A Telehealth Home-Based Exercise Program for Community-Dwelling Older People with Dementia in Indonesia: A Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Study Participants
2.3. Sample Size
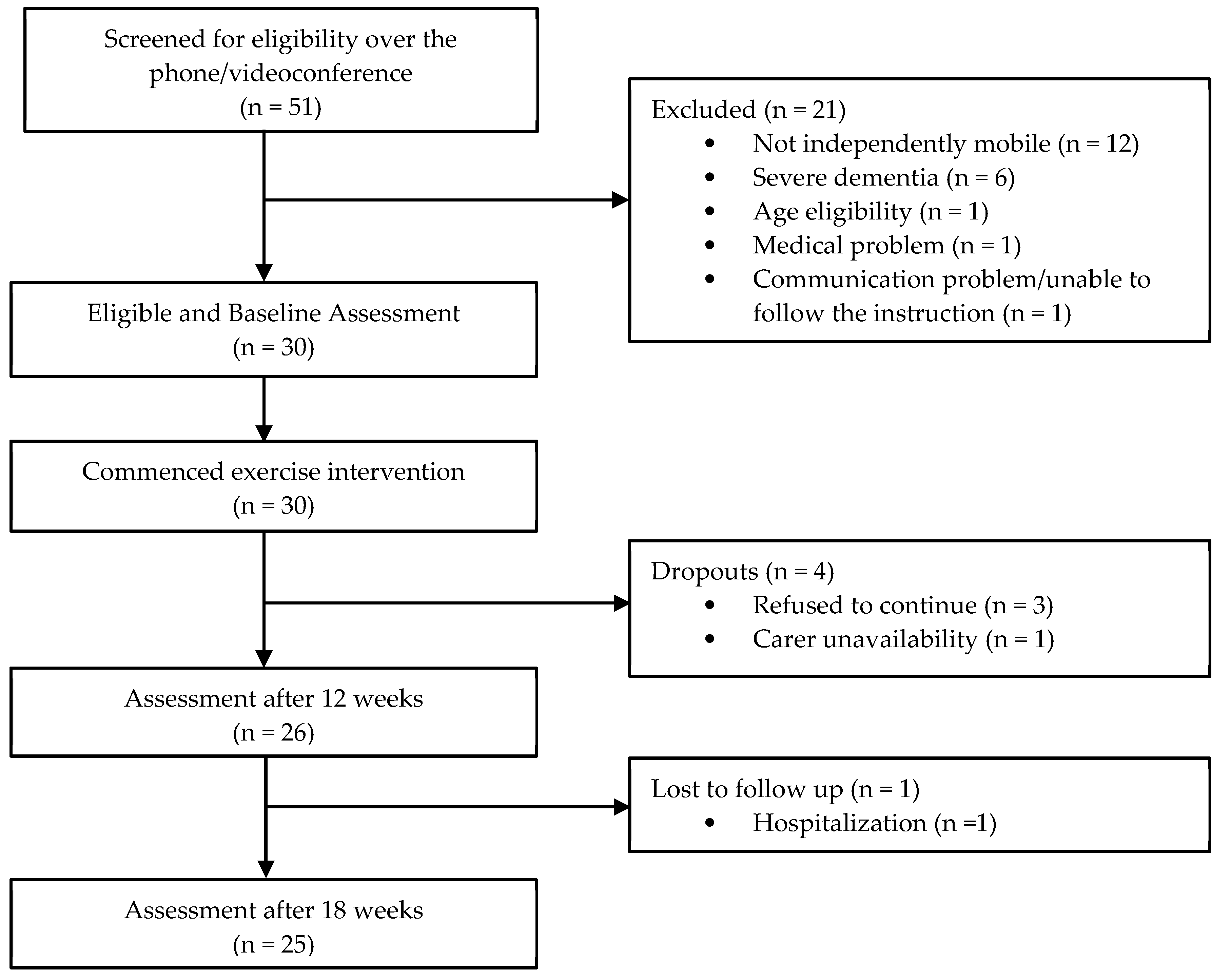
2.4. Recruitment Process
2.5. Data Collection
2.6. Intervention
2.6.1. Orientation Session
2.6.2. Exercise Sessions
2.6.3. Check-Up/Support Session
2.7. Outcomes Measures
2.7.1. Demand
2.7.2. Implementation
2.7.3. Practicality (Including Safety)
2.7.4. Acceptability
2.7.5. Adaptation
2.7.6. Efficacy Testing
- Physical activity level—assessed using the Physical Activity Scale for the Elderly (PASE) [37]. The PASE is a 12-item assessment tool that combines several types of physical activity including household, leisure, and occupational activity over a seven-day period. The range of scores is 0–400, and higher scores indicate higher levels of physical activity [37].
- Function and disability—assessed using the Late-Life Function and Disability Instrument (LLFDI) [38]. The LLFDI is an evaluative outcome measure designed to assess function (ability to perform activities in daily routines) and disability (performance in socially defined tasks) for community-dwelling older people. The function component comprises 32 items and an additional eight device items (for those who use canes and walkers) within three domains (Upper Extremity Functioning, Basic Lower Extremity Functioning and Advanced Lower Extremity Functioning). Both raw scores of function and disability components were transformed to a scaled score (0–100), with higher scores indicating higher level of function. The disability component comprises 16 items over two dimensions (frequency and limitation); The frequency dimension comprises a Social Role domain and Personal Role domain and the limitation dimension comprises the Instrumental Role domain and Management Role domain. Higher scores in frequency indicate high levels in frequency of participating in life tasks and higher score in limitation signify higher levels in capability of participating in life tasks [38].
- Health-related benefits of exercise—assessed using the Vitality Plus Scale [39], a 10-item self-reported Likert scale in which participants rated health domains including sleep, bodily pain, energy, bowel function and appetite with a maximum score of 50 (each item is scored on a 1–5 scale). Higher scores indicate better perceived health [39].
- Fear of falls—assessed using the Iconographical Falls Efficacy Scale (Icon-FES). The Icon-FES is an innovative and valid fear of falling measurement for older people with cognitive impairment [40]. This scale uses pictures to describe the range of situations and activities. Scores range from 10–40, and higher scores indicate greater fear of falls [41].
- Exercise enjoyment—assessed using the 8-item Physical Activity Enjoyment Scale (PACES) [42]. The PACES scale evaluated how the participant felt about physical activity that they had been doing, using a 7-point Likert scale. Scores range from 8–56. Higher scores indicate increased levels of enjoyment from participating in the physical activity.
- Quality of Life—assessed using the Quality of Life in Alzheimer’s Disease (QOL-AD) tool [43]. This scale is designed to assess the quality of life of people with dementia from both people with dementia and their carer’s perspective. It includes 13 items measured (range from 13–52) using a Likert scale, and higher scores indicate better quality of life [43].
- Behavior (neuropsychiatric symptoms)—assessed using the Neuropsychiatric Inventory Questionnaire (NPI-Q). The NPI-Q is a valid and reliable questionnaire to assess neuropsychiatric symptomatology in dementia that includes 12 domains of neuropsychiatry, with scores ranging from 0–36 [44]. This screening questionnaire also provides the Caregiver Distress Scale for identifying the impact of the behaviors on the carer with scores ranging from 0–60. Higher scores indicate higher level of severity of neuropsychiatric symptoms and the distress experienced by the carer [44].
- Impact on informal carer—assessed using the Zarit caregiver burden scale. The Zarit caregiver burden scale is widely used and comprises a 22-item questionnaire with a maximum score of 88; higher scores indicate higher burden [45].
2.8. Data Analysis
3. Results
3.1. Participants and Demand
3.2. Implementation
3.3. Practicality (Including Safety)
3.4. Acceptability
3.5. Adaptation
3.6. Efficacy Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Disease International. World Alzheimer Report 2015. The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends London 2015. Available online: https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf (accessed on 10 September 2022).
- World Health Organization. Global Status Report on the Public Health Response to Dementia: World Health Organization. 2021. Available online: https://www.who.int/publications/i/item/9789240033245 (accessed on 11 April 2022).
- Alzheimer’s Disease International (ADI). World Alzheimer Report 2018. The State of the Art of Dementia Research: New Frontiers. London. 2018. Available online: https://www.alz.co.uk/research/WorldAlzheimerReport2018.pdf (accessed on 10 September 2022).
- World Bank. Live Long and Prosper: Aging in East Asia and Pacific; World Bank Group: Washington, DC, USA, 2016. [Google Scholar] [CrossRef]
- Alzheimer’s Disease International. Dementia in the Asia Pasific Region London. 2014. Available online: https://www.alz.co.uk/adi/pdf/Dementia-Asia-Pacific-2014.pdf (accessed on 23 May 2022).
- Forbes, D.; Forbes, S.C.; Blake, C.M.; Thiessen, E.J.; Forbes, S. Exercise programs for people with dementia. Cochrane Database Syst. Rev. 2015, 2015, CD006489. [Google Scholar] [CrossRef]
- Law, W.; Kwok, T.C. Impacts of a multicomponent intervention programme on neuropsychiatric symptoms in people with dementia and psychological health of caregivers: A feasibility pilot study. Int. J. Geriatr. Psychiatry 2019, 34, 1765–1775. [Google Scholar] [CrossRef]
- Lam, F.M.; Huang, M.-Z.; Liao, L.-R.; Chung, R.C.; Kwok, T.C.; Pang, M.Y. Physical exercise improves strength, balance, mobility, and endurance in people with cognitive impairment and dementia: A systematic review. J. Physiother. 2018, 64, 4–15. [Google Scholar] [CrossRef]
- Cardona, M.I.; Afi, A.; Lakicevic, N.; Thyrian, J.R. Physical activity interventions and their effects on cognitive function in people with dementia: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2021, 18, 8753. [Google Scholar] [CrossRef]
- Orgeta, V.; Miranda-Castillo, C. Does physical activity reduce burden in carers of people with dementia? A literature review. Int. J. Geriatr. Psychiatry 2014, 29, 771–783. [Google Scholar] [CrossRef]
- Suttanon, P.; Hill, K.; Said, C.; Williams, S.B.; Byrne, K.N.; LoGiudice, D.; Lautenschlager, N.T.; Dodd, K.J. Feasibility, safety and preliminary evidence of the effectiveness of a home-based exercise programme for older people with Alzheimer’s disease: A pilot randomized controlled trial. Clin. Rehabil. 2013, 27, 427–438. [Google Scholar] [CrossRef]
- Park, J.; Cohen, I. Effects of Exercise Interventions in Older Adults with Various Types of Dementia: Systematic Review. Act. Adapt. Aging 2018, 43, 83–117. [Google Scholar] [CrossRef]
- Sari, Y.M.; Hill, K.D.; Lee, D.C.A.; Burton, E. Effectiveness of exercise programmes in improving physical function and reducing behavioural symptoms of community living older adults with dementia living in Asia, and impact on their informal carers: A systematic review and meta-analysis. Hong Kong Physiother. J. 2023, 43, 1–15. [Google Scholar] [CrossRef]
- Lu, Z.; Harris, T.B.; Shiroma, E.J.; Leung, J.; Kwok, T. Patterns of Physical Activity and Sedentary Behavior for Older Adults with Alzheimer’s Disease, Mild Cognitive Impairment, and Cognitively Normal in Hong Kong. J. Alzheimers Dis. 2018, 66, 1453–1462. [Google Scholar] [CrossRef]
- Watts, A.S.; Vidoni, E.D.; Loskutova, N.; Johnson, D.K.; Burns, J. Measuring Physical Activity in Older Adults With and Without Early Stage Alzheimer’s Disease. Clin. Gerontol. 2013, 36, 356–374. [Google Scholar] [CrossRef]
- Watts, A.; Walters, R.W.; Hoffman, L.; Templin, J. Intra-Individual Variability of Physical Activity in Older Adults With and Without Mild Alzheimer’s Disease. PloS ONE 2016, 11, e0153898. [Google Scholar] [CrossRef]
- Freeman, S.; Pelletier, C.; Ward, K.; Bechard, L.; Regan, K.; Somani, S.; Middleton, L.E. Factors influencing participation in physical activity for persons living with dementia in rural and northern communities in Canada: A qualitative study. BMJ Open 2022, 12, e060860. [Google Scholar] [CrossRef]
- Prevention USCfDCa. Coronavirus Disease 2019 (COVID-19)—Older Adults 2019. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/older-adults.html (accessed on 16 February 2022).
- Goethals, L.; Barth, N.; Guyot, J.; Hupin, D.; Celarier, T.; Bongue, B. Impact of home quarantine on physical activity among older adults living at home during the COVID-19 pandemic: Qualitative interview study. JMIR Aging 2020, 3, e19007. [Google Scholar] [CrossRef]
- Vernuccio, L.; Sarà, D.; Inzerillo, F.; Catanese, G.; Catania, A.; Vesco, M.; Cacioppo, F.; Dominguez, L.J.; Veronese, N.; Barbagallo, M. Effect of COVID-19 quarantine on cognitive, functional and neuropsychiatric symptoms in patients with mild cognitive impairment and dementia. Aging Clin. Exp. Res. 2022, 34, 1187–1194. [Google Scholar] [CrossRef]
- Lakicevic, N.; Moro, T.; Paoli, A.; Roklicer, R.; Trivic, T.; Cassar, S.; Drid, P. Stay fit, don’t quit: Geriatric Exercise Prescription in COVID-19 Pandemic. Aging Clin. Exp. Res. 2020, 32, 1209–1210. [Google Scholar] [CrossRef]
- Ptomey, L.T.; Vidoni, E.D.; Montenegro-Montenegro, E.; Thompson, M.A.; Sherman, J.R.; Gorczyca, A.M.; Greene, J.L.; Washburn, R.A.; Donnelly, J.E. The Feasibility of Remotely Delivered Exercise Session in Adults With Alzheimer’s Disease and Their Caregivers. J. Aging Phys. Act. 2019, 27, 670–677. [Google Scholar] [CrossRef]
- Suttanon, P.; Hill, K.; Said, C.; Dodd, K. Can balance exercise programmes improve balance and related physical performance measures in people with dementia? A systematic review. Eur. Rev. Aging Phys. Act. 2010, 7, 13–25. [Google Scholar] [CrossRef]
- Juniarti, N.; Mz, I.A.; Sari, C.W.M.; Haroen, H. The Effect of Exercise and Learning Therapy on Cognitive Functions and Physical Activity of Older People with Dementia in Indonesia. J. Aging Res. 2021, 2021, 6647029. [Google Scholar] [CrossRef]
- Chongsuvivatwong, V.; Phua, K.H.; Yap, M.T.; Pocock, N.S.; Hashim, J.H.; Chhem, R.; Wilopo, S.A.; Lopez, A.D. Health and health-care systems in southeast Asia: Diversity and transitions. Lancet 2011, 377, 429–437. [Google Scholar] [CrossRef]
- Hill, K.D.; Suttanon, P.; Lin, S.-I.; Tsang, W.W.; Ashari, A.; Hamid, T.A.A.; Farrier, K.; Burton, E. What works in falls prevention in Asia: A systematic review and meta-analysis of randomized controlled trials. BMC Geriatr. 2018, 18, 3. [Google Scholar] [CrossRef]
- Ptomey, L.T.; Willis, E.A.; Greene, J.L.; Danon, J.C.; Chumley, T.K.; Washburn, R.A.; Donnelly, J.E. The Feasibility of Group Video Conferencing for Promotion of Physical Activity in Adolescents with Intellectual and Developmental Disabilities. Am. J. Intellect. Dev. Disabil. 2017, 122, 525–564. [Google Scholar] [CrossRef]
- Karuncharernpanit, S.; Hendricks, J.; Toye, C. Perceptions of exercise for older people living with dementia in Bangkok, Thailand: An exploratory qualitative study. Int. J. Older People Nurs. 2016, 11, 166–175. [Google Scholar] [CrossRef]
- Newkirk, L.A.; Kim, J.M.; Thompson, J.M.; Tinklenberg, J.R.; Yesavage, J.A.; Taylor, J.L. Validation of a 26-Point Telephone Version of the Mini-Mental State Examination. J. Geriatr. Psychiatry Neurol. 2004, 17, 81–87. [Google Scholar] [CrossRef]
- Billingham, S.A.; Whitehead, A.L.; A Julious, S. An audit of sample sizes for pilot and feasibility trials being undertaken in the United Kingdom registered in the United Kingdom Clinical Research Network database. BMC Med. Res. Methodol. 2013, 13, 104. [Google Scholar] [CrossRef]
- Ellajosyula, R.; Hegde, S. Capacity issues and decision-making in dementia. Ann. Indian Acad. Neurol. 2016, 19, 34–39. [Google Scholar] [CrossRef]
- Gardner, M.M.; Buchner, D.M.; Robertson, M.C.; Campbell, A.J. Practical implementation of an exercise-based falls prevention programme. Age Ageing 2001, 30, 77–83. [Google Scholar] [CrossRef]
- Robertson, M.C.; Campbell, A.J.; Gardner, M.M.; Devlin, N. Preventing Injuries in Older People by Preventing Falls: A Meta-Analysis of Individual-Level Data. J. Am. Geriatr. Soc. 2002, 50, 905–911. [Google Scholar] [CrossRef]
- Bowen, D.J.; Kreuter, M.; Spring, B.; Cofta-Woerpel, L.; Linnan, L.; Weiner, D.; Bakken, S.; Kaplan, C.P.; Squiers, L.; Fabrizio, C.; et al. How We Design Feasibility Studies. Am. J. Prev. Med. 2009, 36, 452–457. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Report on Falls Prevention in Older Age; World Health Organization: Geneva, Switzerland, 2008.
- Sari, Y.M.; Hill, K.D.; Burton, E.; Lee, D.A.; Lalor, A. Experiences of Indonesian people with dementia and carers undertaking a telehealth-delivered exercise program. Gerontologist 2023. under review. [Google Scholar]
- Washburn, R.A.; Smith, K.W.; Jette, A.M.; Janney, C.A. The physical activity scale for the elderly (PASE): Development and evaluation. J. Clin. Epidemiol. 1993, 46, 153–162. [Google Scholar] [CrossRef]
- Sayers, S.P.; Jette, A.M.; Haley, S.M.; Heeren, T.C.; Guralnik, J.M.; Fielding, R.A. Validation of the Late-Life Function and Disability Instrument. J. Am. Geriatr. Soc. 2004, 52, 1554–1559. [Google Scholar] [CrossRef]
- Myers, A.M.; Malott, O.W.; Gray, E.; Tudor-Locke, C.; Ecclestone, N.A.; Cousins, S.O.; Petrella, R. Measuring Accumulated Health-Related Benefits of Exercise Participation for Older Adults: The Vitality Plus Scale. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 1999, 54, M456–M466. [Google Scholar] [CrossRef]
- Delbaere, K.; Close, J.C.T.; Taylor, M.; Wesson, J.; Lord, S.R. Validation of the Iconographical Falls Efficacy Scale in Cognitively Impaired Older People. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2013, 68, 1098–1102. [Google Scholar] [CrossRef]
- Delbaere, K.; Smith, S.; Lord, S.R. Development and Initial Validation of the Iconographical Falls Efficacy Scale. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2011, 66, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Mullen, S.P.; A Olson, E.; Phillips, S.M.; Szabo, A.N.; Wójcicki, T.R.; Mailey, E.L.; Gothe, N.P.; Fanning, J.T.; Kramer, A.F.; McAuley, E. Measuring Enjoyment of Physical Activity in Older Adults: Invariance of the Physical Activity Enjoyment Scale (PACES) across Groups and Time. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 103. [Google Scholar] [CrossRef]
- Logsdon, R.G.; Gibbons, L.E.; McCurry, S.M.; Teri, L. Assessing Quality of Life in Older Adults With Cognitive Impairment. Psychosom. Med. 2002, 64, 510–519. [Google Scholar] [CrossRef]
- Kaufer, D.I.; Cummings, J.L.; Ketchel, P.; Smith, V.; MacMillan, A.; Shelley, T.; Lopez, O.L.; DeKosky, S.T. Validation of the NPI-Q, a Brief Clinical Form of the Neuropsychiatric Inventory. Neuropsychiatry Clin. Neurosci. 2000, 12, 233–239. [Google Scholar] [CrossRef]
- Zarit, S.H.; Reever, M.K.E.; Bach-Peterson, M.J. Relatives of the impaired elderly: Correlates of feelings of burden. Gerontol. 1980, 20, 649–655. [Google Scholar] [CrossRef]
- Washburn, R.A.; McAuley, E.; Katula, J.; Mihalko, S.L.; Boileau, R.A. The Physical Activity Scale for the Elderly (PASE): Evidence for Validity. J. Clin. Epidemiol. 1999, 52, 643–651. [Google Scholar] [CrossRef]
- Lumempouw, S.F.; Misbach, J.; Diatri, N. Efficacy and safety of galantamine in the treatment of Alzheimer’s disease and Alzheimer’s disease with cerebrovascular (mixed dementia). Med. J. Indones. 2007, 16, 94–100. [Google Scholar] [CrossRef]
- Hébert, R.; Bravo, G.; Préville, M. Reliability, validity and reference values of the Zarit Burden Interview for assessing informal caregivers of community-dwelling older persons with dementia. Can. J. Aging 2000, 19, 494–507. [Google Scholar] [CrossRef]
- Utami, Y.H.; Dharmono, S.; Amir, N. The Relationship between Dependency and Caregiver Burden in Geriatric Patient; University of Indonesia: Jakarta, Indonesia, 2013. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Hillsdale, N.J., Ed.; Lawrence Erlbaum Associates Inc.: Mahwah, NJ, USA, 1988. [Google Scholar]
- Aisyah, D.N.; Mayadewi, C.A.; Budiharsana, M.; Solikha, D.A.; Ali, P.B.; Igusti, G.; Kozlakidis, Z.; Manikam, L. Building on health security capacities in Indonesia: Lessons learned from the COVID-19 pandemic responses and challenges. Zoonoses Public Health 2022, 69, 757–767. [Google Scholar] [CrossRef]
- Smith, M.L.; Steinman, L.E.; Casey, E.A. Combatting Social Isolation Among Older Adults in a Time of Physical Distancing: The COVID-19 Social Connectivity Paradox. Front. Public Health 2020, 8, 403. [Google Scholar] [CrossRef]
- Gough, C.; Barr, C.; Lewis, L.K.; Hutchinson, C.; Maeder, A.; George, S. Older adults’ community participation, physical activity, and social interactions during and following COVID-19 restrictions in Australia: A mixed methods approach. BMC Public Health 2023, 23, 172. [Google Scholar] [CrossRef]
- Hoffman, G.J.; Malani, P.N.; Solway, E.; Kirch, M.; Singer, D.C.; Kullgren, J.T. Changes in activity levels, physical functioning, and fall risk during the COVID-19 pandemic. J. Am. Geriatr. Soc. 2022, 70, 49–59. [Google Scholar] [CrossRef]
- Schröder-Butterfill, E.; Fithry, T.S. Care dependence in old age: Preferences, practices and implications in two Indonesian communities. Ageing Soc. 2014, 34, 361–387. [Google Scholar] [CrossRef]
- Sari, Y.M.; Burton, E.; Lee, D.A.; Hill, K.D. Current physiotherapy practice on delivering treatments for older people with dementia in Indonesia: A cross-sectional study. Physiother. Res. Int. 2022, 27, e1931. [Google Scholar] [CrossRef]
- Bello-Haas, D.V.; O’Connell, M.E.; Morgan, D.G.; Crossley, M. Lessons Learned: Feasibility and Acceptability of a Telehealth-Delivered Exercise Intervention for Rural-dwelling Individuals with Dementia and Their Caregivers. Rural. Remote Health 2014, 14, 120–130. [Google Scholar] [CrossRef]
- Angelopoulou, E.; Papachristou, N.; Bougea, A.; Stanitsa, E.; Kontaxopoulou, D.; Fragkiadaki, S.; Pavlou, D.; Koros, C.; Değirmenci, Y.; Papatriantafyllou, J.; et al. How Telemedicine Can Improve the Quality of Care for Patients with Alzheimer’s Disease and Related Dementias? A Narrative Review. Medicina 2022, 58, 1705. [Google Scholar] [CrossRef]
- Elbaz, S.; Cinalioglu, K.; Sekhon, K.; Gruber, J.; Rigas, C.; Bodenstein, K.; Naghi, K.; Lavin, P.; Greenway, K.T.; Vahia, I.; et al. A Systematic Review of Telemedicine for Older Adults With Dementia During COVID-19: An Alternative to In-person Health Services? Front. Neurol. 2021, 12, 761965. [Google Scholar] [CrossRef]
- Taylor, N.F.; Dodd, K.J.; McBurney, H.; Graham, H. Factors influencing adherence to a home-based strength-training programme for young people with cerebral palsy. Physiotherapy 2004, 90, 57–63. [Google Scholar] [CrossRef]
| Consent | Pre Assessment and Orientation | Exercise Intervention Duration (Week) | Post Assessment | Follow-up Assessment | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 18 | ||
| Physiotherapist online-visit sessions | ||||||||||||||||
| Check-up/support sessions | ||||||||||||||||
| Characteristic | Participants with Dementia (n = 30) | Family Carers (n = 30) | Paid Carer (n = 10) |
|---|---|---|---|
| Age (Mean ± SD) | 71.5 ± 8.8 | 42.6 ± 11.3 | 33.2 ± 11.7 |
| Gender (N [%] female) | 19 (63.3%) | 24 (80%) | 9 (90%) |
Family carer relationship with person with dementia—n (%)
| NA | 3 (10%) 25 (83.3%) 2 (6.7%) | NA |
| Having paid carer—n (%) | 10 (33.3%) | NA | NA |
| Living with family carer—n (%) | 25 (83.3%) | NA | NA |
| Days spent (per-week) with older person with dementia (Mean ± SD) | NA | 6.9 (0.4) | 7 (0) |
Dementia Type—n (%)
| 22 (73.3%) 6 (20%) 1 (3.3%) 1 (3.3%) | NA | NA |
| Dementia duration (Mean ± SD) (year) | 2.1 ± 1.2 | NA | NA |
| T-MMSE score (Mean ± SD) | 15.0 ± 3.3 | NA | NA |
| Using walking aid (indoor)—n (%) | 3 (10%) | NA | NA |
Walking aid type (indoor)
| 2 (6.7%) 1 (3.3%) | NA | NA |
| Using walking aid (outdoor)—n (%) | 4 (13.3%) | NA | NA |
Walking aid type (outdoor)
| 2 (50%) 2 (50%) | NA | NA |
| Having fall in the past 12 months—n (%) | 12 (40%) | NA | NA |
Number of falls in the past 12 months—n (%)
| 18 (60%) 8 (26.7%) 1 (3.3%) 2 (6.7%) 0 (0%) 1 (3.3%) 0 (0%) | NA | NA |
Other health condition—n (%) #
| 6 (20%) 1 (3.3%) 4 (13.3%) 6 (20%) 3 (10%) 6 (20%) 3 (10%) 4 (13.3%) 1 (3.3%) 4 (13.3%) | NA | NA |
Participants’ living location—n (%)
| 10 (33.3%) 11 (36.7%) 7 (23.3%) 2 (6.7%) | NA | NA |
| Baseline to 12 Weeks (60 Sessions; n = 30 Participants) | 6-Week Self-Maintenance (i.e., Weeks 13–18) (30 Sessions; n = 26 Participants) | ||||
|---|---|---|---|---|---|
| Median Number of Exercises Undertaken Per Session (IQR [25, 75]) | Median Number of Exercise Sessions Undertaken (IQR [25, 75]) | Median Adherence % (IQR [25, 75]) | Median Number of Exercises Undertaken Per Session (IQR [25, 75]) | Median Number of Exercises Undertaken (IQR [25, 75]) | Median Adherence % (IQR [25, 75]) |
| 7 (3) a | 50.5 (10.5) | 84.1 (17.1) | 6 (2) a | 20 (5) | 66.7 (16.7) |
| Outcome Measures | Baseline | 12 Weeks | 18 Weeks | Group × Time ANOVA Result | Effect Size (Cohen’s d) for Significant Results Baseline—12 Weeks | Effect Size (Cohen’s d) for Significant Results Baseline—18 Weeks | |
|---|---|---|---|---|---|---|---|
| F | p | ||||||
| PASE | 55.1 (55.2) | 94.8 (75.9) a | 96.0 (67.2) b | 3.66 | 0.030 | 0.60 | 0.66 |
| LLDFI–Function Component Total | 50.8 (14.4) | 57.6 (16.4) | 58.7 (15.6) | 2.27 | 0.109 | NS | NS |
| LLDFI–Function (Upper extremity domain) d | 54.6 (16.7) | 63.7 (20.4) | 63.7 (19.0) | 2.33 | 0.104 d | NS | NS |
| LLDFI–Function (Basic lower extremity domain) d | 63.1 (17.9) | 77.9 (20.9) a | 79.5 (18.9) b | 6.61 | 0.002 d | 0.76 | 0.89 |
| LLDFI–Function (Advanced lower extremity domain) d | 41.7 (18.9) | 49.6 (19.4) | 49.9 (19.0) | 1.80 | 0.173 d | NS | NS |
| LLFDI–Disability Component–Frequency Dimension Total | 32.3 (10.4) | 37.3 (9.4) | 38.6 (11.1) b | 3.12 | 0.049 | NS | 0.58 |
| LLFDI–Disability Component–Frequency Dimension–Social Role Domain e | 29.5 (13.6) | 34.9 (10.9) | 35.8 (10.8) | 2.46 | 0.091 e | NS | NS |
| LLFDI–Disability Component–Frequency Dimension–Personal Role Domain e | 28.5 (11.6) | 36.4 (12.2) a | 36.7 (14.8) b | 3.87 | 0.024 e | 0.66 | 0.62 |
| LLFDI–Disability Component–Limitation Dimension Total | 47.0 (13.7) | 53.1 (12.5) | 55.3 (14.3) b | 3.02 | 0.054 | NS | 0.59 |
| LLFDI–Disability Component–Limitation Dimension–Instrumental Role Domain e | 45.6 (16.3) | 52.7 (12.1) | 54.8 (13.2) b | 3.52 | 0.034 e | NS | 0.47 |
| LLFDI–Disability Component–Limitation Dimension–Management Role Domain e | 44.2 (24.6) | 53.6 (21.3) | 55.2 (21.5) | 2.11 | 0.128 e | NS | NS |
| Vitality plus Scale | 35.3 (5.8) | 39.9 (6.1) a | 40.6 (5.3) b | 7.44 | 0.001 | 0.77 | 0.95 |
| Icon-FES | 31.1 (7.7) | 28.0 (7.5) | 28.0 (7.3) | 1.78 | 0.175 | NS | NS |
| 8-item PACES | 24.6 (4.2) | 32.1 (1.9) a | 32.2 (2.6) b | 61.55 | <0.001 | 2.33 | 2.17 |
| QOL-AD (People with Dementia) | 28.8 (5.2) | 32.0 (5.3) a | 32.0 (5.1) b | 3.58 | 0.032 | 0.60 | 0.60 |
| QOL-AD (Carer) | 29.1 (5.3) | 33.8 (4.8) a | 33.6 (4.4) b | 8.68 | <0.001 | 0.91 | 0.90 |
| NPI-Q–Severity | 16.9 (6.9) | 14.1 (7.1) | 14.1 (6.5) | 1.63 | 0.202 | NS | NS |
| NPI-Q–Carer Distress | 19.1 (11.4) | 16.0 (9.2) | 15.3 (9.6) | 1.20 | 0.305 | NS | NS |
| Zarit Caregiver Burden Scale | 34.1 (13.8) | 31.9 (13.7) | 31.1 (12.3) | 0.39 | 0.676 | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sari, Y.M.; Burton, E.; Lee, D.-C.A.; Hill, K.D. A Telehealth Home-Based Exercise Program for Community-Dwelling Older People with Dementia in Indonesia: A Feasibility Study. Int. J. Environ. Res. Public Health 2023, 20, 3397. https://doi.org/10.3390/ijerph20043397
Sari YM, Burton E, Lee D-CA, Hill KD. A Telehealth Home-Based Exercise Program for Community-Dwelling Older People with Dementia in Indonesia: A Feasibility Study. International Journal of Environmental Research and Public Health. 2023; 20(4):3397. https://doi.org/10.3390/ijerph20043397
Chicago/Turabian StyleSari, Yulisna Mutia, Elissa Burton, Den-Ching A. Lee, and Keith D. Hill. 2023. "A Telehealth Home-Based Exercise Program for Community-Dwelling Older People with Dementia in Indonesia: A Feasibility Study" International Journal of Environmental Research and Public Health 20, no. 4: 3397. https://doi.org/10.3390/ijerph20043397
APA StyleSari, Y. M., Burton, E., Lee, D.-C. A., & Hill, K. D. (2023). A Telehealth Home-Based Exercise Program for Community-Dwelling Older People with Dementia in Indonesia: A Feasibility Study. International Journal of Environmental Research and Public Health, 20(4), 3397. https://doi.org/10.3390/ijerph20043397







