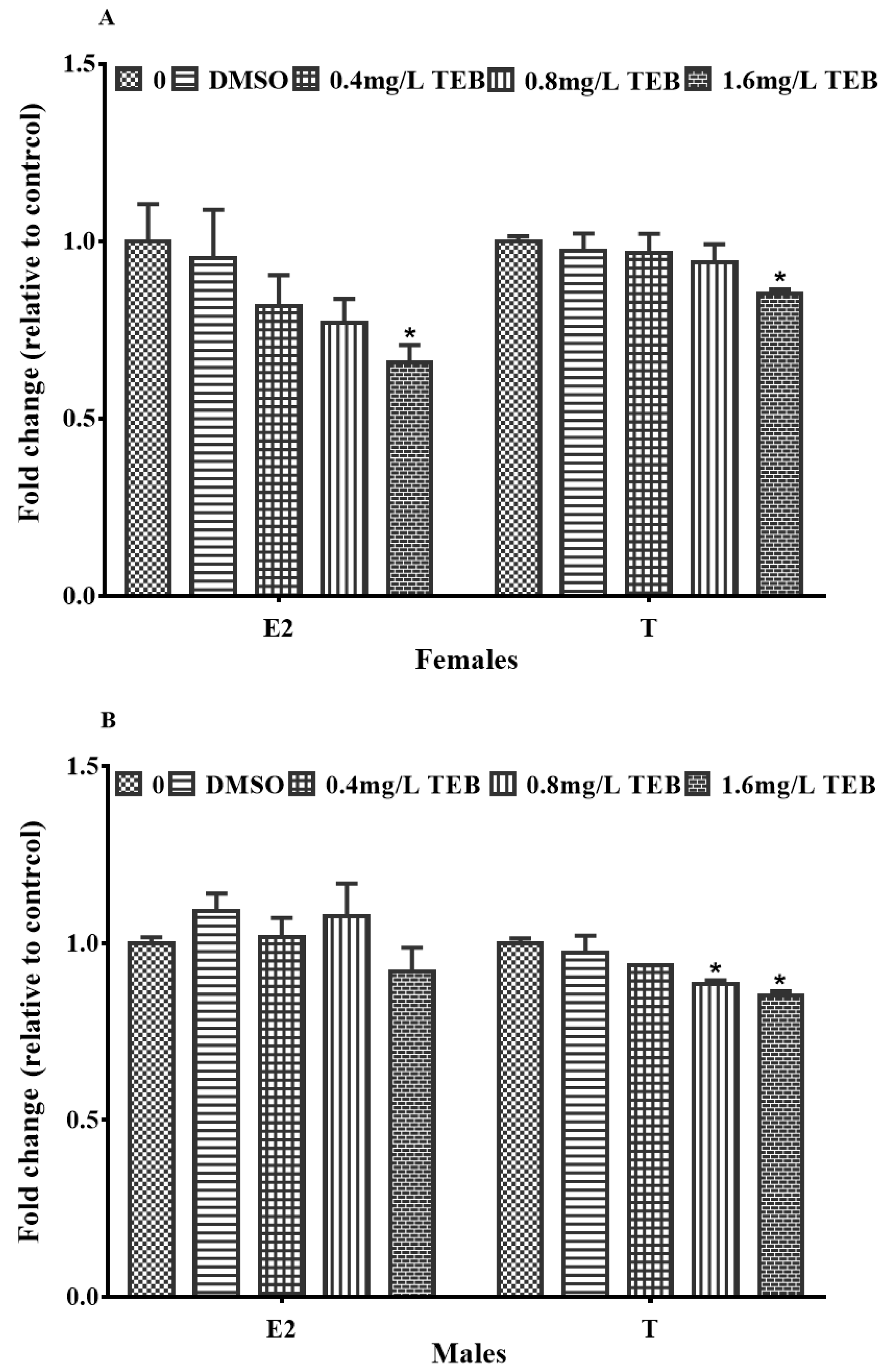
3.6. Plasma Sex Hormones
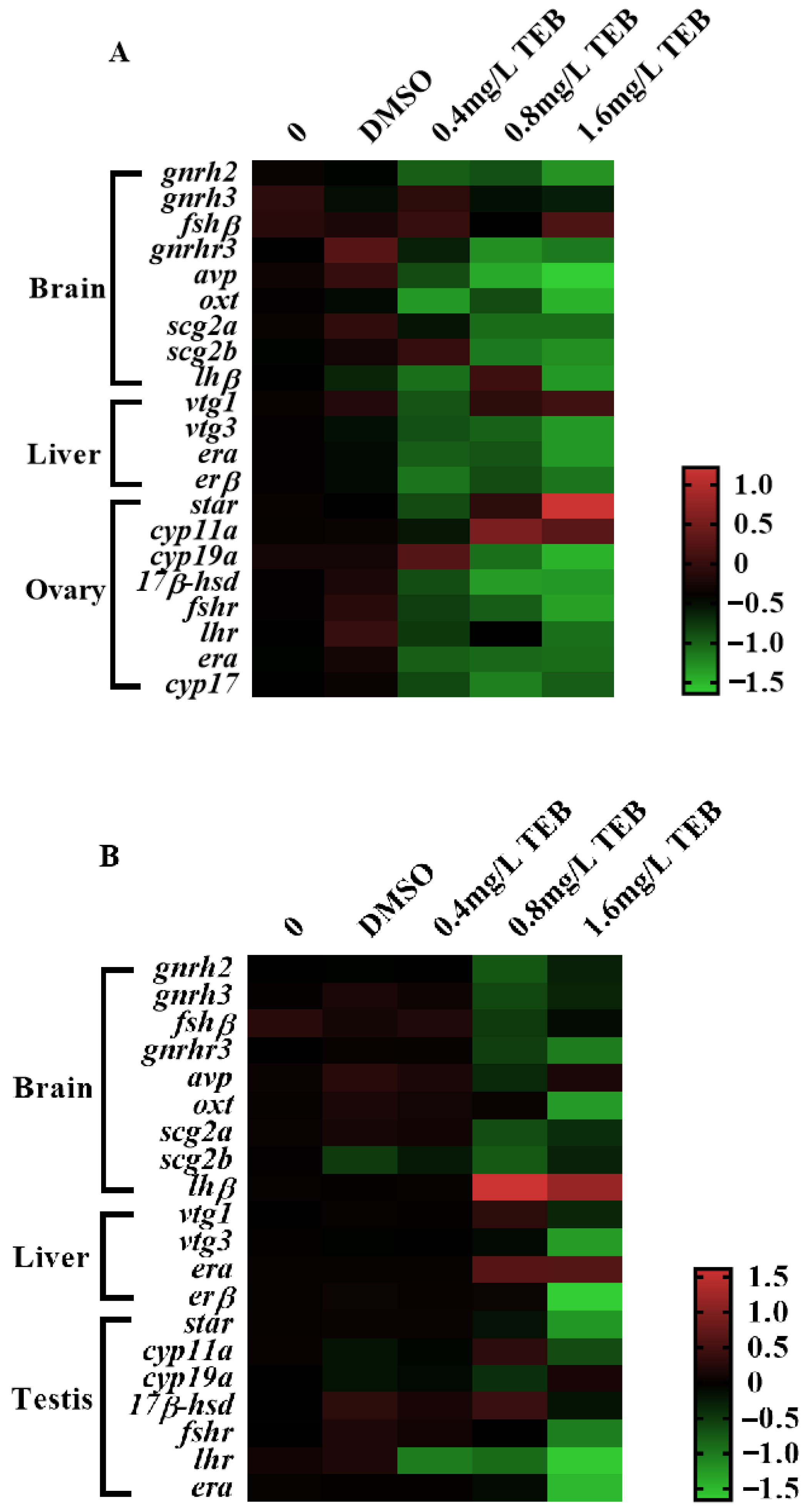
3.7. The Expression Levels of Genes Related to the HPG Axis and Social Behavior
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, N.; Xu, H.; Yao, S.; He, Y.; Zhang, H.; Yu, Y. Chiral triazole fungicide tebuconazole: Enantioselective bioaccumulation, bioactivity, acute toxicity, and dissipation in soils. Environ. Sci. Pollut. Res. 2018, 25, 25468–25475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hua, X.; Yang, Y.; Yin, W.; Tian, M.; Shi, H.; Wang, M. Stereoselective degradation of flutriafol and tebuconazole in grape. Environ. Sci. Pollut. Res. Int. 2015, 22, 4350–4358. [Google Scholar] [CrossRef]
- Carena, L.; Scozzaro, A.; Romagnoli, M.; Pazzi, M.; Martone, L.; Minero, C.; Vione, D. Phototransformation of the fungicide tebuconazole, and its predicted fate in sunlit surface freshwaters. Chemosphere 2022, 303, 134895. [Google Scholar] [CrossRef]
- Castro, T.; Souza, J.; Carvalho, A.; Assis, I.; Palmieri, M.; Vieira, L.; Marcussi, S.; Machado, M.; Murgas, L. Anxiety-associated behavior and genotoxicity found in adult, Danio rerio, exposed to tebuconazole-based commercial product. Environ. Toxicol. Phar. 2018, 62, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Mao, L.; Zhang, L.; Zhang, Y.; Jiang, H. Combined toxicity of imidacloprid, acetochlor, and tebuconazole to zebrafish (Danio rerio): Acute toxicity and hepatotoxicity assessment. Environ. Sci. Pollut. Res. 2020, 27, 10286–10295. [Google Scholar] [CrossRef] [PubMed]
- Lefrancq, M.; Jadas-Hecart, A.; La Jeunesse, I.; Landry, D.; Payraudeau, S. High frequency monitoring of pesticides in runoff water to improve understanding of their transport and environmental impacts. Sci. Total Environ. 2017, 587, 75–86. [Google Scholar] [CrossRef]
- Clasen, B.; Loro, V.L.; Murussi, C.R.; Tiecher, T.L.; Moraes, B.; Zanella, R. Bioaccumulation and oxidative stress caused by pesticides in Cyprinus carpio reared in a rice-fish system. Sci. Total Environ. 2018, 626, 737–743. [Google Scholar] [CrossRef]
- Chen, G.; Liu, F.; Zhang, X.; Dong, J.; Qiao, Y.; Zhang, R.; Liao, H. Dissipation rates, residue distribution and dietary risk assessment of isoprothiolane and tebuconazole in paddy field using UPLC-MS/MS. Int. J. Environ. Anal. Chem. 2020, 102, 5200–5212. [Google Scholar] [CrossRef]
- Mercadante, R.; Polledri, E.; Scurati, S.; Moretto, A.; Fustinoni, S. Identification and quantification of metabolites of the fungicide tebuconazole in human urine. Chem. Res. Toxicol. 2014, 27, 1943–1949. [Google Scholar] [CrossRef]
- Poulsen, R.; Luong, X.; Hansen, M.; Styrishave, B.; Hayes, T. Tebuconazole disrupts steroidogenesis in Xenopus laevis. Aquat. Toxicol. 2015, 168, 28–37. [Google Scholar] [CrossRef]
- Li, S.; Wu, Q.; Sun, Q.; Coffin, S.; Gui, W.; Zhu, G. Parental exposure to tebuconazole causes thyroid endocrine disruption in zebrafish and developmental toxicity in offspring. Aquat. Toxicol. 2019, 211, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, Y.; Sun, Q.; Coffin, S.; Chen, L.; Qiao, K.; Zhu, G. Tebuconazole induced oxidative stress related hepatotoxicity in adult and larval zebrafish (Danio rerio). Chemosphere 2022, 241, 125129. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Chen, G.; Zeng, J.; Liu, F.; Liao, X.; Guo, C.; Lu, H. Low trifloxystrobin-tebuconazole concentrations induce cardiac and developmental toxicity in zebrafish by regulating notch mediated-oxidative stress generation. Ecotoxicol. Environ. Saf. 2022, 241, 113752. [Google Scholar] [CrossRef]
- Li, G.; Li, D.; Rao, H. Potential neurotoxicity, immunotoxicity, and carcinogenicity induced by metribuzin and tebuconazole exposure in earthworms (Eisenia fetida) revealed by transcriptome analysis. Sci. Total Environ. 2022, 807, 150760. [Google Scholar] [CrossRef] [PubMed]
- Taxvig, C.; Hass, U.; Axelstad, M.; Dalgaard, M.; Boberg, J.; Andeasen, H.R.; Vinggaard, A.M. Endocrine-disrupting activities in vivo of the fungicides tebuconazole and epoxiconazole. Toxicol. Sci. 2007, 100, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Antia, A.; Ortiz-Santaliestra, M.E.; Mougeot, F.; Camarero, P.R.; Mateo, R. Birds feeding on tebuconazole treated seeds have reduced breeding output. Environ. Pollut. 2021, 271, 116292. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, Z.; Zhu, W. Evaluating the effects of the tebuconazole on the earthworm, Eisenia fetida by H-1 NMR-Based untargeted metabolomics and mRNA assay. Ecotoxicol. Environ. Saf. 2020, 194, 110370. [Google Scholar] [CrossRef]
- Kohn, G.M.; King, A.P.; Dohme, R.; Meredith, G.R.; West, M.J. In the company of cowbirds, Molothrus ater: Robust patterns of sociability predict reproductive performance. J. Comp. Psychol. 2013, 127, 40–48. [Google Scholar] [CrossRef]
- De Oliveira, J.; Chadili, E.; Piccini, B.; Turies, C.; Maillot-Marechal, E.; Palluel, O.; Budzinski, H.; Cousin, X.; Brion, F.; Hinfray, N. Refinement of an OECD test guideline for evaluating the effects of endocrine disrupting chemicals on aromatase gene expression and reproduction using novel transgenic cyp19a1a-eGFP zebrafish. Aquat. Toxicol. 2020, 220, 105403. [Google Scholar] [CrossRef]
- Salahinejad, A.; Naderi, M.; Attaran, A.; Meuthen, D.; Niyogi, S.; Chivers, D.P. Effects of chronic exposure to bisphenol-S on social behaviors in adult zebrafish: Disruption of the neuropeptide signaling pathways in the brain. Environ. Pollut. 2020, 262, 113992. [Google Scholar] [CrossRef]
- Xiao, W.Y.; Li, Y.W.; Chen, Q.L.; Liu, Z.H. Tributyltin impaired reproductive success in female zebrafish through disrupting oogenesis, reproductive behaviors and serotonin synthesis. Aquat. Toxicol. 2018, 200, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; An, J.; Xie, D.; Gong, S.; Li, Y. Suppression and recovery of reproductive behavior induced by early life exposure to mercury in zebrafish. Com. Biochem. Phys. C 2020, 239, 108876. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.R.; Li, Y.W.; Chen, Q.L.; Shen, Y.J.; Liu, Z.H. Tributyltin impaired spermatogenesis and reproductive behavior in male zebrafish. Aquat. Toxicol. 2020, 224, 105503. [Google Scholar] [CrossRef] [PubMed]
- Thore, E.S.J.; Philippe, C.; Brendonck, L.; Pinceel, T. Antidepressant exposure reduces body size, increases fecundity and alters social behavior in the short-lived killifish Nothobranchius furzeri. Environ. Pollut. 2020, 265 (Pt A), 115068. [Google Scholar] [CrossRef]
- Chen, S.; Lin, C.; Tan, J.; Wang, Y.; Wang, X.; Wang, X.; Liu, L.; Li, J.; Hou, L.; Liu, J.; et al. Reproductive potential of mosquitofish is reduced by the masculinizing effect of a synthetic progesterone, gestodene: Evidence from morphology, courtship behaviour, ovary histology, sex hormones and gene expressions. Sci. Total Environ. 2021, 769, 144570. [Google Scholar] [CrossRef] [PubMed]
- Hussar, G.J.; Paradela, A.L.; Jonas, T.C.; Serra, W.; Gomes, J.P.R.; Peres, M.R. Ensaio para a determinação de dosagem tóxica do fungicida tebuconazole (folicur 200 ce) sobre alevinos e juvenis de tilápia (tilapia rendalli) e de pacu (piaractus mesopotamicus). Eng. Ambient. Espírito St. Do Pinhal. 2004, 1, 35–44. [Google Scholar]
- Sancho, E.; Villarroel, M.J.; Fernandez, C.; Andreu, E.; Ferrando, M.D. Short-term exposure to sublethal tebuconazole induces physiological impairment in male zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2010, 73, 370–376. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 229: Fish Short Term Reproduction Assay. In OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2012. [Google Scholar]
- Vieira, R.S.; Venâncio, C.A.; Félix, L.M. Behavioural impairment and oxidative stress by acute exposure of zebrafish to a commercial formulation of tebuconazole. Environ. Toxicol. Pharm. 2022, 91, 103823. [Google Scholar] [CrossRef]
- Wu, Q.; Yan, W.; Liu, C.; Hung, T.C.; Li, G. Co-exposure with titanium dioxide nanoparticles exacerbates MCLR-induced brain injury in zebrafish. Sci. Total Environ. 2019, 693, 133540. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, J.; Yu, L.; Liu, C.; Wang, J. Gonadal impairment and parental transfer of tris (2-butoxyethyl) phosphate in zebrafish after long-term exposure to environmentally relevant concentrations. Chemosphere 2019, 218, 449–457. [Google Scholar] [CrossRef]
- Lu, J.; Wu, Q.; Yang, Q.; Li, G.; Wang, R.; Liu, Y.; Jiang, J. Molecular mechanism of reproductive toxicity induced by beta-cypermethrin in zebrafish. Com. Biochem. Phys. C 2021, 239, 108894. [Google Scholar] [CrossRef] [PubMed]
- Bernabo, I.; Guardia, A.; Macirella, R.; Sesti, S.; Tripepi, S.; Brunelli, E. Tissues injury and pathological changes in Hyla intermedia juveniles after chronic larval exposure to tebuconazole. Ecotoxicol. Environ. Saf. 2020, 205, 111367. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Bu, Y.; Ma, L.; Liu, R. Transgenerational reproductive and developmental toxicity of tebuconazole in Caenorhabditis elegans. J. Appl. Toxicol. 2020, 40, 578–591. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, H.; Wang, F.; Chen, C.; Zeng, X. Sperm motility modulated by trpv1 regulates zebrafish fertilization. Theriogenology 2020, 151, 41–51. [Google Scholar] [CrossRef]
- Yang, F.X.; Xu, Y.; Hui, Y. Reproductive effects of prenatal exposure to nonylphenol on zebrafish (Danio rerio). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006, 142, 77–84. [Google Scholar] [CrossRef]
- Cao, J.; Wang, G.; Wang, T.; Chen, J.; Guo, W.; Wu, P.; He, X.; Xie, L. Copper caused reproductive endocrine disruption in zebrafish (Danio rerio). Aquat. Toxicol. 2019, 211, 124–136. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, H.; He, J.; Wu, X.; Wang, L.; Liu, X.; Lin, H. Interaction of nuclear ERs and GPER in vitellogenesis in zebrafish. J. Steroid. Biochem. Mol. Biol. 2019, 189, 10–18. [Google Scholar] [CrossRef]
- Hou, J.; Li, L.; Wu, N.; Su, Y.; Lin, W.; Li, G.; Gu, Z. Reproduction impairment and endocrine disruption in female zebrafish after long-term exposure to MCLR: A life cycle assessment. Environ. Pollut. 2016, 208, 477–485. [Google Scholar] [CrossRef]
- Liu, N.; Dong, F.; Xu, J.; Liu, X.; Chen, Z.; Tao, Y.; Pan, X.; Chen, X.; Zheng, Y. Stereoselective Determination of Tebuconazole in Water and Zebrafish by Supercritical Fluid Chromatography Tandem Mass Spectrometry. J. Agric. Food Chem. 2015, 63, 6297–6303. [Google Scholar] [CrossRef]
- Zhao, Y.; Xie, L.; Yan, Y. Microcystin-LR impairs zebrafish reproduction by affecting oogenesis and endocrine system. Chemosphere 2015, 120, 115–122. [Google Scholar] [CrossRef]
- Maharajan, K.; Muthulakshmi, S.; Karthik, C.; Nataraj, B.; Nambirajan, K.; Hemalatha, D.; Ji, S.; Kadirvelu, K.; Liu, K.C.; Ramesh, M. Pyriproxyfen induced impairment of reproductive endocrine homeostasis and gonadal histopathology in zebrafish (Danio rerio) by altered expression of hypothalamus-pituitary-gonadal (HPG) axis genes. Sci. Total Environ. 2020, 735, 139496. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Sun, X.; Yu, M.; Fu, W.; Chen, H.; Chen, J. Chronic exposure to environmental concentrations of phenanthrene impairs zebrafish reproduction. Ecotoxicol. Environ. Saf. 2019, 182, 109376. [Google Scholar] [CrossRef] [PubMed]
- Baatrup, E.; Henriksen, P.G. Disrupted reproductive behavior in unexposed female zebrafish (Danio rerio) paired with males exposed to low concentrations of 17alpha-ethinylestradiol (EE2). Aquat. Toxicol. 2015, 160, 197–204. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.; Chadili, E.; Turies, C.; Brion, F.; Cousin, X.; Hinfray, N. A comparison of behavioral and reproductive parameters between wild-type, transgenic and mutant zebrafish: Could they all be considered the same “zebrafish” for reglementary assays on endocrine disruption? Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 239, 108879. [Google Scholar] [CrossRef] [PubMed]
- Parhar, I.S.; Ogawa, S.; Ubuka, T. Reproductive neuroendocrine pathways of social behavior. Front. Endocrinol. 2016, 7, 28. [Google Scholar] [CrossRef]
- Spence, R. Zebrafish ecology and behaviour. In Zebrafish Models in Neurobehavioral Research; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–46. [Google Scholar] [CrossRef]
- Nasiadka, A.; Clark, M.D. Zebrafish breeding in the laboratory environment. ILAR J. 2012, 53, 161–168. [Google Scholar] [CrossRef]
- Neumann, I.D.; Landgraf, R. Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012, 35, 649–659. [Google Scholar] [CrossRef]
- Pouso, P.; Quintana, L.; Lopez, G.C.; Somoza, G.M.; Silva, A.C.; Trudeau, V.L. The secretogranin-II derived peptide secretoneurin modulates electric behavior in the weakly pulse type electric fish, Brachyhypopomus gauderio. Gen. Comp. Endocrinol. 2015, 222, 158–166. [Google Scholar] [CrossRef]
- Mitchell, K.; Zhang, W.S.; Lu, C.; Tao, B.; Chen, L.; Hu, W.; Trudeau, V.L. Targeted mutation of secretogranin-2 disrupts sexual behavior and reproduction in zebrafish. Proc. Natl. Acad. Sci. USA 2020, 117, 12772–12783. [Google Scholar] [CrossRef]
- Godwin, J.; Thompson, R. Nonapeptides and social behavior in fishes. Horm. Behav. 2012, 61, 230–238. [Google Scholar] [CrossRef]
- Mitchell, K.; Mikwar, M.; Da Fonte, D.; Lu, C.; Tao, B.; Peng, D.; Erandani, W.K.C.U.; Hu, W.; Trudeau, V.L. Secretoneurin is a secretogranin-2 derived hormonal peptide in vertebrate neuroendocrine systems. Gen. Comp. Endocrinol. 2020, 299, 113588. [Google Scholar] [CrossRef] [PubMed]
- Zempo, B.; Tanaka, N.; Daikoku, E.; Ono, F. High-speed camera recordings uncover previously unidentified elements of zebrafish mating behaviors integral to successful fertilization. Sci. Rep. 2021, 11, 20228. [Google Scholar] [CrossRef] [PubMed]
- Maruska, K.P.; Fernald, R.D. Social regulation of gene expression in the hypothalamic-pituitary-gonadal axis. Physiology 2011, 26, 412–423. [Google Scholar] [CrossRef] [PubMed]
| Groups | Ovary (µg/g WW) | Testis (µg/g WW) | Before Water Renewed | After Water Renewed |
|---|---|---|---|---|
| Control | ND | ND | ND | ND |
| Solvent Control | ND | ND | ND | ND |
| 0.4 mg/L TEB | 5.65 ± 0.06 | 5.78 ± 0.05 | 0.32 ± 0.02 | 0.39 ± 0.01 |
| 0.8 mg/L TEB | 8.87 ± 0.07 | 7.95 ± 0.09 | 0.74 ± 0.04 | 0.79 ± 0.01 |
| 1.6 mg/L TEB | 14.72 ± 0.04 | 13.83 ± 0.07 | 1.53 ± 0.01 | 1.58 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, W.; Li, G.; Lu, Q.; Hou, J.; Pan, M.; Peng, M.; Peng, X.; Wan, H.; Liu, X.; Wu, Q. Molecular Mechanisms of Tebuconazole Affecting the Social Behavior and Reproduction of Zebrafish. Int. J. Environ. Res. Public Health 2023, 20, 3928. https://doi.org/10.3390/ijerph20053928
Yan W, Li G, Lu Q, Hou J, Pan M, Peng M, Peng X, Wan H, Liu X, Wu Q. Molecular Mechanisms of Tebuconazole Affecting the Social Behavior and Reproduction of Zebrafish. International Journal of Environmental Research and Public Health. 2023; 20(5):3928. https://doi.org/10.3390/ijerph20053928
Chicago/Turabian StyleYan, Wei, Guangyu Li, Qiqi Lu, Jianjun Hou, Meiqi Pan, Maomin Peng, Xitian Peng, Hui Wan, Xixia Liu, and Qin Wu. 2023. "Molecular Mechanisms of Tebuconazole Affecting the Social Behavior and Reproduction of Zebrafish" International Journal of Environmental Research and Public Health 20, no. 5: 3928. https://doi.org/10.3390/ijerph20053928
APA StyleYan, W., Li, G., Lu, Q., Hou, J., Pan, M., Peng, M., Peng, X., Wan, H., Liu, X., & Wu, Q. (2023). Molecular Mechanisms of Tebuconazole Affecting the Social Behavior and Reproduction of Zebrafish. International Journal of Environmental Research and Public Health, 20(5), 3928. https://doi.org/10.3390/ijerph20053928






