Abstract
To stimulate the integration of chronic care across disciplines, the Netherlands has implemented single-disease management programmes (SDMPs) in primary care since 2010; for example, for COPD, type 2 diabetes mellitus, and cardiovascular diseases. These disease-specific chronic care programmes are funded by bundled payments. For chronically ill patients with multimorbidity or with problems in other domains of health, this approach was shown to be less fit for purpose. As a result, we are currently witnessing several initiatives to broaden the scope of these programmes, aiming to provide truly person-centred integrated care (PC-IC). This raises the question if it is possible to design a payment model that would support this transition. We present an alternative payment model that combines a person-centred bundled payment with a shared savings model and pay-for-performance elements. Based on theoretical reasoning and results of previous evaluation studies, we expect the proposed payment model to stimulate integration of person-centred care between primary healthcare providers, secondary healthcare providers, and the social care domain. We also expect it to incentivise cost-conscious provider-behaviour, while safeguarding the quality of care, provided that adequate risk-mitigating actions, such as case-mix adjustment and cost-capping, are taken.
1. Introduction
In many countries, the prevalence of chronic diseases, and in particular people with multimorbidity, i.e., two or more chronic diseases, is increasing [1]. Two thirds of people over 45 will develop multimorbidity in their remaining lifetime [2]. To address their needs, many countries are now implementing different models of integrated care [3]. As the Netherlands were among the first countries to do so on a very large scale, there are lessons to be learned for other countries from how this evolved in the Netherlands, in particular with regards to possible incentives for truly person-centred and integrated care.
Historically, the Dutch healthcare system has had a strong primary care sector, in which general practitioners (GPs) act as gatekeepers to secondary care (i.e., patients need a referral by the GP) [4]. To improve the quality of care to people with chronic diseases, single-disease management programmes (SDMPs) have been introduced in Dutch primary care since 2010, for diabetes type-2 (DM2) [5], cardiovascular risk management (CVR) [6], and chronic obstructive pulmonary disease (COPD) [7]. Therefore, the GP is the main caregiver for many patients with chronic diseases in the Netherlands. These SDMPs were based on chronic care standards, which are essentially clinical guidelines for providing high quality, multidisciplinary, integrated care.
To coordinate the implementation of the SDMPs in a region, a new organisational entity, the primary care cooperative (care group), was introduced. Today, there are 130 primary care cooperatives in the Netherlands based on the collaborations of general practices [8]. For the daily execution of the SDMP’s and to reduce the workload of GPs, a new professional role was introduced in the GP practice, namely that of the nurse practitioner. The nurse practitioner regularly monitors symptoms and physiological parameters of patients with the chronic diseases mentioned above, and provides lifestyle and coping advice [9].
To further incentivise the integration of multidisciplinary care, the implementation of the SDMPs was supported by a bundled payment model [10]. The bundled payment covers the costs of coordination, the costs of regular check-ups by the nurse practitioner or the GP, three hours with the dietician for people with DM2, the foot therapist for patients with DM2, the physiotherapist for patients with more severe COPD, and a single (tele)consultation with a medical specialist when necessary. Health insurers contract primary care cooperatives, which in turn subcontract GPs and other healthcare providers for providing the services in the bundle [11]. The fee of the bundled payment results from the negotiation between the primary care cooperative and the health insurer about the content and price of the services in the bundle, which thus varies between primary care cooperatives.
Compared to other countries, the scope of the bundled payment in the Netherlands is limited, both in terms of target population and services included in the bundle. For instance, in the United States, accountable care organisations are generally responsible for all healthcare expenditures of a delineated patient population [12,13]. In the Gesundes Kinzigtal programme in Germany, the target population includes a group of 33,000 patients from Baden-Württemberg, who are insured by two public health insurers. Key characteristics focus on prevention, self-management, reduction of polypharmacy, patient-centred care, and shared decision-making. The programme is funded by a capitation-based payment combined with a shared savings model [14,15]. In the United Kingdom (UK), general practices receive a lump sum for all GP-care, some specialist care, and generic medication [16,17]. In the UK, integrated care organisations are introduced to stimulate integration between primary care physicians and specialists. The integrated care organisations are responsible for a case-mix corrected budget per capita [18].
As a result of the introduction of the SDMPs in the Netherlands, the vast majority of patients with DM2, CVR, and COPD are now treated in primary care. The quality of chronic care is monitored by InEeN, a primary care interest organisation, which annually publishes process- and outcome-indicators at the care-group level [19]. These indicators were found to improve over time [20], but the clinical relevance and long-term impact of these improvements are uncertain [14,20,21]. Improvements in the work experience of GPs were also reported [9,14,20].
However, the SDMPs have several limitations. First, the chronic care programmes focus on a single chronic disease, rather than adopting a holistic approach that considers the social context of the chronically ill patient (e.g., family, living environment, financial resources, and the work situation) [21,22]. The programmes mainly aim to improve clinical disease-specific indicators, and there is less attention paid to psychological and social aspects. This does not match well with how our perspective on disease and health has evolved. In the Netherlands, many primary care cooperatives have recently embraced the new concept of so-called positive health (‘health as the ability to adapt and to self-manage, in the face of social, physical, and emotional challenges’) that was introduced in 2011 by Huber et al. [23,24]. Second, the scope of the services included in the current bundles is limited. The bundled payment does not cover care that transcends the chronic disease [25,26]. The bundled payment does not include all primary healthcare, no secondary care, no mental health care, and no social services. It might stimulate collaboration between healthcare providers in primary care (e.g., between the GP and the dietician), but less so between the GP and the specialist or between the GP and the social worker.
The introduction of the SDMP and the bundled payments were expected to improve the efficiency of care delivery and reduce healthcare expenditures or the growth thereof [27]. However, there is evidence that they increased the total costs of healthcare, especially in patients with multimorbidity [14,28]. This cost increase probably results from a combination of the detection of unmet needs in patients with multimorbidity, double declarations, and an incentive to refer the more complex patients to secondary care to avoid costs exceeding the bundled payment [28]. The currently used SDMPs and bundled payments are not suitable for patients with multiple chronic diseases.
As a result, we are currently witnessing several initiatives to broaden the scope of the SDMPs aiming to provide person-centred and integrated care (PC-IC) [28,29]. This raises the question of which payment model would best support this transition [29]. As a first step, InEeN proposed merging the current bundled payments for people with multiple of the respective chronic diseases to remove duplication [30]. However, that proposal would still not fully incentivise PC-IC. This paper aims to present an alternative payment model that incentivises the integrated nature of a PC-IC programme for people with chronic diseases. It is based on a targeted literature review of (incentives in) traditional and more recent payment models in different countries and inspired by a specific PC-IC initiative in the Netherlands.
2. Methods
2.1. Case Example: OPTIMA FORMA
The proposed payment model was specifically designed to match with one of the initiatives to move towards PC-IC in the Netherlands, i.e., the project, OPTIMA FORMA—Towards a patient-centred multimorbidity approach for chronic disease management in primary care. In this project, healthcare providers, patients, GP experts with a special interest in DM2, COPD, or CVD, primary care cooperatives coordinators, and researchers developed a new integrated care programme that goes beyond the disease-specific clinical domain. The new care plan has a quadruple aim: (1) enhancing patient experience, (2) improving population health, (3) reducing costs, and (4) improving the work life of health care providers [31].
In the PC-IC programme, a holistic assessment of the health status is performed, personal goals are set, and interventions to achieve these goals are put in place [32,33]. The first step in this programme is assessing the integral health status of the patient (health across multiple domains—Figure 1), using a (preferably digital) questionnaire at home and physical measurements (i.e., blood pressure, weight, and glucose levels). The second step is an appointment in which the results are discussed with the patient in a semi-structured way. The case manager asks if the patient recognizes himself in the results of the assessment, if there are other issues that the patient would like to discuss, and the priorities of the patient. Personal goals are formulated in the third step, which can range from purely medical goals to social goals. In the fourth step, the healthcare provider and patient will together choose the right interventions to achieve these goals, based on the experience of the healthcare provider, the ideas of the patient, and a list of regional options. Different methods can be used to achieve these goals (i.e., through self-management, with e-health, with coaching from a non-medical care provider, with coaching from a healthcare provider within the GP practice, or with coaching from a healthcare provider outside the GP practice). The goals and interventions are documented in a personal healthcare plan, which is preferably digitally available to all relevant healthcare providers and the patient. Then, referrals are made if necessary, and the treatment is started. An evaluation is planned and carried out, if necessary multiple times. If a treatment goal is reached or another treatment goal is more urgent, the cycle can be repeated. The development of this PC-IC approach is described elsewhere in this issue [34].
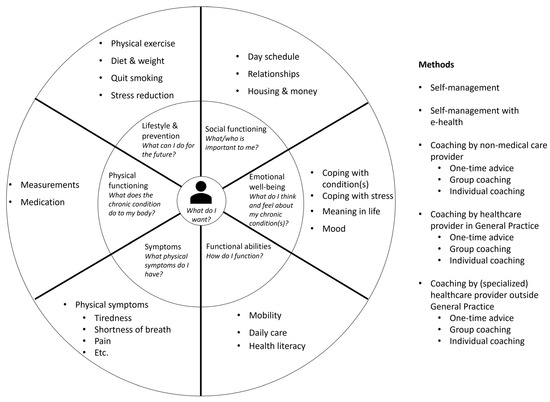
Figure 1.
Person-centred and integrated care programme for people with chronic diseases.
2.2. Incentives in Payment Models
To design a payment model that would match the PC-IC programme of OPTIMA FORMA, we first studied the incentives for providers and other stakeholders that are present in the current Dutch healthcare system for all types of healthcare services used by patients with chronic disease. We classified these payment models according to the typology of Quinn (2015) [35] and identified the incentives related to these payment methods. Quinn (2015) [35] classifies eight basic payment methods in health care: (1) Per time period (budget/salary), (2) Per beneficiary (capitation), (3) Per recipient (contact capitation), (4) Per episode (case rates/per stay/bundled payments), (5) Per day (per diem/per visit), (6) Per service (fee for service (FFS)), (7) Per dollar of costs (cost reimbursement), and (8) Per dollar of charges (percentage of charges).
Secondly, we studied incentives for stakeholders in innovative payment models. These innovative payment models were identified through the alternative payment model (APM) framework described by the Health Care Payment Learning and Action Network (HCP-LAN) [36]. The identified alternative payment models were: (1) pay for performance, (2) shared savings models, and (3) (sub)population-based bundled payment. We combined elements of these models to design an alternative payment model to stimulate PC-IC care for people with chronic diseases.
2.3. Design of an Alternative Payment Model
In the next step, we selected three alternative payment models and explicitly focused on the distinctive elements in their design. Since we aimed to propose an alternative payment model for the Dutch setting, the selection was based on two criteria, namely comprehensiveness and origin in the Dutch setting. The selection included:
- a population-based bundled payment model with an explicit incentive for quality of care of Cattel and Eijkenaar [37].
- a shared savings model of Hayen et al. [38].
- the alternative payment model of Steenhuis et al. [39].
We combined the design elements and design choices that were mentioned by these models into Table 1. Table 1 was used to guide the design of an alternative payment model that would fit the PC-IC programme OPTIMA FORMA. The design choices made were primarily informed by theory on provider-incentives and results from previous evaluation studies of the identified innovative payment models: (1) pay-for-performance [40,41], (2) shared-savings models [13,15,42,43], and (3) (sub)population—based bundled payments [37,44,45].

Table 1.
Design elements for the implementation of an alternative payment model.
2.4. Expected Impact on Integration of Care
In the last step, we projected the expected impact of the innovative payment model on the integration of care, using the spider-web linked to the typology of Stokes et al [46]. This typology classifies the level of integrated care on eight domains: (1) Target population, (2) Time, (3) Sectors, (4) Provider coverage, (5) Financial pooling/sharing, (6) Income, (7) Multiple disease/needs focus, and (8) Quality measurements [46]. The higher the number, the higher the level of integration (1 = integration is poorly stimulated, 2 = integration is mediately stimulated, and 3 = integration is highly stimulated).
3. Results
3.1. Incentives Induced by Different Payment Models
In Table 2, we provide a summary of current and alternative payment models to fund care for patients with chronic diseases in the Netherlands.

Table 2.
Current Dutch payment models based on the typology of Quinn (2015) [35] and the HCP-LAN (2017) [36] and their (financial) incentives.
Table 2 provides insight into the incentives induced by each payment model. None of the presented payment models above fully incentivises PC-IC. The SDMPs are currently funded by a fixed annual fee, which is paid in three monthly instalments (chronic care episode). The bundle primarily includes the GP, practice nurses, and a few paramedics working in the primary care sector. Hence, it stimulates collaboration between these service providers, but not beyond. It is likely to improve the quality and efficiency in primary care, but it also creates an incentive for adverse selection and referral of complex patients to secondary care. This is present, even though the fixed fee is based on a weighted average of resources used by patients with different severities. It also stimulates so-called ‘over-bundling’, referring to the incentive to enrol more patients than necessary. These undesired incentives can be mitigated by carefully combining elements of different payment models [37]. From a theoretical perspective, a bundled payment with a broader scope in terms of target population and services, in combination with a shared savings model and a pay-for-performance model seems promising [37].
3.2. Proposed Payment Model for Person-Centred and Integrated Care
Figure 2 shows the proposed payment model for all patients with one or more chronic conditions, starting with those that are currently included in the existing bundles for DM2, CVR, and COPD. The patient population is delineated by diagnosed chronic disease (at least DM2, CVR, or COPD), insurance (the patient has to be insured at one of the participating health insurers), and GP-practice (GP-practice has to collaborate with one of the participating primary care cooperatives). The payment model consists of three parts: (1) a person-centred bundled payment, (2) a shared savings model that pertains to all healthcare costs, and (3) a pay-for-performance part.
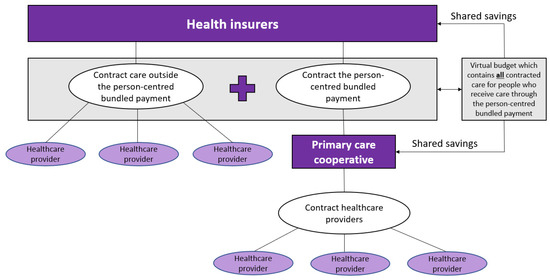
Figure 2.
Conceptualization of the person-centred bundled payment in combination with the virtual budget and the shared savings.
Part one is a person-centred bundled payment that will be prospectively paid to the primary care cooperatives. For each patient, a personal healthcare plan is designed within the OPTIMA FORMA project (Figure 1). The services that can be included in the personal healthcare plan are shown in Figure 3. The bundled payment is based on the weighted average sum of all included services. The weighting is based on the number of patients that use a service and the costs of the service. The primary care cooperative is responsible for the coordination, organization, and financing of all subcontracted participating providers since the primary care cooperative is the main contractor.
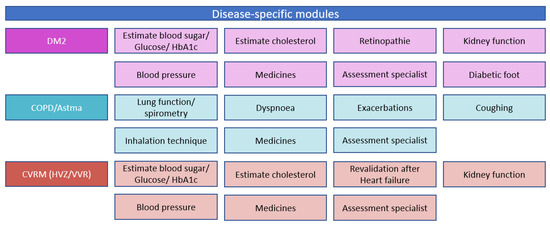
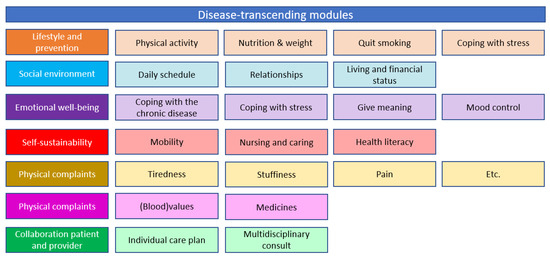
Figure 3.
Disease-specific and disease-transcending modules provided through the new care plan and delivered if needed by the patient.
Part two is a virtual budget that contains all expected (healthcare) costs of these patients (the contracted bundled payment and the contracted expenditures outside the bundled payment). The case-mix adjusted weighted virtual budget will be compared to the realised expenditures to estimate the savings or losses. It is important to cap the expenditures, so the primary care cooperative does not bear the risk for patients with extreme high (unexpected) expenditures. One could start with a one-sided shared savings model, meaning that only the savings and not the losses will be shared between the health insurer and the primary care cooperative in the region, to mitigate risks for the primary care cooperative and avoid adverse behaviour. The savings will be distributed in a prespecified ratio between the primary care cooperative and the health insurer.
In part three, the prespecified ratio to share the savings depends on the quality of the delivered care. This pay-for-performance part depends on the measured performance of the monitored quality indicators. It is important to avoid time-consuming checklists and process indicators and adopt a small set of key outcome indicators. This requires trust from the health insurers and leads to more flexibility for providers to only provide services that are applicable for a patient instead of ticking boxes to show that they followed the correct process. Quality indicators are measured at primary care cooperative level.
More details are provided in Appendix A.
The contract between the insurer and the primary care cooperatives should be signed for multiple years, preferably for three to five years. This provides the opportunity to explore the potentials of the alternative payment model (stimulate integration of care, improve quality of care, and reduce overall healthcare costs) and to gain mutual trust between the different stakeholders [39,43,75]. When the contract is renewed, changes can be made accordingly. For instance, after three or five years the one-sided shared savings model could be transformed into a two-sided shared savings model (the primary care cooperative also shares in the potential losses). A two-sided shared savings model stimulates cost-conscious behaviour better, but also increases the financial risk for the primary care cooperative [12,13,76].
3.3. Consequences of the Proposed Payment Model
The suggested alternative payment model is expected to be associated with incentives presented in Table 3. Each of the three parts of the proposed payment model has desirable and undesirable consequences, and the latter can be mitigated by the other part(s).

Table 3.
Consequences which are generated by the proposed alternative payment model.
As the range of services that can be included in the individual care plan (Figure 3) is much wider than in the current bundle for SDMP, the person-centred bundled payment is expected to stimulate the holistic approach that is aimed for by the PC-IC programme. The primary care cooperative and its associated care providers will have an incentive to improve efficiency by better coordination and collaboration because the budget extends over a wider range of services. This increases mutual responsibility. One of the perverse incentives of a bundled payment that may not cover the full care path of a patient, is that patients are referred to services outside the bundle [50]. The shared savings model mitigates this perverse incentive because the comparison of the actual and the expected expenditure (i.e., the virtual budget) pertains to the total healthcare expenditure. This could result in cost-conscious behaviour [38,77]. The current bundles for SDMPs do not incorporate a shared savings model. If the shared savings model stimulates cost savings through increased efforts to slow down the progression of disease and prevent acute hospital admissions, it also improves health outcomes. However, to mitigate financial risks for the primary care cooperative, a one-sided shared savings model is preferred over a two-sided shared savings model to avoid adverse behaviour of the primary care cooperative, especially at the beginning [78]. A perverse incentive of the person-centred bundled payment model and a shared savings model is cutting costs on necessary care. The pay-for-performance part of the model aims to reduce this risk by stimulating a high quality of care.
Like all payment methods, this alternative payment model still induces some undesirable consequences which are hard to eliminate by one of the three parts of the payment model. The risk of reducing costs by cutting necessary care might be there to some extent. Furthermore, the threshold can be lowered to include patients in the person-centred bundled payment for whom one may expect little cost. However, adequate case-mix adjustment and capping costs could reduce these risks. To some extent, the person-centred bundled payment also reduces the choice of the patient because certain care providers are contracted, and others might not. As the personal healthcare plan is based on the needs, capabilities, and wishes of the patients, it is important that the contracted providers are able to provide the services shown in Figure 3 [39]. Another undesired consequence is that the primary care cooperative bears too much risk because all expenditures are included in the virtual budget. The primary care cooperative might not be able to control all of these expenditures. The incentives of providers outside the person-centred bundled payment are not well aligned because these physicians are mostly paid FFS. It is important that these providers feel motivated to collaborate. This might be achieved by investing part of the savings in joint quality improvement and innovation plans, which are attractive to these providers as well. Every pay-for-performance model introduces a risk for gaming behaviour, but the size of that risk depends on the proportion of a provider’s income that comes from the quality-payment. The challenge is to strike a balance between a sufficiently large proportion to incentivize quality improvement and a sufficiently small proportion to avoid gaming [77].
3.4. Impact on Integration
Figure 4 shows the degree of integration of the proposed payment model and the currently used bundled payments for the SDMPs on the eight dimensions of the framework based on Stokes et al [46]. Table 4 explains the levels that were expected for each domain.
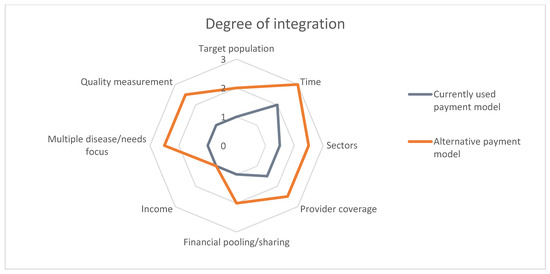
Figure 4.
Degree of integration.

Table 4.
Description of the level of integration expected to result from the proposed payment model.
4. Discussion
The aim of this paper was to design a bundled payment model that incentivises the transition from single-disease management to PC-IC for patients with chronic diseases. Based on a targeted literature review, we identified the incentives which are (theoretically) generated by the eight basic payment methods classified by Quinn [35] and the alternative payment models identified through the APM framework [36]. Based on the identified incentives, we designed an alternative payment model for PC-IC that consists of three main elements, i.e., (1) a person-centred bundled payment, (2) shared savings, and (3) pay-for-performance. The combination of these elements is expected to provide well-aligned, desired incentives towards multi-disciplinary collaboration to meet a patient’s needs, capabilities, and preferences. Each element is necessary to mitigate the undesired incentives of other elements. Furthermore, adequate risk-adjustment and cost-capping are prerequisites to mitigate large risks for providers and to mitigate adverse behaviour.
The implementation of this alternative payment model comes with certain challenges. The first challenge pertains to the investment of resources needed for implementation, which mainly include financial investments (e.g., transition costs to the alternative payment model) and time investments (e.g., to expand collaborations) [39]. To manage the alternative payment model, the software in place should be adapted to monitor the costs and quality of care over time [39]. Administrative costs of monitoring quality of care and negotiating about the conditions of the contract may increase, but this may be offset by a reduction in administrative costs when the services no longer have to be separately claimed [39].
The second challenge is to define the patient population that will be included in the person-centred bundled payment. The population of patients with DM2, CVR, and/or COPD is very heterogenous in terms of patient-characteristics, disease-severity, and co-existing morbidity patterns. For an adequate estimation of the expected expenditures, necessary to determine the savings or losses, clear inclusion and exclusion criteria need to be defined.
The third challenge is to estimate an appropriate budget for the person-centred bundle. The budget will be estimated by a weighted sum of the costs of all (health)care modules provided in the bundle. The weighting will be carried out by predicting the number of patients that would use the various modules. As time after implementation progresses, figures regarding the relative use of the modules will become more reliable. Specifically, for OPTIMA FORMA, a clinical and economic evaluation study is planned that will provide the first estimates of the utilization of specific services. Micro-costing studies are necessary to determine the costs per module.
For an appropriate comparison of expected and actual expenditures and to avoid extreme savings or catastrophic losses [79], adequate adjustment for differences in case-mix is important. Many countries with a multiple payer system (e.g., multiple social health insurers) like in the Netherlands, apply some form of risk equalization to distribute [part of] the budget among the payers. Whether variables included in the risk equalization formula of the health insurance system can also be used to adjust for differences in the case-mix of providers remains to be investigated. It is obvious that variables that are influenced by the PC-IC programme cannot be used in the case-mix adjustment because that would diminish/eliminate the estimated effects [38].
Another challenge when designing the alternative payment model is to determine the quality indicators for the pay-for-performance part of the alternative payment model. It is important to select quality indicators that are sensitive to improvements by the PC-IC programme and the alternative payment model. Based on a systematic literature review, specific design features that contribute to the desired effect of pay-for-performance are: (1) using outcome measures that are very specific and easy to track; (2) targeting individuals or small teams; (3) using absolute rather than relative targets; (4) frequently paying with little delay after delivery; and (5) involving providers from the start in the design [40]. Primary care cooperatives are reluctant to accept financial responsibility for indicators they cannot influence [80]. Conceptually, one would like to have one or more indicators for each of the four aims of PC-IC, but the challenge is to find the right balance between registration burden [79] and information need.
To increase the chances of the successful implementation of PC-IC, several requirements need to be met. In their paper on the successful implementation of integrated care for people with multimorbidity, Looman et al [29] stressed the importance of ten mechanisms, of which one is securing long-term funding and adopting an innovative payment model that overcomes fragmentation. However, most important is constructive alignment, meaning that simultaneous measures at the micro, meso, and macro levels are needed to support the implementation of PC-IC [29]. With respect to the payment model, this implies that the incentives for all participating healthcare providers, as well as with existing financial streams, have to be aligned [39,80].
A more fundamental question that arises is whether a population-based payment model that would extend to the entire population in a geographically defined area (e.g., a region) and all care providers within that area would not be a more appropriate alternative compared to the alternative payment model proposed here. Especially, because that would stimulate prevention of disease and network care for the entire population in the catchment area, all of which is paid for from one bundled budget [50]. On one hand, it could fit the integrated nature of the PC-IC programme, but on the other hand, the step from the currently used bundled payments to a population-based payment might be too big. As it currently stands, the PC-IC programme OPTIMA FORMA focusses on people with the mentioned chronic diseases. If the population of interest were defined as the entire population of insured people in a region, the effect of the PC-IC programme could easily be diluted. That does not alter the fact the PC-IC programmes would benefit from economies of scale, which could reduce the financial risks for primary care cooperatives.
5. Conclusions
To conclude, we designed a payment model with well-aligned incentives to support the adoption of PC-IC. This model consists of: (1) a person-centred bundled payment; (2) a shared savings model; and (3) a pay-for-performance part in which the sharing ratio between insurer and provider is conditional on the performance of the provider. This alternative model is likely to be an adequate alternative for the relatively limited bundled payment model that is currently used to fund the SDMPs in the Netherlands.
Author Contributions
Conceptualization, S.S.B. and M.P.M.H.R.-v.M.; methodology, S.S.B. and M.P.M.H.R.-v.M.; investigation, S.S.B., L.H.A.R., E.W.M.A.B. and M.P.M.H.R.-v.M.; writing—original draft preparation, S.S.B. and M.P.M.H.R.-v.M.; writing—S.S.B., L.H.A.R., E.W.M.A.B., L.M.A.G. and M.P.M.H.R.-v.M.; visualization, S.S.B.; supervision, L.M.A.G. and M.P.M.H.R.-v.M.; project administration, L.H.A.R. and E.W.M.A.B.; funding acquisition, E.W.M.A.B. and M.P.M.H.R.-v.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ZonMw, grant number 839110024 and by a subsidy of VEZN (Versterking Eerstelijn Zuid-Nederland).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A
- Target population: The care programme focuses on people with DM2, COPD/asthma, and/or CVRM, and who are subscribed for at least one of these current DMPs at one of the participating GP practices and who is insured at one of the participating health insurers. This was used to delineate the patient population. The patient population is heterogeneous because people with these chronic diseases can be of all ages and can have multiple other chronic diseases. The care plan has the potential to broaden the scope and include other chronic diseases (i.e., people with rheumatological disorders). The payment model could be extended as well.
- Main contractor of the bundled payment: The main contractor is the primary care cooperative. The care group finances all participating healthcare providers participating in the care programme. The primary care cooperative is responsible for a fair allocation. The healthcare providers are subcontracted by the primary care cooperative [i.e., the GP practice, the dietician, the physiotherapist, the foot therapist, the medical specialist, the primary mental health physician, and the district nurse].
- Care coordinator and provider coverage: The general practitioner (GP) is responsible and the practice nurse (POH-S) is the coordinator of care. The POH-S and the patient formulate the care goals, based on the integral health status of the patient. The POH-S coordinates and organizes the chosen health interventions.
- Provided care through the person-centred care plan: All the interventions provided in the different modules (Figure 1) are covered in either the person-centred bundled payment or included in the virtual budget. The disease-specific and disease-transcending modules focus on (1) lifestyle and prevention, (2) social environment, (3) emotional well-being, (4) self-sustainability, (5) physical complaints, and (6) physical functioning.
- Budget for quarterly bundled payment: the bundled payment is a fixed budget which is paid every three months. The budget is based on a weighted average sum of all provided care activities. The weighting is based on the number of patients that use a certain care activity and the price of the intervention. Each goal of the patient can be reached in different ways:
- The patient can do something his/herself.
- The patient could use E-health to reach a goal.
- The patient could receive help from a non-medical caregiver (once, in a group, or long-term individual help).
- The patient could receive help from a GP or a practice nurse (once, in a group, or long-term individual help).
- The patient could receive help from a (specialized) provider outside the GP practice (once, in a group, or long-term individual help).
The average tariff per module could be calculated with the following formula:
The average budget for the person-centred bundled payment could be calculated with the following formula:
- 6.
- Retrospective or prospective payment: The average tariff of the person-centred bundled payment will be prospectively determined. The virtual budget will also be prospectively calculated and compared to the real expenditures. These real expenditures will be retrospectively determined and compared with the estimated virtual budget.
- 7.
- Calculation of the virtual budget [38]: The virtual budget will be determined by the average [health]care expenditures of one year. All patients who were included in at least one of the existing DMPs for DM2, CVRM, and or COPD in 2017, will be included in the virtual budget. The average expenditures per patient will be calculated with the following formula [38]:
The maximum budget = 5% of the highest costs will be ignored [the patients with the 5% highest costs could account for more than 50% of the total expenditures]. If the total healthcare costs of the patient exceed this threshold, the costs are not counted. We used the total expenditures from 2017 because this is the most recent year in which all billable costs are registered in the system of the health insurer.
To calculate the virtual budget (benchmark), we must correct for the trend in healthcare costs. The trend reflects the expenditures that would have been there if there would not have been a new care plan. We will calculate the trend with the following formula [38]:
The virtual budget (benchmark) will be calculated with the following formula:
This benchmark must be adjusted for differences in case-mix variables between the treatment and the control group. In step 9, we will explain how this can be done.
- 8.
- Usage of a one-sided or two-sided shared savings model and the duration of the contract: We would advise to start with a one-sided shared savings contract. In this case, the primary care cooperative and health insurer only share when the care group achieves to save money. When there are more expenditures in 2021 compared to the virtual budget, these will be paid by the health insurer. It is undesirable to start with a two-sided shared savings model because the care groups need some time to get used to the new delivery model. If the risks are too high for the primary care cooperative, it could induce adverse behaviour and reduce costs by saving on needed care. From the literature, we know that a two-sided shared savings model could eventually increase savings [12,13,76], which could make it desirable to switch to a two-sided contract after some time, for instance, after three years. For this reason, we would advise to sign a contract for at least three to five years, to have enough time to recoup the investments.
- 9.
- Distribution if there are any savings: the primary care cooperative and preferential health insurer should negotiate about the distribution of any savings. We would advise to let the distribution depend on the quality of the delivered care. Step 10 will describe a pay-for-performance model in more detail. It is important to clarify how possible savings will be spent. The care group is responsible for their part of the savings. The care group could divide all savings equally across participating providers. It is also possible to use any savings to innovate or to use the savings in a region where it is needed the most.
- 10.
- Case-mix adjustment: First, it is important to avoid risk-selection. If the GP practice only selects relatively healthy patients and we would not adjust for case-mix variables, this practice can easily generate savings, because the average costs of a relatively healthy patient population are lower compared to the average costs. The average budget must be adjusted to the included patient population of that region/ practice. It is important to adjust for case-mix differences because the patient population is heterogeneous [patients who are just diagnosed with one chronic disease, or patients with multimorbidity and psychosocial problems].
Second, we calculate the virtual budget with the total expenditure of 2017 times the trend in expenditures. To be able to compare the benchmark population to the included population, we should adjust for case-mix differences. Furthermore, adjusting between the benchmark and included population, we should also adjust for differences between treatment and the control group. Hayen et al [42] adjusted for age, gender, percentage of patients who extent the maximum budget, percentage of patients who did not make any healthcare costs beside the bundled payment, percentage of patients with an additional insurance, and health status of the patient [expenditures of pharmacy (FKGs) and diagnosis-related groups (DRGs)]. It is also important to take periodic effects into account, like differences in the basic health care package.
- 11.
- Pay-for-performance: To keep track of the quality of care, we would advise to implement a form of pay-for-performance. We suggest making the sharing distribution dependent on the quality of care. The bottleneck is selecting which quality indicators to use. It is important that the indicators reflect the care plan and that they can be improved by the new way of working. Care groups, health insurers, and researchers should discuss which indicators would fit this purpose. We would advise to mainly focus on outcome indicators because these are clinically most relevant. The currently used indicators do not seem to fit because these indicators are mainly focused on the process of care instead of outcomes. Actions just need to be ticked off a checklist.
An example of how a pay-for-performance model could be structured [38]: There could be different quality domains determined. For each quality domain, an absolute quality score between 0% and 100% will be estimated. The practice receives several points which depend on the performance. A score between 0–25% will be rewarded with 0 points, between 25–50% = 3 points, between 50–75% = 6 points, 75–90% = 9 points, and above 90% = 12 points. The practice can also earn points for quality improvement compared to the previous year. An improvement of 0–2.5% = 1 point, of 2.5–5% = 2 points, of 5–7.5% = 3 points, and more than 7.5% = 4 points. If there are, for instance, ten indicators, the maximum number of points per indicator = 16 points. The maximum total score is 10 indicators × 16 = 160 points. Imagine that the care group receives 120 points. In this case, the care group receives 120/160 × 100 = 62.5% of the agreed sharing percentage.
It would also be possible to directly link some of the quality indicators to the tariff of the person-centred bundled payment. For example, −5% to +5% of the tariff could depend on the delivered quality of care.
Another possibility would be to explicitly [financially or non-financially] incentivise patients to stimulate the desired behaviour. The patient must work him/herself towards set health goals. An incentive could stimulate the patient to achieve these goals.
References
- Chen, Y.H.; Karimi, M.; Rutten-Van Mölken, M.P.M.H. The disease burden of multimorbidity and its interaction with educational level. PLoS ONE 2020, 15, e0243275. [Google Scholar] [CrossRef] [PubMed]
- Velek, P.; Luik, A.I.; Brusselle, G.G.O.; Stricker, B.C.; Bindels, P.J.E.; Kavousi, M.; Kieboom, B.C.T.; Voortman, T.; Ruiter, R.; Ikram, M.A.; et al. Sex-specific patterns and lifetime risk of multimorbidity in the general population: A 23-year prospective cohort study. BMC Med. 2022, 20, 304. [Google Scholar] [CrossRef] [PubMed]
- Struckmann, V.; Leijten, F.R.; van Ginneken, E.; Kraus, M.; Reiss, M.; Spranger, A.; Boland, M.R.; Czypionka, T.; Busse, R.; Mölken, M.R.-V. Relevant models and elements of integrated care for multi-morbidity: Results of a scoping review. Health Policy 2017, 122, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Macinko, J.; Starfield, B.; Shi, L. The Contribution of Primary Care Systems to Health Outcomes within Organization for Economic Cooperation and Development (OECD) Countries, 1970–1998. Health Serv. Res. 2003, 38, 831–865. [Google Scholar] [CrossRef]
- NHG-Standaard Diabetes Mellitus Type 2 (M01). 2021. Available online: https://richtlijnen.nhg.org/files/pdf/63_Diabetesmellitustype2_november-2021.pdf (accessed on 11 November 2021).
- Platform Vitale Vaten. Zorgstandaard Vasculair Risicomanagement Deel I (Voor Zorgverleners). 2009. pp. 1–27. Available online: http://www.vitalevaten.nl/uploads/media/Zorgstandaard_deel_I_voor_zorgverleners.pdf (accessed on 11 November 2021).
- Long Alliantie Nederland. Zorgstandaard COPD. 1991. pp. 1–30. Available online: https://www.longalliantie.nl/content/LAN_Zorgstandaard_COPD-2016-2.pdf (accessed on 11 November 2021).
- Out, K.E.M.; de Jong, J.D. Het Perspectief van Zorggroepen en Gezondheidscentra op Onderhandelingen en Contracten Met Zorgverzekeraars. Nivel. 2016. Available online: https://www.nivel.nl/sites/default/files/bestanden/Rapport_Contractering_van_zorggroepen_en_gezondheidscentra.pdf (accessed on 8 November 2021).
- van den Berg, M.; de Bakker, D. Meta-Analyse Introductie: Introductie Praktijkondersteuning op HBO-Niveau in de Huisartsenpraktijk in Nederland. Nivel. 2003. Available online: https://www.nivel.nl/sites/default/files/bestanden/LINH-praktijkondersteuning-hbo-huisartsenpraktijk.pdf (accessed on 8 November 2021).
- de Bakker, D.; Raams, J.; Schut, E.; Vrijhoef, B.; de Wildt, J. Eindrapport van de Evaluatiecommissie—Integrale Bekostiging Integrale bekostiging van zorg: Werk in uitvoering. Nivel. 2012. Available online: https://www.nivel.nl/sites/default/files/bestanden/Eindrapport-integrale-bekostiging-zorg.pdf (accessed on 10 December 2021).
- Tsiachristas, A.; Hipple-Walters, B.; Lemmens, K.M.; Nieboer, A.P.; Rutten-van Mölken, M.P. Towards integrated care for chronic conditions: Dutch policy developments to overcome the (financial) barriers. Health Policy 2011, 101, 122–132. [Google Scholar] [CrossRef]
- McWilliams, J.M.; Chernew, M.E.; Landon, B.E.; Schwartz, A.L. Performance Differences in Year 1 of Pioneer Accountable Care Organizations. N. Engl. J. Med. 2015, 372, 1927–1936. [Google Scholar] [CrossRef]
- Song, Z.; Ji, Y.; Safran, D.G.; Chernew, M.E. Health Care Spending, Utilization, and Quality 8 Years into Global Payment. N. Engl. J. Med. 2019, 381, 252–263. [Google Scholar] [CrossRef]
- Busse, R.; Stahl, J. Integrated Care Experiences And Outcomes In Germany, The Netherlands, and England. Health Aff. 2014, 33, 1549–1558. [Google Scholar] [CrossRef]
- Hildebrandt, H.; Hermann, C.; Knittel, R.; Richter-Reichhelm, M.; Siegel, A.; Witzenrath, W. Gesundes Kinzigtal Integrated Care: Improving population health by a shared health gain approach and a shared savings contract. Int. J. Integr. Care 2010, 10, e046. [Google Scholar] [CrossRef]
- Coulter, A. Evaluating general practice fundholding in the United Kingdom. Eur. J. Public Health 1995, 5, 233–239. [Google Scholar] [CrossRef]
- Gosden, T.; Torgerson, D.J. The effect of fundholding on prescribing and referral costs: A review of the evidence. Health Policy 1997, 40, 103–114. [Google Scholar] [CrossRef]
- Where next for Integrated Care Organisations in the English NHS? Available online: https://www.nuffieldtrust.org.uk/files/2017-01/where-next-integrated-care-english-nhs-web-final.pdf%0Ahttp://nuffield.dh.bytemark.co.uk/sites/files/nuffield/publication/where_next_for_integrated_care_organisations_in_the_english_nhs_230310.pdf%0Ahttp://ww (accessed on 15 March 2022).
- Transparante Ketenzorg. 2021. pp. 4–7. Available online: https://ineen.nl/wp-content/uploads/2021/06/Benchmark-Transparante-Ketenzorg-2020.pdf (accessed on 14 September 2022).
- Struijs, J.N.; de Jong-van Til, J.T.; Lemmens, L.C.; Drewes, H.W.; de Bruin, S.R.; Baan, C.A. Drie Jaar Integrale Bekostiging van Diabeteszorg. Effecten Op Zorgproces En Kwaliteit van Zorg. RIVM. 2017. Available online: http://www.narcis.nl/publication/RecordID/oai%3Arivm.openrepository.com%3A10029%2F257271 (accessed on 14 March 2022).
- Murtagh, S.; McCombe, G.; Broughan, J.; Carroll, Á.; Casey, M.; Harrold, Á.; Dennehy, T.; Fawsitt, R.; Cullen, W. Integrating Primary and Secondary Care to Enhance Chronic Disease Management: A Scoping Review. Int. J. Integr. Care 2021, 21, 4. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Soubhi, H.; Fortin, M.; Hudon, C.; O’Dowd, T. Managing patients with multimorbidity: Systematic review of interventions in primary care and community settings. BMJ 2012, 345, e5205. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Knottnerus, J.A.; Green, L.; van der Horst, H.; Jadad, A.R.; Kromhout, D.; Leonard, B.; Lorig, K.; Loureiro, M.I.; van der Meer, J.W.M.; et al. How should we define health? BMJ 2011, 343, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; van Vliet, M.; Giezenberg, M.; Winkens, B.; Heerkens, Y.; Dagnelie, P.C.; Knottnerus, J.A. Towards a ‘patient-centred’ operationalisation of the new dynamic concept of health: A mixed methods study. BMJ Open 2016, 6, e010091. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, B.; Payne, K.; Alderson, P.; McMurdo, M.E.T.; Mercer, S. Adapting clinical guidelines to take account of multimorbidity. BMJ 2012, 345, e6341. [Google Scholar] [CrossRef]
- Wallace, E.; Salisbury, C.; Guthrie, B.; Lewis, C.; Fahey, T.; Smith, S.M. Managing patients with multimorbidity in primary care. BMJ 2015, 350, 6–11. [Google Scholar] [CrossRef]
- Tsiachristas, A.; Dikkers, C.; Boland, M.R.; Mölken, M.P.R.-V. Exploring payment schemes used to promote integrated chronic care in Europe. Health Policy 2013, 113, 296–304. [Google Scholar] [CrossRef]
- Karimi, M.; Tsiachristas, A.; Looman, W.; Stokes, J.; van Galen, M.; Rutten-van Mölken, M. Bundled payments for chronic diseases increased health care expenditure in the Netherlands, especially for multimorbid patients. Health Policy 2021, 125, 751–759. [Google Scholar] [CrossRef]
- Looman, W.; Struckmann, V.; Köppen, J.; Baltaxe, E.; Czypionka, T.; Huic, M.; Pitter, J.; Ruths, S.; Stokes, J.; Bal, R.; et al. Drivers of successful implementation of integrated care for multi-morbidity: Mechanisms identified in 17 case studies from 8 European countries. Soc. Sci. Med. 2021, 277, 113728. [Google Scholar] [CrossRef]
- Denkraam Integratie Zorgprogramma’s Voor Chronische Aandoeningen. 2019. pp. 1–17. Available online: https://ineen.nl/wp-content/uploads/2020/06/200528-05-01-denkraam-integratie-zorgprogrammas.pdf (accessed on 18 January 2022).
- Bodenheimer, T.; Sinsky, C. From Triple to Quadruple Aim: Care of the Patient Requires Care of the Provider. Ann. Fam. Med. 2014, 12, 573–576. [Google Scholar] [CrossRef]
- Vercoulen, J.H. A simple method to enable patient-tailored treatment and to motivate the patient to change behaviour. Chronic Respir. Dis. 2012, 9, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, E.; Vercoulen, J.; Elbers, L.; Behr, R.; Schermer, T. De NCSI-Methode: Maatwerk Voor COPD-Zorg. Huisarts-en Wet. 2016, 59, 242–247. [Google Scholar] [CrossRef]
- Raaijmakers, L.H.A.; Schermer, T.R.; Wijnen, M.; Van Bommel, H.E.; Michielsen, L.; Boone, F.; Vercoulen, J.H.; Bischoff, E.W.M.A. Development of a person-centred integrated care approach for chronic disease management in Dutch primary care: A mixed-method study. Int. J. Environ. Res. Public Health, 2023; in press. [Google Scholar]
- Quinn, K. The 8 Basic Payment Methods in Health Care. Ann. Intern. Med. 2015, 163, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Alternative Payment Model: APM Framework Refreshed for 2017. Available online: https://hcp-lan.org/workproducts/apm-refresh-whitepaper-final.pdf (accessed on 16 September 2021).
- Cattel, D.; Eijkenaar, F. Value-Based Provider Payment Initiatives Combining Global Payments With Explicit Quality Incentives: A Systematic Review. Med Care Res. Rev. 2019, 77, 511–537. [Google Scholar] [CrossRef] [PubMed]
- Hayen, A.P.; van den Berg, M.J.; Meijboom, B.R.; Struijs, J.N.; Westert, G.P. Incorporating shared savings programs into primary care: From theory to practice. BMC Health Serv. Res. 2015, 15, 580. [Google Scholar] [CrossRef] [PubMed]
- Steenhuis, S.; Struijs, J.; Koolman, X.; Ket, J.; van der Hijden, E. Unraveling the Complexity in the Design and Implementation of Bundled Payments: A Scoping Review of Key Elements From a Payer’s Perspective. Milbank Q. 2020, 98, 197–222. [Google Scholar] [CrossRef]
- Eijkenaar, F.; Emmert, M.; Scheppach, M.; Schöffski, O. Effects of pay for performance in health care: A systematic review of systematic reviews. Health Policy 2013, 110, 115–130. [Google Scholar] [CrossRef]
- Mendelson, A.; Kondo, K.; Damberg, C.; Low, A.; Motúapuaka, M.; Freeman, M.; O’Neil, M.; Relevo, R.; Kansagara, D. The Effects of Pay-for-Performance Programs on Health, Health Care Use, and Processes of Care: A systematic review. Ann. Intern. Med. 2017, 166, 341–353. [Google Scholar] [CrossRef]
- Hayen, A. Shared Savings and Patient Cost Sharing in the Dutch Health Care System. Ph.D. Thesis, Tilburg University, Tilburg, The Netherlands, 30 November 2018. [Google Scholar]
- McWilliams, J.M.; Hatfield, L.A.; Landon, B.E.; Hamed, P.; Chernew, M.E. Medicare Spending after 3 Years of the Medicare Shared Savings Program. N. Engl. J. Med. 2018, 379, 1139–1149. [Google Scholar] [CrossRef]
- Agarwal, R.; Liao, J.M.; Gupta, A.; Navathe, A.S. The Impact Of Bundled Payment On Health Care Spending, Utilization, And Quality: A Systematic Review. Health Aff. 2020, 39, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Struijs, J.N.; de Vries, E.F.; van Dorst, H.D.C.A.; Over, E.A.B.; Baan, C.A. Geboortezorg in Beeld—Een Nulmeting En Eerste Ervaringen Met Het Werken Met Integrale Bekostiging. RIVM. 2018. Available online: https://www.rivm.nl/bibliotheek/rapporten/2018-0109.pdf (accessed on 8 February 2023).
- Stokes, J.; Struckmann, V.; Kristensen, S.R.; Fuchs, S.; van Ginneken, E.; Tsiachristas, A.; van Mölken, M.R.; Sutton, M. Towards incentivising integration: A typology of payments for integrated care. Health Policy 2018, 122, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Kralj, B.; Kantarevic, J. Quality and quantity in primary care mixed-payment models: Evidence from family health organizations in Ontario. Can. J. Econ./Rev. Can. D’économique 2013, 46, 208–238. [Google Scholar] [CrossRef]
- Krasnik, A.; Groenewegen, P.P.; Pedersen, P.A.; von Scholten, P.; Mooney, G.; Gottschau, A.; Flierman, H.A.; Damsgaard, M.T. Changing remuneration systems: Effects on activity in general practice. BMJ 1990, 300, 1698–1701. [Google Scholar] [CrossRef] [PubMed]
- Iversen, T.; Luras, H. The effect of capitation on GPs’ referral decisions. Health Econ. 2000, 9, 199–210. [Google Scholar] [CrossRef]
- van der Hijden, E.; Steenhuis, S.; Hofstra, G.; van der Wolk, J.; Bijlsma, W.; Struijs, J.; Koolman, X. Ontwikkelingen in zorginkoop: Van inkoop van verrichtingen naar inkoop van zorgbundels. Maandbl. Voor Account. en Bedrijfsecon. 2019, 93, 223–239. [Google Scholar] [CrossRef]
- Tsiachristas, A. Payment and economic evaluation of integrated care. Int. J. Integr. Care 2015, 15, e013. [Google Scholar] [CrossRef]
- Gosden, T.; Forland, F.; Kristiansen, I.; Sutton, M.; Leese, B.; Giuffrida, A.; Sergison, M.; Pedersen, L. Capitation, salary, fee-for-service and mixed systems of payment: Effects on the behaviour of primary care physicians. Cochrane Database Syst. Rev. 2000, 2000, CD002215. [Google Scholar] [CrossRef]
- Gosden, T.; Forland, F.; Kristiansen, I.S.; Sutton, M.; Leese, B.; Giuffrida, A.; Sergison, M.; Pedersen, L. Impact of payment method on behaviour of primary care physicians: A systematic review. J. Health Serv. Res. Policy 2001, 6, 44–55. [Google Scholar] [CrossRef]
- Simoens, S.; Giuffrida, A. The Impact of Physician Payment Methods on Raising the Efficiency of the Healthcare System: An international comparison. Appl. Health Econ. Health Policy 2004, 3, 39–46. [Google Scholar] [CrossRef]
- Barros, P.P. Cream-skimming, incentives for efficiency and payment system. J. Health Econ. 2003, 22, 419–443. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.C. Theory and Practice in the Design of Physician Payment Incentives. Milbank Q. 2001, 79, 149–177. [Google Scholar] [CrossRef] [PubMed]
- Andrawis, J.P.; Koenig, K.M.; Bozic, K.J. Bundled payment care initiative: How this all started. Semin. Arthroplast. JSES 2016, 27, 188–192. [Google Scholar] [CrossRef]
- Cutler, D.M.; Ghosh, K. The Potential for Cost Savings through Bundled Episode Payments. N. Engl. J. Med. 2012, 366, 1075–1077. [Google Scholar] [CrossRef]
- Weeks, W.B.; Rauh, S.S.; Wadsworth, E.B.; Weinstein, J.N. The Unintended Consequences of Bundled Payments. Ann. Intern. Med. 2013, 158, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Struijs, J.N.; Mohnen, S.M.; Molema, C.C.M.; de Jong-van Til, J.T.; Baan, C.A. Effect van Integrale Bekostiging Op Curatieve. RIVM. 2010. Available online: https://www.rivm.nl/bibliotheek/rapporten/260131005.pdf (accessed on 16 September 2021).
- De Invloed van Financiele Prikkels op de Behandeltijd in de GGZ. Me Judice. 2015. Available online: https://www.mejudice.nl/artikelen/detail/de-invloed-van-financiele-prikkels-op-de-behandeltijd-in-de-ggz (accessed on 16 September 2021).
- Advies Zorgprestatiemodel Ggz en, fz. Advies Zorgprestatiemodel Ggz en fz. 2019. pp. 1–60. Available online: https://www.vgn.nl/system/files/article/file/Advies%2Bzorgprestatiemodel%2Bggz%2Ben%2Bfz%2B-%2BDEFINITIEF.pdf (accessed on 16 September 2021).
- Belonen van Zorg Die Waarde Toevoegt Inhoud. 2018. Available online: https://puc.overheid.nl/nza/doc/PUC_252732_22/1/ (accessed on 16 September 2021).
- Invoering Abonnementstarief in de Wmo per 2020 Uitvoeringstoets naar de Gemeenten. 2018. Available online: https://www.eerstekamer.nl/overig/20181219/invoering_abonnementstarief_in_de/document (accessed on 16 September 2021).
- Doorontwikkeling Bekostiging Wlz. 2017. Available online: https://puc.overheid.nl/nza/doc/PUC_3566_22/1/ (accessed on 16 September 2021).
- Eijkenaar, F.; Schut, E. Uitkomstbekostiging in de Zorg: Een (on) Begaanbare Weg? 2015. pp. 1–126. Available online: https://www.eur.nl/sites/corporate/files/Onderzoeksrapport_uitkomstbekostiging_in_de_zorg_def_24032015__FE3__0.pdf (accessed on 6 December 2021).
- Holmstrom, B.; Milgrom, P. Multitask Principal-Agent Analyses: Incentive Contracts, Asset Ownership, and Job Design. J. Law, Econ. Organ. 1991, 7, 24–52. [Google Scholar] [CrossRef]
- Smith, P.C.; York, N. Quality incentives: The case of U.K. general practitioners—An ambitious U.K. quality improvement initiative offers the potential for enormous gains in the quality of primary health care. Health Aff. 2004, 23, 112–118. [Google Scholar] [CrossRef]
- Kirschner, K.; Braspenning, J.; Akkermans, R.P.; Jacobs, J.E.A.; Grol, R. Assessment of a pay-for-performance program in primary care designed by target users. Fam. Pract 2013, 30, 161–171. [Google Scholar] [CrossRef]
- Campbell, S.M.; Reeves, D.; Kontopantelis, E.; Sibbald, B.; Roland, M. Effects of Pay for Performance on the Quality of Primary Care in England. N. Engl. J. Med. 2009, 361, 368–378. [Google Scholar] [CrossRef]
- Longzorg Gestuurd op Uitkomsten. 2018. pp. 24–25. Available online: https://www.gc-nijkerk.nl/wp-content/uploads/2018/02/longzorg-eerstelijns.pdf (accessed on 12 April 2022).
- LongZorg Nijkerk Doelstelling. 2018. Available online: https://www.rug.nl/cpheb/docs/jurriaanpropper.pdf (accessed on 12 April 2022).
- McWilliams, J.M.; Hatfield, L.A.; Chernew, M.E.; Landon, B.E.; Schwartz, A.L. Early Performance of Accountable Care Organizations in Medicare. N. Engl. J. Med. 2016, 374, 2357–2366. [Google Scholar] [CrossRef]
- Song, Z.; Rose, S.; Safran, D.G.; Landon, B.E.; Day, M.P.; Chernew, M.E. Changes in Health Care Spending and Quality 4 Years into Global Payment. N. Engl. J. Med. 2014, 371, 1704–1714. [Google Scholar] [CrossRef]
- Ouayogodé, M.H.; Colla, C.H.; Lewis, V.A. Determinants of success in Shared Savings Programs: An analysis of ACO and market characteristics. Healthcare 2016, 5, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Nyweide, D.J.; Lee, W.; Cuerdon, T.T.; Pham, H.H.; Cox, M.; Rajkumar, R.; Conway, P.H. Association of Pioneer Accountable Care Organizations vs Traditional Medicare Fee for Service With Spending, Utilization, and Patient Experience. JAMA—J. Am. Med. Assoc. 2015, 313, 2152–2161. [Google Scholar] [CrossRef] [PubMed]
- Cattel, D.; Eijkenaar, F.; Schut, F.T. Value-based provider payment: Towards a theoretically preferred design. Health Econ. Policy Law 2018, 15, 94–112. [Google Scholar] [CrossRef]
- Berwick, D.M. Launching Accountable Care Organizations—The Proposed Rule for the Medicare Shared Savings Program. N. Engl. J. Med. 2011, 364, e32. [Google Scholar] [CrossRef]
- Overheveling van Zorg? Of Overheveling van Problemen? 2020. pp. 34–36. Available online: https://www.medischcontact.nl/nieuws/laatste-nieuws/artikel/overheveling-van-zorg-of-overheveling-van-problemen.htm (accessed on 15 June 2022).
- de Vries, E.F.; Drewes, H.W.; Struijs, J.N.; Heijink, R.; Baan, C.A. Barriers to payment reform: Experiences from nine Dutch population health management sites. Health Policy 2019, 123, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).