A Novel Bioinspired Algorithm for Mixed and Incomplete Breast Cancer Data Classification
Abstract
1. Introduction
2. Related Works
3. Materials and Methods
3.1. Datasets
- Breast Cancer Digital Repository (BCDR) [22]. This dataset is composed of data extracted from Portuguese women after being tested with biopsies to identify breast lesions. As stated in [22], “BCDR-F01 has a total of 362 segmentations from which 187 are from benign findings and the remainder 175 from malignant findings. In addition to the patient age and breast density, the data set includes a set of selected binary attributes for indicating abnormalities observed by radiologists, namely masses, microcalcifications, calcifications (other than microcalcifications), axillary adenopathies, architectural distortions, and stroma distortions. Thus, the clinical data for each instance of the BCDR-F01 data set include a total of eight attributes per instance: six binary attributes related to observed abnormalities, an ordinal attribute for breast density, and a numerical attribute that contains the patient age at the time of the study.”
- Breast Cancer Wisconsin (Original) Data Set (BCWO) [23]. This dataset was provided by the UCI repository [24] and is available at http://archive.ics.uci.edu/ml/datasets/Breast+Cancer+Wisconsin+%28Original%29, accessed on 11 January 2021. It consists of patients treated by Dr. Wolberg, offering valuable information on clinical cases of breast cancer. BCWO contains 699 records of tissue samples, with each record characterized by the following attributes: Clump Thickness, Uniformity of Cell Size, Uniformity of Cell Shape, Marginal Adhesion, Single Epithelial Cell Size, Bare Nuclei, Bland Chromatin, Normal Nucleoli, and Mitoses. All attributes were manually measured on a scale of 1 to 10.
- Breast Cancer SEER (BCSEER) [25]. The National Cancer Institute provides this dataset, which consists of real patients from 1973 to 2013 who underwent breast cancer-related studies. The institute provides the surveillance, epidemiology, and end results (SEER) database. The SEER database classifies cancer histology and topography information based on the third edition of the International Classifications of Diseases for Oncology (ICD-O-3). In our study, we used the version of the dataset available on the Kaggle website (https://www.kaggle.com/code/jnegrini/breast-cancer-dataset, accessed on 11 January 2021).
- Breast Cancer Wisconsin (Diagnostic) Data Set (BCWD) [26]. This binary dataset, provided by Dr. Wolberg in 1995, consists of data obtained from breast analysis and subsequently confirmed by biopsy. Features are computed from a digitized image of a fine needle aspirate (FNA) of a breast mass. They describe the characteristics of the cell nuclei present in the image. These features include radius (mean of distances from center to points on the perimeter), texture (standard deviation of gray-scale values), perimeter, area, smoothness (local variation in radius lengths), compactness (perimeter^2/area − 1.0), concavity (severity of concave portions of the contour), concave points (number of concave portions of the contour), symmetry, and fractal dimension (“coastline approximation” − 1). The dataset is available at http://archive.ics.uci.edu/ml/datasets/Breast+Cancer+Wisconsin+%28Diagnostic%29, accessed on 11 January 2021.
- Breast Cancer Wisconsin (Prognostic) Data Set (BCWP) [27]. This dataset was provided by Dr. Wolberg and contained data on breast cancer patients with invasive breast cancer. This dataset was donated in the same year as the BCWD. Each record represents follow-up data on one breast cancer case. These are consecutive patients seen by Dr. Wolberg since 1984 and include only those cases exhibiting invasive breast cancer and no evidence of distant metastases at the time of diagnosis. The dataset has 32 predictive attributes, with the first 30 computed from a digitized image of a fine needle aspirate (FNA) of a breast mass. They describe the characteristics of the cell nuclei present in the image. The other two attributes are recurrence time (in case of recurrence) and disease-free time (in case of non-recurrence). This dataset is available at http://archive.ics.uci.edu/ml/datasets/Breast+Cancer+Wisconsin+%28Prognostic%29, accessed on 11 January 2021.
- Lung Cancer Data Set (LCDS) [18]. This dataset was chosen as it contains information on patients who had surgeries. The dataset, which was donated in 1999, focuses on the survival of these patients after surgery. It is an interesting dataset due to the scarcity of the data (only 32 subjects) and the large amount of predictive features (55). It is available at http://archive.ics.uci.edu/ml/datasets/Lung+Cancer, accessed on 11 January 2021.
- Mammographic Mass Data Set (MMDS) [28]. Donated in 2007, this dataset contains patterns of mammography studies carried out on 961 German patients. It contains a BI-RADS assessment, the patient’s age, and three BI-RADS attributes. It also contains the ground truth (severity field) for 516 benign and 445 malignant masses identified on full-field digital mammograms, collected at the Institute of Radiology of the University Erlangen-Nuremberg between 2003 and 2006. Each instance has an associated BI-RADS assessment ranging from 1 (definitely benign) to 5 (highly suggestive of malignancy) assigned in a double-review process by physicians. The dataset is available at http://archive.ics.uci.edu/ml/datasets/Mammographic+Mass, accessed on 11 January 2021.
- Breast Cancer Data Set (BCDS) [29]. This dataset, which contains data on patients with recurrent breast cancer, was provided by Milan Soklic and Matjaz Zwitter at the Institute of Oncology in Yugoslavia. The dataset contains eight attributes: age, menopause, premenopausal, tumor size, inv-nodes, node-caps (yes, no), degree of malignancy (1, 2, 3), breast (left, right), breast quad (left-up, left-low, right-up, right-low, central), and irradiation (yes, no). The dataset is available at http://archive.ics.uci.edu/ml/datasets/Breast+Cancer, accessed on 11 January 2021.
- Haberman’s Survival Data Set (HSDS) [30]. This dataset was donated in 1999 to the Machine Learning repository of the University of California [18]. It contains data on the survival of patients with breast cancer who had surgical removal of lesions. It has only four predictive features: age of patient at the time of operation (numerical), patient’s year of operation, and number of positive axillary nodes detected. The decision attribute is survival status (1 if the patient survived 5 years or longer or 2 if the patient died within 5 years). The dataset is available at http://archive.ics.uci.edu/ml/datasets/Haberman%27s+Survival, accessed on 11 January 2021.
- Thoracic Surgery Data Set (TSDS) [31]. The data was collected retrospectively at the Wrocław Thoracic Surgery Centre for patients who underwent major lung resections for primary lung cancer in the years 2007–2011. The Centre is associated with the Department of Thoracic Surgery of the Medical University of Wrocław and the Lower-Silesian Centre for Pulmonary Diseases, Poland. The research database constitutes a part of the National Lung Cancer Registry, administered by the Institute of Tuberculosis and Pulmonary Diseases in Warsaw, Poland. The goal of the dataset is to predict whether the patient will or will not survive surgery. The dataset has 16 predictive attributes: forced vital capacity; volume that has been exhaled at the end of the first second of forced expiration; performance status (Zubrod scale); pain before surgery; hemoptysis before surgery; dyspnea before surgery; cough before surgery; weakness before surgery; size of the original tumor, from OC11 (smallest) to OC14 (largest); type 2 DM—diabetes mellitus; MI up to 6 months; peripheral arterial diseases; smoking; asthma; and age at surgery. The dataset can be found at http://archive.ics.uci.edu/ml/datasets/Thoracic+Surgery+Data, accessed on 11 January 2021.
3.2. Algorithms
- K-Nearest Neighbors (NN) was proposed by Cover and Hart in 1967 [33]. This algorithm is based on assigning a class according to the k nearest pattern. If the pattern belongs to different classes, a majority voting process will be carried out to obtain a single class.
- Naïve Bayes [36] is a classifier based on probability and the independence of each attribute. It is derived from Bayes’ theorem.
- ALVOT is a general purpose classification model that uses different views of information based on a Support Set System [37]. This model uses a voting schema based on aggregation procedures. The model has a high computational cost when using all typical testors, but it can obtain good results with mixed and incomplete data.
- NAC was proposed in 2017 by Villuendas-Rey et al. [38] as a learning model for classifying mixed and incomplete data. It is based on a similarity operator named MIDSO, and is a particular case of both the ALVOT and NN classifiers. It has low computational complexity and yields good results when applied to financial data.
- AIRS1 is a classification algorithm based on the Artificial Immune System, The algorithm was proposed in 2001 [39], based on the principle of clonal selection and affinity maturation.
- Immunos1 is another algorithm that reduces information in one training iteration. It was proposed in 2005 [40].
- CLONALG is an algorithm based on the principle of clonal selection for classification. Each prototype improves the recognition of patterns in each iteration due to the affinity function. This algorithm was proposed in 2002 [41].
3.3. Performance Measure
4. Results
5. Discussion
6. Conclusions
- Its creation of a reduced prototype set; this decreases storage complexity, making it suitable for hardware implementation in devices associated with other medical devices, such as mammographs, etc.
- Its ease of use and good performance, which allows doctors to make decisions when there is high demand in the analysis of mammographic studies.
- The main limitation of the proposal is that, as with most metaheuristics, it has several parameters. This helps to improve the algorithm’s performance by varying the values of the parameters.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Tiwari, R.C.; Murray, T.; Ghafoor, A.; Samuels, A.; Ward, E.; Feuer, E.J.; Thun, M.J. Cancer statistics, 2004. Cancer J. Clin. 2004, 54, 8–29. [Google Scholar] [CrossRef]
- Hassanpour, S.H.; Dehghani, M. Review of cancer from perspective of molecular. J. Cancer Res. Pract. 2017, 4, 127–129. [Google Scholar] [CrossRef]
- Breast Cancer Risk Factors You Cannot Change. Available online: https://www.cancer.org/cancer/breast-cancer/risk-and-prevention/breast-cancer-risk-factors-you-cannot-change.html (accessed on 17 March 2019).
- Wolpert, D.H.; Macready, W.G. No free lunch theorems for optimization. IEEE Trans. Evol. Comput. 1997, 1, 67–82. [Google Scholar] [CrossRef]
- Amrane, M.; Oukid, S.; Gagaoua, I.; Ensar, T. Breast cancer classification using machine learning. In Proceedings of the 2018 Electric Electronics, Computer Science, Biomedical Engineerings’ Meeting (EBBT), Istanbul, Turkey, 18–19 April 2018. [Google Scholar]
- Saritas, M.M.; Yasar, A. Performance analysis of ANN and Naive Bayes classification algorithm for data classification. Int. J. Intell. Syst. Appl. Eng. 2019, 7, 88–91. [Google Scholar] [CrossRef]
- Ting, F.F.; Tan, Y.J.; Sim, K.S. Convolutional neural network improvement for breast cancer classification. Expert Syst. Appl. 2019, 120, 103–115. [Google Scholar] [CrossRef]
- Yuan, Y.; Qin, W.; Buyyounouski, M.; Ibragimov, B.; Hancock, S.; Han, B.; Xing, L. Prostate cancer classification with multiparametric MRI transfer learning model. Med. Phys. 2019, 46, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Alsadoon, A.; Prasad, P.W.C.; Abdullah, S.; Deva, A. Deep convolutional network for breast cancer classification: Enhanced loss function (ELF). J. Supercomput. 2020, 76, 8548–8565. [Google Scholar] [CrossRef]
- Arif, M.; Niessen, W.J.; Schoots, I.G.; Veenland, J.F. Automated classification of significant prostate cancer on MRI: A systematic review on the performance of machine learning applications. Cancers 2020, 12, 1606. [Google Scholar]
- Devarriya, D.; Gulati, C.; Mansharamani, V.; Sakalle, A.; Bhardwaj, A. Unbalanced breast cancer data classification using novel fitness functions in genetic programming. Expert Syst. Appl. 2020, 140, 112866. [Google Scholar] [CrossRef]
- Binitha, S.; Sathya, S.S. A survey of bio inspired optimization algorithms. Int. J. Soft Comput. Eng. 2012, 2, 137–151. [Google Scholar]
- Mendoza, J.E.; Rousseau, L.-M.; Villegas, J.G. A hybrid metaheuristic for the vehicle routing problem with stochastic demand and duration constraints. J. Heuristics 2016, 22, 539–566. [Google Scholar] [CrossRef]
- Salhi, S.; Thompson, J. An overview of heuristics and metaheuristics. In The Palgrave Handbook of Operations Research; Salhi, S., Boylan, J.., Eds.; Palgrave Macmillan: Cham, Switzerland, 2022; pp. 353–403. [Google Scholar]
- González-Patiño, D.; Villuendas-Rey, Y.; Argüelles-Cruz, A.J.; Camacho-Nieto, O.; Yáñez-Márquez, C. AISAC: An Artificial Immune System for Associative Classification Applied to Breast Cancer Detection. Appl. Sci. 2020, 10, 515. [Google Scholar] [CrossRef]
- Madani, M.; Behzadi, M.M.; Nabavi, S. The Role of Deep Learning in Advancing Breast Cancer Detection Using Different Imaging Modalities: A Systematic Review. Cancers 2022, 14, 5334. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ahmad, I.; Javeed, D.; Zaidi, S.A.; Alotaibi, F.M.; Ghoneim, M.E.; Daradkeh, Y.I.; Asghar, J.; Eldin, E.T. Intelligent Hybrid Deep Learning Model for Breast Cancer Detection. Electronics 2022, 11, 2767. [Google Scholar] [CrossRef]
- Aljuaid, H.; Alturki, N.; Alsubaie, N.; Cavallaro, L.; Liotta, A. Computer-aided diagnosis for breast cancer classification using deep neural networks and transfer learning. Comput. Methods Programs Biomed. 2022, 223, 106951. [Google Scholar] [CrossRef]
- Chatterjee, S.; Biswas, S.; Majee, A.; Sen, S.; Oliva, D.; Sarkar, R. Breast cancer detection from thermal images using a Grunwald-Letnikov-aided Dragonfly algorithm-based deep feature selection method. Comput. Biol. Med. 2022, 141, 105027. [Google Scholar] [CrossRef]
- Bourouis, S.; Band, S.S.; Mosavi, A.; Agrawal, S.; Hamdi, M. Meta-heuristic algorithm-tuned neural network for breast cancer diagnosis using ultrasound images. Front. Oncol. 2022, 12, 834028. [Google Scholar]
- Badr, Y.A.; Abou El-Naga, A.H. A Hybrid Metaheuristic Approach for Automatic Clustering of Breast Cancer. In Proceedings of the 2022 5th International Conference on Computing and Informatics (ICCI), Cairo, Egypt, 9–10 March 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 392–399. [Google Scholar]
- Moura, D.C.; López, M.A.G. An evaluation of image descriptors combined with clinical data for breast cancer diagnosis. Int. J. Comput. Assist. Radiol. Surg. 2013, 8, 561–574. [Google Scholar] [CrossRef]
- Wolberg, W.H.; Mangasarian, O.L. Multisurface method of pattern separation for medical diagnosis applied to breast cytology. Proc. Natl. Acad. Sci. USA 1990, 87, 9193–9196. [Google Scholar] [CrossRef]
- Dua, D.; Graff, C. UCI Machine Learning Repository; University of California: Irvine, CA, USA, 2019; Available online: http://archive.ics.uci.edu/ml (accessed on 11 January 2021).
- Rajesh, K.; Anand, S. Analysis of SEER dataset for breast cancer diagnosis using C4. 5 classification algorithm. Int. J. Adv. Res. Comput. Commun. Eng. 2012, 1, 1021–2278. [Google Scholar]
- Mangasarian, O.L.; Street, W.N.; Wolberg, W.H. Breast cancer diagnosis and prognosis via linear programming. Oper. Res. 1995, 43, 570–577. [Google Scholar] [CrossRef]
- Street, W.N.; Mangasarian, O.L.; Wolberg, W.H. An inductive learning approach to prognostic prediction. In Proceedings of the Twelfth International Conference on Machine Learning, Tahoe City, CA, USA, 9–12 July 1995; Prieditis, A., Russell, S., Eds.; Morgan Kaufmann: San Francisco, CA, USA, 1995; pp. 522–530. [Google Scholar]
- Elter, M.; Schulz-Wendtland, R.; Wittenberg, T. The prediction of breast cancer biopsy outcomes using two CAD approaches that both emphasize an intelligible decision process. Med. Phys. 2007, 34, 4164–4172. [Google Scholar] [CrossRef] [PubMed]
- Michalski, R.S.; Mozetic, I.; Hong, J.; Lavrac, N. The Multi-Purpose Incremental Learning System AQ15 and its Testing Application to Three Medical Domains. In Proceedings of the Fifth National Conference on Artificial Intelligence, Philadelphia, PA, USA, 11–15 August 1986; Morgan Kaufmann: Philadelphia, PA, USA, 1986; pp. 1041–1045. [Google Scholar]
- Haberman, S.J. Generalized Residuals for Log-Linear Models. In Proceedings of the 9th International Biometrics Conference, Boston, MA, USA, 22–27 August 1976; pp. 104–122. [Google Scholar]
- Zięba, M.; Tomczak, J.M.; Lubicz, M.; Świątek, J. Boosted SVM for extracting rules from imbalanced data in application to prediction of the post-operative life expectancy in the lung cancer patients. Appl. Soft Comput. 2014, 14, 99–108. [Google Scholar] [CrossRef]
- Alcalá-Fdez, J.; Sánchez, L.; Garcia, S.; del Jesus, M.J.; Ventura, S.; Garrell, J.M.; Otero, J.; Romero, C.; Bacardit, J.; Rivas, V.M. KEEL: A software tool to assess evolutionary algorithms for data mining problems. Soft Comput. 2009, 13, 307–318. [Google Scholar] [CrossRef]
- Cover, T.; Hart, P. Nearest neighbor pattern classification. IEEE Trans. Inf. Theory 1967, 13, 21–27. [Google Scholar] [CrossRef]
- Quinlan, J.R. C4.5: Programs for Machine Learning; Morgan Kaufmann Publishers: San Mateo, CA, USA, 1993. [Google Scholar]
- Quinlan, J.R. Induction of decision trees. Mach. Learn. 1986, 1, 81–106. [Google Scholar] [CrossRef]
- John, G.H.; Langley, P. Estimating Continuous Distributions in Bayesian Classifiers. In Proceedings of the Eleventh Conference on Uncertainty in Artificial Intelligence, Quebec, QC, Canada, 18–19 August 1995. [Google Scholar]
- Ruiz-Shulcloper, J. Pattern recognition with mixed and incomplete data. Pattern Recognit. Image Anal. 2008, 18, 563–576. [Google Scholar] [CrossRef]
- Villuendas-Rey, Y.; Rey-Benguría, C.F.; Ferreira-Santiago, Á.; Camacho-Nieto, O.; Yáñez-Márquez, C. The Naïve Associative Classifier (NAC): A novel, simple, transparent, and accurate classification model evaluated on financial data. Neurocomputing 2017, 265, 105–115. [Google Scholar] [CrossRef]
- Watkins, A.B. AIRS: A Resource Limited Artificial Immune Classifier. Master’s Thesis, Mississippi State University, Mississippi, MS, USA, 2001. [Google Scholar]
- Brownlee, J. Immunos-81, the Misunderstood Artificial Immune System; Technical Report 1-02; Faculty of Information & Communication Technologies (ICT), Swinburne University of Technology (SUT): Melbourne, Australia, 2005. [Google Scholar]
- De Castro, L.N.; Von Zuben, F.J. Learning and optimization using the clonal selection principle. IEEE Trans. Evol. Comput. 2002, 6, 239–251. [Google Scholar] [CrossRef]
- Ferri, C.; Hernández-Orallo, J.; Modroiu, R. An experimental comparison of performance measures for classification. Pattern Recognit. Lett. 2009, 30, 27–38. [Google Scholar] [CrossRef]
- Demšar, J. Statistical comparisons of classifiers over multiple data sets. J. Mach. Learn. Res. 2006, 7, 1–30. [Google Scholar]
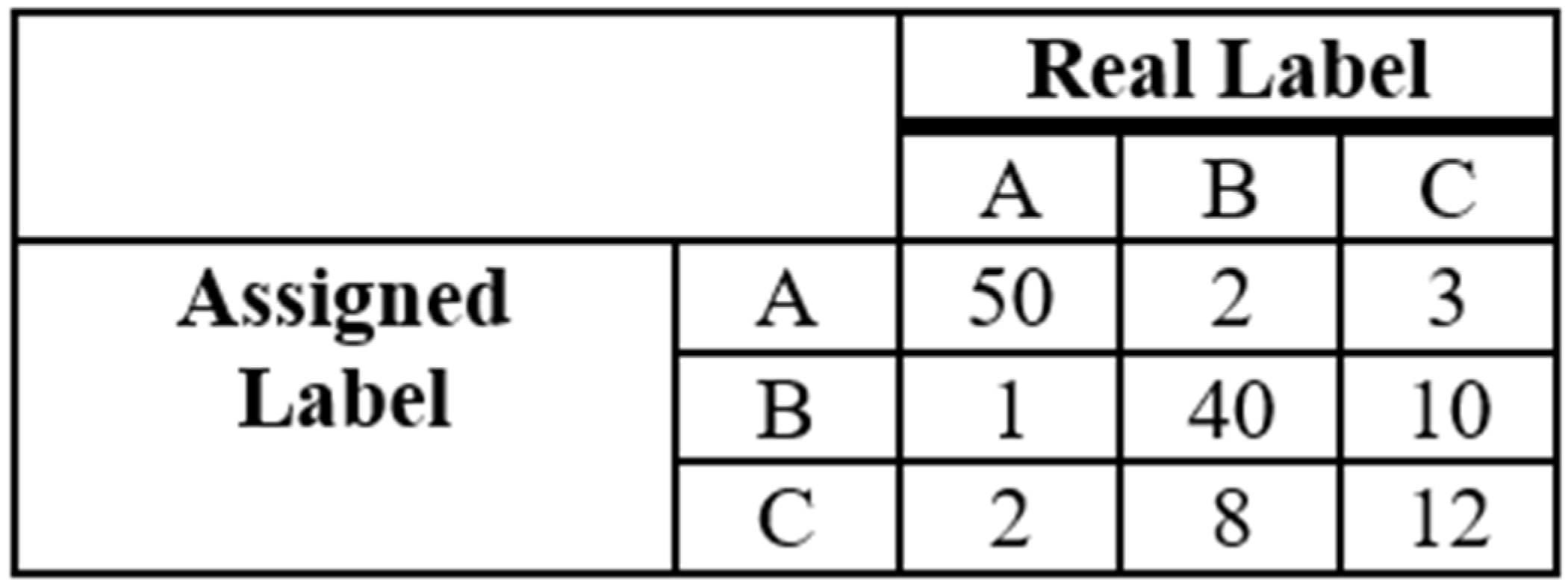



| Dataset | Attributes | Instances | Imbalance Ratio | Missing Values |
|---|---|---|---|---|
| BCDR | 38 | 362 | 1.06 | Yes |
| BCWO | 9 | 699 | 1.90 | Yes |
| BCSEER | 5 | 1405 | 5.41 | No |
| BCWD | 30 | 569 | 1.60 | No |
| BCWP | 33 | 198 | 3.21 | Yes |
| LCDS | 56 | 32 | 1.44 | Yes |
| MMDS | 5 | 961 | 1.15 | Yes |
| BCDS | 9 | 286 | 2.36 | Yes |
| HSDS | 3 | 306 | 2.77 | No |
| TSDS | 14 | 470 | 5.71 | No |
| Algorithm | Parameters |
|---|---|
| NN | K: 1; Dissimilarity: HEOM |
| C4.5 | BinarySplits: False; collapseTree: True; confidenceFactor: 0.25; minNumObj: 2; numFolds: 3; unpruned: False; useLaplace: False; useMDLcorrection: True; |
| Naïve Bayes | - |
| ALVOT | Dissimilarity: HEOM, Support Set System: All attributes |
| NAC | Dissimilarity: HEOM |
| AIRS1 | seed = 1; affinityThresholdScalar = 0.2; mutationRate = 0.1; totalResources = 150; stimulationValue = 0.9; clonalRate = 10; hypermutationRate = 2.0; numInstancesAffinityThreshold = −1; arbInitialPoolSize = 1; memInitialPoolSize = 1; knn = 3; |
| Immunos1 | - |
| CLONALG | clonalFactor = 0.1; antibodyPoolSize = 30; selectionPoolSize = 20; totalReplacement = 0; numGenerations = 10; seed = 1; remainderPoolRatio = 0.1 |
| Dataset | ALVOT | C4.5 | NAC | Naïve Bayes | NN | AISAC-MMD |
|---|---|---|---|---|---|---|
| BCDR | 0.770 | 0.749 | 0.678 | 0.727 | 0.729 | 0.784 |
| BCWO | 0.941 | 0.951 | 0.975 | 0.960 | 0.953 | 0.969 |
| BCSEER | 0.834 | 1.000 | 0.908 | 0.972 | 0.984 | 1.000 |
| BCWD | 0.934 | 0.931 | 0.894 | 0.930 | 0.960 | 0.965 |
| BCWP | 0.563 | 0.727 | 0.699 | 0.667 | 0.707 | 0.767 |
| LCDS | 0.542 | 0.469 | 0.450 | 0.594 | 0.531 | 0.688 |
| MMDS | 0.789 | 0.823 | 0.806 | 0.778 | 0.752 | 0.797 |
| BCDS | 0.728 | 0.741 | 0.731 | 0.727 | 0.682 | 0.731 |
| HSDS | 0.748 | 0.703 | 0.733 | 0.748 | 0.660 | 0.765 |
| TSDS | 0.728 | 0.845 | 0.774 | 0.745 | 0.760 | 0.845 |
| Dataset | AIRS1 | CLONALG | Immunos1 | AISAC-MMD |
|---|---|---|---|---|
| BCDR | 0.732 | 0.577 | 0.561 | 0.784 |
| BCWO | 0.967 | 0.941 | 0.847 | 0.969 |
| BCSEER | 0.945 | 0.965 | 0.954 | 1.000 |
| BCWD | 0.938 | 0.889 | 0.905 | 0.965 |
| BCWP | 0.641 | 0.742 | 0.566 | 0.767 |
| LCDS | 0.531 | 0.469 | 0.563 | 0.688 |
| MMDS | 0.634 | 0.700 | 0.743 | 0.797 |
| BCDS | 0.675 | 0.671 | 0.734 | 0.731 |
| HSDS | 0.637 | 0.732 | 0.568 | 0.765 |
| TSDS | 0.774 | 0.745 | 0.760 | 0.845 |
| AISAC-MMD vs. | R+ | R− | p-Value | Decision |
|---|---|---|---|---|
| NN | 55 | 0 | 0.004317 | Reject H0 |
| C4.5 | 49 | 6 | 0.024932 | Reject H0 |
| Naïve Bayes | 55 | 0 | 0.004317 | Reject H0 |
| ALVOT | 39 | 6 | 0.044011 | Reject H0 |
| NAC | 55 | 0 | 0.004317 | Reject H0 |
| AIRS1 | 55 | 0 | 0.004317 | Reject H0 |
| Immunos1 | 54 | 1 | 0.005922 | Reject H0 |
| CLONALG | 55 | 0 | 0.004317 | Reject H0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Patiño, D.; Villuendas-Rey, Y.; Saldaña-Pérez, M.; Argüelles-Cruz, A.-J. A Novel Bioinspired Algorithm for Mixed and Incomplete Breast Cancer Data Classification. Int. J. Environ. Res. Public Health 2023, 20, 3240. https://doi.org/10.3390/ijerph20043240
González-Patiño D, Villuendas-Rey Y, Saldaña-Pérez M, Argüelles-Cruz A-J. A Novel Bioinspired Algorithm for Mixed and Incomplete Breast Cancer Data Classification. International Journal of Environmental Research and Public Health. 2023; 20(4):3240. https://doi.org/10.3390/ijerph20043240
Chicago/Turabian StyleGonzález-Patiño, David, Yenny Villuendas-Rey, Magdalena Saldaña-Pérez, and Amadeo-José Argüelles-Cruz. 2023. "A Novel Bioinspired Algorithm for Mixed and Incomplete Breast Cancer Data Classification" International Journal of Environmental Research and Public Health 20, no. 4: 3240. https://doi.org/10.3390/ijerph20043240
APA StyleGonzález-Patiño, D., Villuendas-Rey, Y., Saldaña-Pérez, M., & Argüelles-Cruz, A.-J. (2023). A Novel Bioinspired Algorithm for Mixed and Incomplete Breast Cancer Data Classification. International Journal of Environmental Research and Public Health, 20(4), 3240. https://doi.org/10.3390/ijerph20043240








