Alterations in Pregnenolone and Testosterone Levels in Male Shift Workers
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Sampling
2.2. Clinical Parameters Collection
2.3. Hormones Assays
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parent-Thirion, A.; Biletta, I.; Cabrita, J.; Llave, O.V.; Vermeylen, G.; Wilczynska, A.; Wilkens, M. 6th European Working Conditions Survey: 2017 Update; Publications Office of the European Union: Luxembourg, 2017. [Google Scholar]
- Alterman, T.; Luckhaupt, S.E.; Dahlhamer, J.M.; Ward, B.W.; Calvert, G.M. Prevalence rates of work organization characteristics among workers in the U.S.: Data from the 2010 National Health Interview Survey. Am. J. Ind. Med. 2013, 56, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Lu, L.; Idris, S.U. Working Time in Transition: The Dual Task of Standardization and Flexibilization in China; International Labour Office: Geneva, Switzerland, 2005. [Google Scholar]
- Copertaro, A.; Bracci, M. Working against the biological clock: A review for the Occupational Physician. Ind. Health 2019, 57, 557–569. [Google Scholar] [CrossRef]
- Martelli, M.; Zingaretti, L.; Salvio, G.; Bracci, M.; Santarelli, L. Influence of Work on Andropause and Menopause: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 10074. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Huang, D.; Wang, H.; Yang, Z. Associations of shift work and night work with risk of all-cause, cardiovascular and cancer mortality: A meta-analysis of cohort studies. Sleep Med. 2021, 86, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Di, W.; Zeng, Y.; Liu, D.; Han, M.; Qie, R.; Huang, S.; Zhao, Y.; Feng, Y.; Hu, D.; et al. Association between shift work and risk of metabolic syndrome: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2792–2799. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Chen, W.; Lin, X. Night-shift work, breast cancer incidence, and all-cause mortality: An updated meta-analysis of prospective cohort studies. Sleep Breath. 2022, 26, 1509–1526. [Google Scholar] [CrossRef]
- Grosser, L.; Knayfati, S.; Yates, C.; Dorrian, J.; Banks, S. Cortisol and shiftwork: A scoping review. Sleep Med. Rev. 2022, 64, 101581. [Google Scholar] [CrossRef]
- Sen, A.; Sellix, M.T. The Circadian Timing System and Environmental Circadian Disruption: From Follicles to Fertility. Endocrinology 2016, 157, 3366–3373. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Huang, T.; Schernhammer, E.S.; Sun, Q.; Wang, M. Rotating Night Shift Work and Healthy Aging After 24 Years of Follow-up in the Nurses’ Health Study. JAMA Netw. Open 2022, 5, e2210450. [Google Scholar] [CrossRef]
- Koop, S.; Oster, H. Eat, sleep, repeat—Endocrine regulation of behavioural circadian rhythms. FEBS J. 2022, 289, 6543–6558. [Google Scholar] [CrossRef]
- Bracci, M.; Copertaro, A.; Manzella, N.; Staffolani, S.; Strafella, E.; Nocchi, L.; Barbaresi, M.; Copertaro, B.; Rapisarda, V.; Valentino, M.; et al. Influence of night-shift and napping at work on urinary melatonin, 17-β-estradiol and clock gene expression in pre-menopausal nurses. J. Biol. Regul. Homeost. Agents 2013, 27, 267–274. [Google Scholar] [PubMed]
- Bracci, M.; Manzella, N.; Copertaro, A.; Staffolani, S.; Strafella, E.; Barbaresi, M.; Copertaro, B.; Rapisarda, V.; Valentino, M.; Santarelli, L. Rotating-shift nurses after a day off: Peripheral clock gene expression, urinary melatonin, and serum 17-β-estradiol levels. Scand. J. Work. Environ. Health 2014, 40, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Schernhammer, E.S.; Rosner, B.; Willett, W.C.; Laden, F.; Colditz, G.A.; Hankinson, S.E. Epidemiology of urinary melatonin in women and its relation to other hormones and night work. Cancer Epidemiol. Biomark. Prev. 2004, 13, 936–943. [Google Scholar] [CrossRef]
- Nagata, C.; Nagao, Y.; Yamamoto, S.; Shibuya, C.; Kashiki, Y.; Shimizu, H. Light exposure at night, urinary 6-sulfatoxymelatonin, and serum estrogens and androgens in postmenopausal Japanese women. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.S.; Eliassen, A.H.; Hankinson, S.E.; Lenart, E.B.; Willett, W.C.; Tamimi, R.M. Breast Cancer Research in the Nurses’ Health Studies: Exposures Across the Life Course. Am. J. Public Health 2016, 106, 1592–1598. [Google Scholar] [CrossRef]
- Eliassen, A.H.; Missmer, S.A.; Tworoger, S.S.; Spiegelman, D.; Barbieri, R.L.; Dowsett, M.; Hankinson, S.E. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J. Natl. Cancer Inst. 2006, 98, 1406–1415. [Google Scholar] [CrossRef]
- IARC Working Group on the Identification of Carcinogenic Hazards to Humans. Night Shift Work; International Agency for Research on Cancer: Lyon, France, 2020; (IARC Monographs on the Identification of Carcinogenic Hazards to Humans, No. 124.). Available online: https://www.ncbi.nlm.nih.gov/books/NBK568195/ (accessed on 8 February 2023).
- Zhang, Y.; Papantoniou, K. Night shift work and its carcinogenicity. Lancet Oncol. 2019, 20, e550. [Google Scholar] [CrossRef]
- National Toxicology Program. NTP Cancer Hazard Assessment Report on Night Shift Work and Light at Night; National Toxicology Program: Research Triangle Park, NC, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK571598/ (accessed on 8 February 2023).
- Bracci, M.; Ciarapica, V.; Zabaleta, M.E.; Tartaglione, M.F.; Pirozzi, S.; Giuliani, L.; Piva, F.; Valentino, M.; Ledda, C.; Rapisarda, V.; et al. BRCA1 and BRCA2 Gene Expression: Diurnal Variability and Influence of Shift Work. Cancers 2019, 11, 1146. [Google Scholar] [CrossRef] [PubMed]
- Cordina-Duverger, E.; Menegaux, F.; Popa, A.; Rabstein, S.; Harth, V.; Pesch, B.; Brüning, T.; Fritschi, L.; Glass, D.C.; Heyworth, J.S.; et al. Night shift work and breast cancer: A pooled analysis of population-based case—Control studies with complete work history. Eur. J. Epidemiol. 2018, 33, 369–379. [Google Scholar] [CrossRef]
- Costa, G.; Haus, E.; Stevens, R. Shift work and cancer—Considerations on rationale, mechanisms, and epidemiology. Scand. J. Work. Environ. Health 2010, 36, 163–179. [Google Scholar] [CrossRef]
- Manzella, N.; Bracci, M.; Strafella, E.; Staffolani, S.; Ciarapica, V.; Copertaro, A.; Rapisarda, V.; Ledda, C.; Amati, M.; Valentino, M.; et al. Circadian Modulation of 8-Oxoguanine DNA Damage Repair. Sci. Rep. 2015, 5, 13752. [Google Scholar] [CrossRef] [PubMed]
- Samulin Erdem, J.; Notø, H.; Skare, Ø.; Lie, J.S.; Petersen-Øverleir, M.; Reszka, E.; Pepłońska, B.; Zienolddiny, S. Mechanisms of breast cancer risk in shift workers: Association of telomere shortening with the duration and intensity of night work. Cancer Med. 2017, 6, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Folkerd, E.J.; Dowsett, M. Influence of sex hormones on cancer progression. J. Clin. Oncol. 2010, 28, 4038–4044. [Google Scholar] [CrossRef] [PubMed]
- Yassin, A.; AlRumaihi, K.; Alzubaidi, R.; Alkadhi, S.; Al Ansari, A. Testosterone, testosterone therapy and prostate cancer. Aging Male 2019, 22, 219–227. [Google Scholar] [CrossRef]
- Markozannes, G.; Tzoulaki, I.; Karli, D.; Evangelou, E.; Ntzani, E.; Gunter, M.J.; Norat, T.; Ioannidis, J.P.; Tsilidis, K.K. Diet, body size, physical activity and risk of prostate cancer: An umbrella review of the evidence. Eur. J. Cancer 2016, 69, 61–69. [Google Scholar] [CrossRef]
- Gan, Y.; Li, L.; Zhang, L.; Yan, S.; Gao, C.; Hu, S.; Qiao, Y.; Tang, S.; Wang, C.; Lu, Z. Association between shift work and risk of prostate cancer: A systematic review and meta-analysis of observational studies. Carcinogenesis 2018, 39, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Krstev, S.; Knutsson, A. Occupational Risk Factors for Prostate Cancer: A Meta-analysis. J. Cancer Prev. 2019, 24, 91–111. [Google Scholar] [CrossRef]
- Rivera-Izquierdo, M.; Martínez-Ruiz, V.; Castillo-Ruiz, E.M.; Manzaneda-Navío, M.; Pérez-Gómez, B.; Jiménez-Moleón, J.J. Shift Work and Prostate Cancer: An Updated Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 1345. [Google Scholar] [CrossRef]
- Wendeu-Foyet, M.G.; Menegaux, F. Circadian Disruption and Prostate Cancer Risk: An Updated Review of Epidemiological Evidences. Cancer Epidemiol. Biomark. Prev. 2017, 26, 985–991. [Google Scholar] [CrossRef]
- Dasari, S.S.; Archer, M.; Mohamed, N.E.; Tewari, A.K.; Figueiro, M.G.; Kyprianou, N. Circadian Rhythm Disruption as a Contributor to Racial Disparities in Prostate Cancer. Cancers 2022, 14, 5116. [Google Scholar] [CrossRef] [PubMed]
- Papantoniou, K.; Pozo, O.J.; Espinosa, A.; Marcos, J.; Castaño-Vinyals, G.; Basagaña, X.; Pagès, E.J.; Mirabent, J.; Martín, J.; Faro, P.S.; et al. Increased and mistimed sex hormone production in night shift workers. Cancer Epidemiol. Biomark. Prev. 2015, 24, 854–863. [Google Scholar] [CrossRef]
- Sigurdardottir, L.G.; Valdimarsdottir, U.A.; Fall, K.; Rider, J.R.; Lockley, S.W.; Schernhammer, E.; Mucci, L.A. Circadian disruption, sleep loss, and prostate cancer risk: A systematic review of epidemiologic studies. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1002–1011. [Google Scholar] [CrossRef]
- Liu, K.; Hou, G.; Wang, X.; Chen, H.; Shi, F.; Liu, C.; Zhang, X.; Han, F.; Yang, H.; Zhou, N.; et al. Adverse effects of circadian desynchrony on the male reproductive system: An epidemiological and experimental study. Hum. Reprod. 2020, 35, 1515–1528. [Google Scholar] [CrossRef]
- Demirkol, M.K.; Yıldırım, A.; Gıca, Ş.; Doğan, N.T.; Resim, S. Evaluation of the effect of shift working and sleep quality on semen parameters in men attending infertility clinic. Andrologia 2021, 53, e14116. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, K.M.; Kohn, T.P.; Kohn, J.R.; Sigalos, J.T.; Kirby, E.W.; Pickett, S.M.; Pastuszak, A.W.; Lipshultz, L.I. Shift Work Sleep Disorder and Night Shift Work Significantly Impair Erectile Function. J. Sex. Med. 2020, 17, 1687–1693. [Google Scholar] [CrossRef] [PubMed]
- Piper, T.; Schlug, C.; Mareck, U.; Schänzer, W. Investigations on changes in ¹³C/¹²C ratios of endogenous urinary steroids after pregnenolone administration. Drug Test. Anal. 2011, 3, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ren, J.; Li, N.; Liu, J.; Tan, S.C.; Low, T.Y.; Ma, Z. A dose-response and meta-analysis of dehydroepiandrosterone (DHEA) supplementation on testosterone levels: Perinatal prediction of randomized clinical trials. Exp. Gerontol. 2020, 141, 111110. [Google Scholar] [CrossRef] [PubMed]
- Collomp, K.; Buisson, C.; Gravisse, N.; Belgherbi, S.; Labsy, Z.; Do, M.C.; Gagey, O.; Dufay, S.; Vibarel-Rebot, N.; Audran, M. Effects of short-term DHEA intake on hormonal responses in young recreationally trained athletes: Modulation by gender. Endocrine 2018, 59, 538–546. [Google Scholar] [CrossRef]
- Horne, J.A.; Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar]
- Rodríguez-González-Moro, M.T.; Rodríguez-González-Moro, J.M.; Rivera-Caravaca, J.M.; Vera-Catalán, T.; Simonelli-Muñoz, A.J.; Gallego-Gómez, J.I. Work Shift and Circadian Rhythm as Risk Factors for Poor Sleep Quality in Public Workers from Murcia (Spain). Int. J. Environ. Res. Public Health 2020, 17, 5881. [Google Scholar] [CrossRef]
- Razavi, P.; Devore, E.E.; Bajaj, A.; Lockley, S.W.; Figueiro, M.G.; Ricchiuti, V.; Gauderman, W.J.; Hankinson, S.E.; Willett, W.C.; Schernhammer, E.S. Shift Work, Chronotype, and Melatonin Rhythm in Nurses. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1177–1186. [Google Scholar] [CrossRef]
- Bracci, M.; Eléxpuru Zabaleta, M.; Tartaglione, M.F.; Ledda, C.; Rapisarda, V.; Santarelli, L. Exosomal miR-92a Concentration in the Serum of Shift Workers. Appl. Sci. 2020, 10, 430. [Google Scholar] [CrossRef]
- Wittmann, M.; Dinich, J.; Merrow, M.; Roenneberg, T. Social jetlag: Misalignment of biological and social time. Chronobiol. Int. 2006, 23, 497–509. [Google Scholar] [CrossRef]
- Copertaro, A.; Bracci, M.; Gesuita, R.; Carle, F.; Amati, M.; Baldassari, M.; Mocchegiani, E.; Santarelli, L. Influence of shift-work on selected immune variables in nurses. Ind. Health 2011, 49, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Gold, D.R.; Rogacz, S.; Bock, N.; Tosteson, T.D.; Baum, T.M.; Speizer, F.E.; Czeisler, C.A. Rotating shift work, sleep, and accidents related to sleepiness in hospital nurses. Am. J. Public Health 1992, 82, 1011–1014. [Google Scholar] [CrossRef]
- Bracci, M.; Copertaro, A.; Ciarapica, V.; Barbaresi, M.; Esposito, S.; Albanesi, A.; Valentino, M.; Ledda, C.; Rapisarda, V.; Santarelli, L. NOCTURNIN Gene Diurnal Variation in Healthy Volunteers and Expression Levels in Shift Workers. BioMed Res. Int. 2019, 2019, 7582734. [Google Scholar] [CrossRef]
- Miller, W.L. Role of mitochondria in steroidogenesis. Endocr. Dev. 2011, 20, 1–19. [Google Scholar] [CrossRef]
- Sarti, P.; Magnifico, M.C.; Altieri, F.; Mastronicola, D.; Arese, M. New evidence for cross talk between melatonin and mitochondria mediated by a circadian-compatible interaction with nitric oxide. Int. J. Mol. Sci. 2013, 14, 11259–11276. [Google Scholar] [CrossRef]
- Witzig, M.; Grimm, A.; Schmitt, K.; Lejri, I.; Frank, S.; Brown, S.A.; Eckert, A. Clock-Controlled Mitochondrial Dynamics Correlates with Cyclic Pregnenolone Synthesis. Cells 2020, 9, 2323. [Google Scholar] [CrossRef] [PubMed]
- Mannowetz, N.; Miller, M.R.; Lishko, P.V. Regulation of the sperm calcium channel CatSper by endogenous steroids and plant triterpenoids. Proc. Natl. Acad. Sci. USA 2017, 114, 5743–5748. [Google Scholar] [CrossRef]
- Caetano, G.; Bozinovic, I.; Dupont, C.; Léger, D.; Lévy, R.; Sermondade, N. Impact of sleep on female and male reproductive functions: A systematic review. Fertil. Steril. 2021, 115, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Sleep Disorders Are Associated with Testosterone Deficiency and Erectile Dysfunction-a U.S. Claims Database Analysis. Available online: https://www.nature.com/articles/s41443-022-00649-2 (accessed on 27 December 2022).
- Zhang, F.; Xiong, Y.; Qin, F.; Yuan, J. Short Sleep Duration and Erectile Dysfunction: A Review of the Literature. Nat. Sci. Sleep 2022, 14, 1945–1961. [Google Scholar] [CrossRef] [PubMed]
- Michaud, J.E.; Billups, K.L.; Partin, A.W. Testosterone and prostate cancer: An evidence-based review of pathogenesis and oncologic risk. Ther. Adv. Urol. 2015, 7, 378–387. [Google Scholar] [CrossRef]
- Xia, B.W.; Zhao, S.C.; Chen, Z.P.; Chen, C.; Liu, T.S.; Yang, F.; Yan, Y. Relationship between serum total testosterone and prostate volume in aging men. Sci. Rep. 2021, 11, 14122. [Google Scholar] [CrossRef]
- Morgentaler, A.; Traish, A.M. Shifting the paradigm of testosterone and prostate cancer: The saturation model and the limits of androgen-dependent growth. Eur. Urol. 2009, 55, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Harman, S.M.; Metter, E.J.; Tobin, J.D.; Pearson, J.; Blackman, M.R. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J. Clin. Endocrinol. Metab. 2001, 86, 724–731. [Google Scholar] [CrossRef]
- Touitou, Y.; Motohashi, Y.; Reinberg, A.; Touitou, C.; Bourdeleau, P.; Bogdan, A.; Auzéby, A. Effect of shift work on the night-time secretory patterns of melatonin, prolactin, cortisol and testosterone. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 60, 288–292. [Google Scholar] [CrossRef]
- Harding, B.N.; Castaño-Vinyals, G.; Palomar-Cros, A.; Papantoniou, K.; Espinosa, A.; Skene, D.J.; Middleton, B.; Gomez-Gomez, A.; Navarrete, J.M.; Such, P.; et al. Changes in melatonin and sex steroid hormone production among men as a result of rotating night shift work—The HORMONIT study. Scand. J. Work. Environ. Health 2022, 48, 41–51. [Google Scholar] [CrossRef]
- Kelly, M.R.; Yuen, F.; Satterfield, B.C.; Auchus, R.J.; Gaddameedhi, S.; Van Dongen, H.P.A.; Liu, P.Y. Endogenous Diurnal Patterns of Adrenal and Gonadal Hormones During a 24-Hour Constant Routine after Simulated Shift Work. J. Endocr. Soc. 2022, 6, bvac153. [Google Scholar] [CrossRef]
- Jensen, M.A.; Hansen, Å.M.; Kristiansen, J.; Nabe-Nielsen, K.; Garde, A.H. Changes in the diurnal rhythms of cortisol, melatonin, and testosterone after 2, 4, and 7 consecutive night shifts in male police officers. Chronobiol. Int. 2016, 33, 1280–1292. [Google Scholar] [CrossRef]
- Erickson, M.L.; Wang, W.; Counts, J.; Redman, L.M.; Parker, D.; Huebner, J.L.; Dunn, J.; Kraus, W.E. Field-Based Assessments of Behavioral Patterns during Shiftwork in Police Academy Trainees Using Wearable Technology. J. Biol. Rhythm. 2022, 37, 260–271. [Google Scholar] [CrossRef]
- Pavlovic, M.V.; Marinkovic, D.Z.; Andric, S.A.; Kostic, T.S. The cost of the circadian desynchrony on the Leydig cell function. Sci. Rep. 2022, 12, 15520. [Google Scholar] [CrossRef]
- Briguglio, G.; Teodoro, M.; Italia, S.; Verduci, F.; Pollicino, M.; Coco, M.; De Vita, A.; Micali, E.; Alibrandi, A.; Lembo, G.; et al. Salivary Biomarkers and Work-Related Stress in Night Shift Workers. Int. J. Environ. Res. Public Health 2021, 18, 3184. [Google Scholar] [CrossRef]
- Burek, K.; Rabstein, S.; Kantermann, T.; Vetter, C.; Rotter, M.; Wang-Sattler, R.; Lehnert, M.; Pallapies, D.; Jöckel, K.H.; Brüning, T.; et al. Night work, chronotype and cortisol at awakening in female hospital employees. Sci. Rep. 2022, 12, 6525. [Google Scholar] [CrossRef] [PubMed]
- Cannizzaro, E.; Cirrincione, L.; Mazzucco, W.; Scorciapino, A.; Catalano, C.; Ramaci, T.; Ledda, C.; Plescia, F. Night-Time Shift Work and Related Stress Responses: A Study on Security Guards. Int. J. Environ. Res. Public Health 2020, 17, 562. [Google Scholar] [CrossRef]
- Minelli, A.; Di Palma, M.; Rocchi, M.B.L.; Ponzio, E.; Barbadoro, P.; Bracci, M.; Pelusi, G.; Prospero, E. Cortisol, chronotype, and coping styles as determinants of tolerance of nursing staff to rotating shift work. Chronobiol. Int. 2021, 38, 666–680. [Google Scholar] [CrossRef] [PubMed]
- Bracci, M.; Ciarapica, V.; Copertaro, A.; Barbaresi, M.; Manzella, N.; Tomasetti, M.; Gaetani, S.; Monaco, F.; Amati, M.; Valentino, M.; et al. Peripheral Skin Temperature and Circadian Biological Clock in Shift Nurses after a Day off. Int. J. Mol. Sci. 2016, 17, 623. [Google Scholar] [CrossRef] [PubMed]
- Aedo, A.R.; Nuñez, M.; Landgren, B.M.; Cekan, S.Z.; Diczfalusy, E. Studies on the pattern of circulating steroids in the normal menstrual cycle. Euro. J. Endocrinol. 1977, 84, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg, B.; de la Torre, B.; Hedman, M.; Falkay, G.; Diczfalusy, E. Circadian variation in systemic hormone levels in healthy men. J. Endocrinol. Investig. 1979, 2, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Turcu, A.F.; Zhao, L.; Chen, X.; Yang, R.; Rege, J.; Rainey, W.E.; Veldhuis, J.D.; Auchus, R.J. Circadian rhythms of 11-oxygenated C19 steroids and ∆5-steroid sulfates in healthy men. Eur. J. Endocrinol. 2021, 185, K1–K6. [Google Scholar] [CrossRef] [PubMed]
- Havlíková, H.; Hill, M.; Hampl, R.; Stárka, L. Sex- and age-related changes in epitestosterone in relation to pregnenolone sulfate and testosterone in normal subjects. J. Clin. Endocrinol. Metab. 2002, 87, 2225–2231. [Google Scholar] [CrossRef] [PubMed]
- Anawalt, B.D.; Matsumoto, A.M. Aging and androgens: Physiology and clinical implications. Rev. Endocr. Metab. Disord. 2022, 23, 1123–1137. [Google Scholar] [CrossRef] [PubMed]
- Penell, J.C.; Kushnir, M.M.; Lind, L.; Bergquist, J.; Bergquist, J.; Lind, P.M.; Naessen, T. Concentrations of nine endogenous steroid hormones in 70-year-old men and women. Endocr. Connect. 2021, 10, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Isidori, A.M.; Aversa, A.; Calogero, A.; Ferlin, A.; Francavilla, S.; Lanfranco, F.; Pivonello, R.; Rochira, V.; Corona, G.; Maggi, M. Adult- and late-onset male hypogonadism: The clinical practice guidelines of the Italian Society of Andrology and Sexual Medicine (SIAMS) and the Italian Society of Endocrinology (SIE). J. Endocrinol. Investig. 2022, 45, 2385–2403. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.M. Diagnosis of hypogonadism in ageing men. Rev. Endocr. Metab. Disord. 2022, 23, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Porcaro, A.B.; Amigoni, N.; Tafuri, A.; Rizzetto, R.; Shakir, A.; Tiso, L.; Cerrato, C.; Lacola, V.; Antoniolli, S.Z.; Gozzo, A.; et al. Endogenous testosterone as a predictor of prostate growing disorders in the aging male. Int. Urol. Nephrol. 2021, 53, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, S.S.; Nindl, B.C.; Vaara, J.P.; Santtila, M.; Häkkinen, K.; Kyröläinen, H. Basal Endogenous Steroid Hormones, Sex Hormone-Binding Globulin, Physical Fitness, and Health Risk Factors in Young Adult Men. Front. Physiol. 2018, 9, 1005. [Google Scholar] [CrossRef] [PubMed]
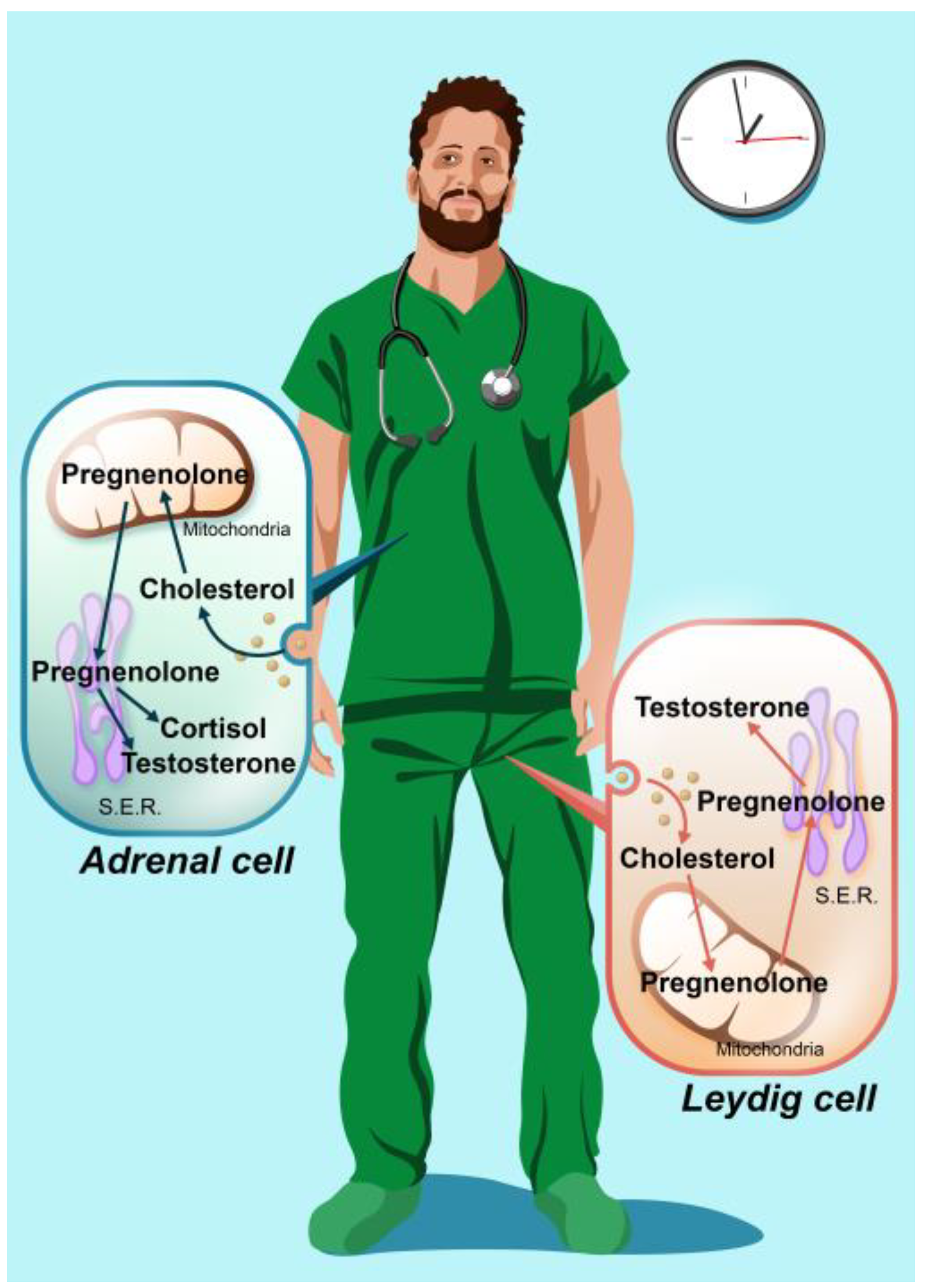
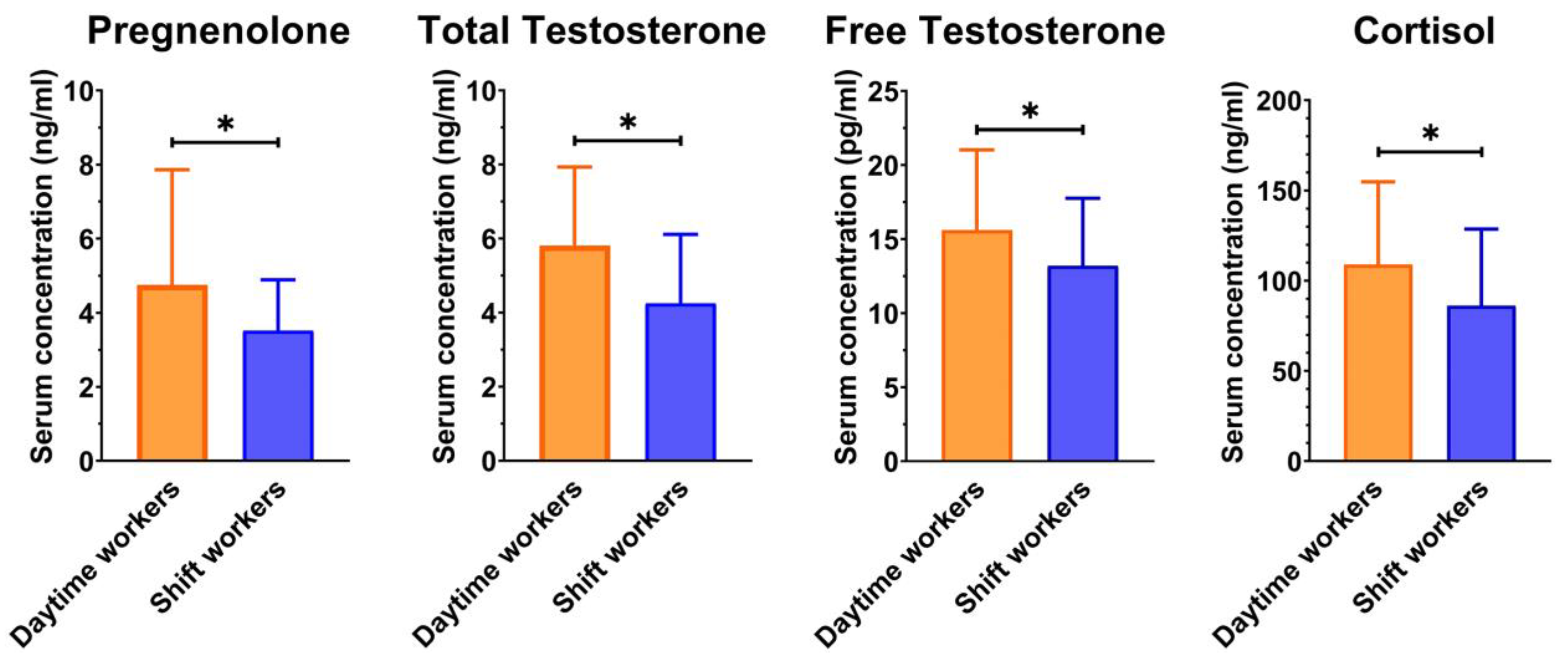
| Parameters | Daytime Workers (n = 46) | Shift Workers (n = 42) | p-Value | ||
|---|---|---|---|---|---|
| Mean ± SD | % | Mean ± SD | % | ||
| Age (years) | 34.2 ± 5.4 | 30.7 ± 5.5 | 0.003 | ||
| Shift work seniority (years) | 7.7 ± 5.1 | ||||
| Night shift works (nights per month) | 5.6 ± 1.4 | ||||
| Smokers (%) | 23.9 | 40.5 | 0.096 | ||
| BMI (Kg/m2) | 24.6 ± 3.4 | 25.1 ± 2.8 | 0.456 | ||
| Physical activity (%) | 60.9 | 51.5 | 0.422 | ||
| Chronotype (MEQ score) * | 54.1 ± 7.7 | 51.8 ± 6.9 | 0.145 | ||
| Wake-up time on the blood sampling day | 6:44 ± 0:40 | 6:32 ± 0:47 | 0.199 | ||
| Social jet lag (minutes) | 52.8 ± 47.1 | 51.8 ± 49.6 | 0.923 | ||
| Use of video display devices after dinner (minutes) | 76.4 ± 49.0 | 69.1 ± 42.5 | 0.459 | ||
| Cholesterol (mg/dL) | 174.9 ± 27.3 | 176.4 ± 34.5 | 0.820 | ||
| Triglycerides (mg/dL) | 87.4 ± 38.1 | 89.2 ± 47.0 | 0.843 | ||
| Glucose (mg/dL) | 84.2 ± 10.6 | 83.4 ± 8.4 | 0.698 | ||
| Parameters | Pregnenolone | Total Testosterone | Free Testosterone | Cortisol |
|---|---|---|---|---|
| Pregnenolone | 1 | 0.26 * | 0.33 ** | 0.25 * |
| Total testosterone | 0.26 * | 1 | 0.29 ** | 0.08 |
| Free testosterone | 0.33 ** | 0.29 ** | 1 | 0.25 * |
| Cortisol | 0.25 * | 0.08 | 0.25 * | 1 |
| Pregnenolone | Total Testosterone | Free Testosterone | Cortisol | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R * | β | p-Value | R * | β | p-Value | R * | β | p-Value | R * | β | p-Value | |
| Type of work (shift work vs. daytime work) | 0.31 | −0.265 | 0.036 | 0.44 | −0.304 | 0.008 | 0.38 | −0.123 | 0.285 | 0.36 | −0.132 | 0.257 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bracci, M.; Zingaretti, L.; Martelli, M.; Lazzarini, R.; Salvio, G.; Amati, M.; Milinkovic, M.; Ulissi, A.; Medori, A.R.; Vitale, E.; et al. Alterations in Pregnenolone and Testosterone Levels in Male Shift Workers. Int. J. Environ. Res. Public Health 2023, 20, 3195. https://doi.org/10.3390/ijerph20043195
Bracci M, Zingaretti L, Martelli M, Lazzarini R, Salvio G, Amati M, Milinkovic M, Ulissi A, Medori AR, Vitale E, et al. Alterations in Pregnenolone and Testosterone Levels in Male Shift Workers. International Journal of Environmental Research and Public Health. 2023; 20(4):3195. https://doi.org/10.3390/ijerph20043195
Chicago/Turabian StyleBracci, Massimo, Laura Zingaretti, Margherita Martelli, Raffaella Lazzarini, Gianmaria Salvio, Monica Amati, Marijana Milinkovic, Alfio Ulissi, Anna Rita Medori, Ermanno Vitale, and et al. 2023. "Alterations in Pregnenolone and Testosterone Levels in Male Shift Workers" International Journal of Environmental Research and Public Health 20, no. 4: 3195. https://doi.org/10.3390/ijerph20043195
APA StyleBracci, M., Zingaretti, L., Martelli, M., Lazzarini, R., Salvio, G., Amati, M., Milinkovic, M., Ulissi, A., Medori, A. R., Vitale, E., Ledda, C., & Santarelli, L. (2023). Alterations in Pregnenolone and Testosterone Levels in Male Shift Workers. International Journal of Environmental Research and Public Health, 20(4), 3195. https://doi.org/10.3390/ijerph20043195










