Assessment of the Effectiveness, Socio-Economic Impact and Implementation of a Digital Solution for Patients with Advanced Chronic Diseases: The ADLIFE Study Protocol
Abstract
1. Rationale
2. Methods and Analysis
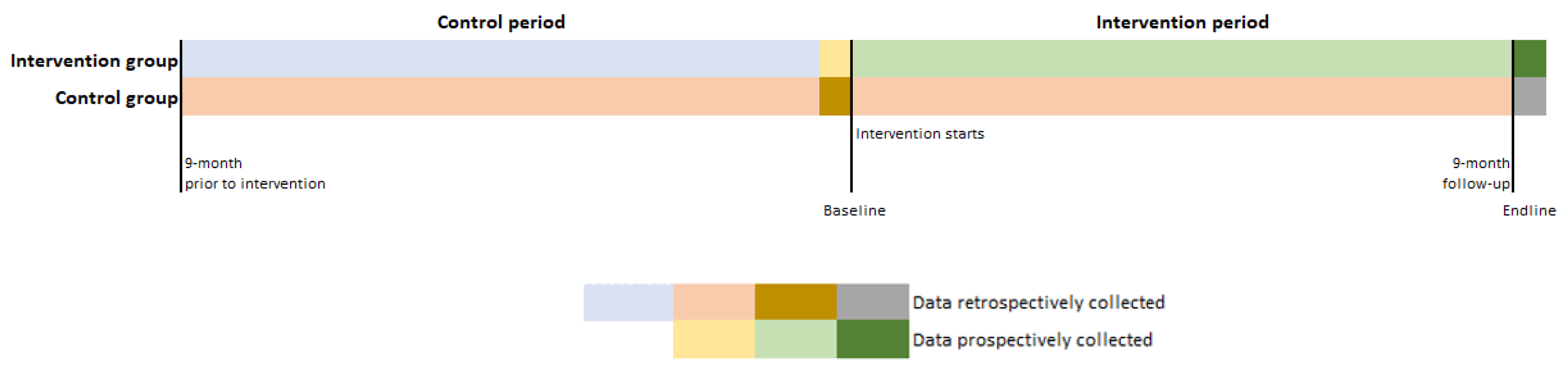
2.1. Study Design
2.1.1. Non-Randomized, Non-Concurrent, and Unblinded
2.2. Study Population
- Aged over 55;
- HF in functional stage III/IV according to the NYHA scale and/or stages C and D of the ACCF/AHA classification. Stable phase (at least two months without decompensation requiring hospital care);
- And/or COPD GOLD scale > 2 (FEV1 < 50) and/or mMRC ≥ 2 and/or CAT ≥ 10 and/or use of oxygen at home;
- With or without comorbidities;
- They are able to provide informed consent;
- They still live and generally plan on living in their home for the intervention duration;
- They or their informal caregiver(s) are able to use digital technology, communication tools, and/or networks and have access to a computer, laptop, tablet or smartphone and wifi/internet connection;
- They or their informal caregiver(s) understand, read, and talk the native language.
2.3. Recruitment
2.4. The ADLIFE Intervention and Standard of Care
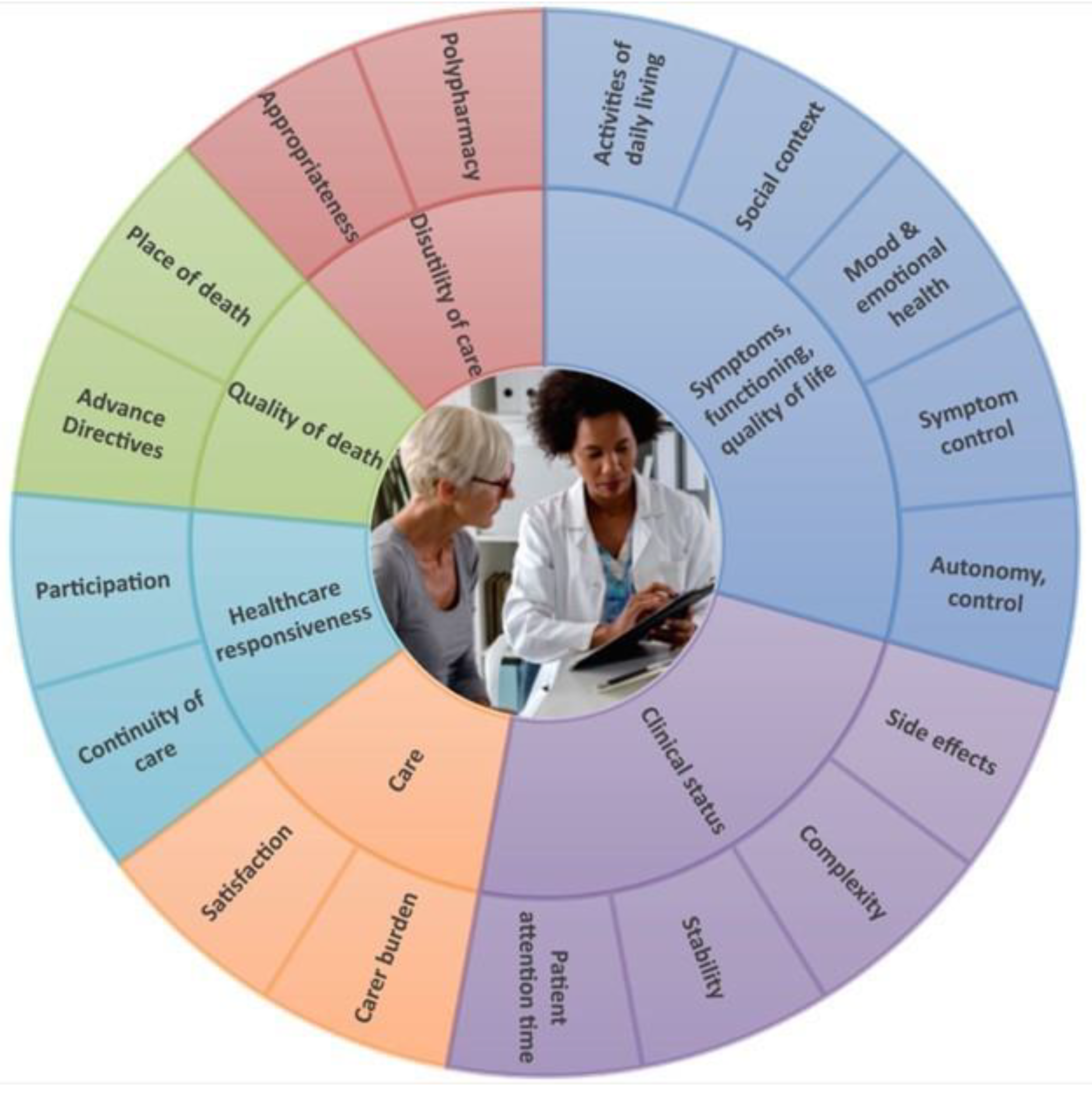
2.5. Outcomes
2.5.1. Primary
2.5.2. Secondary
- Patient-Reported Outcome Measurements (PROMs):
- Technology acceptance and future adoption of the ADLIFE intervention (the Unified Theory of Acceptance and Use of Technology, UTAUT) [37];
- Resource use and their associated costs will be also assessed on patients [38] (see data collection guide 2 in Supplementary File S4);
- Caregiver will be assessed on:
- Burden (Zarit Burden Interview, ZBI) [39];
- Mental well-being (Warwick-Edinburgh Mental Wellbeing Scale, WEMWBS) [40].
- Healthcare professionals will be qualitatively assessed on:
- Perceived coordination among settings;
- Quality of the integration of care;
- Decision making process;
- Working conditions.
- All stakeholders will be qualitatively assessed on:
- Perceived communication;
- Satisfaction with accessibility, security, and personalized care plans;
- Barriers/facilitators related to the implementation process.
2.6. Sample Size
2.7. Data Collection
2.8. Data Management
2.9. Analysis
2.9.1. Effectiveness Assessment
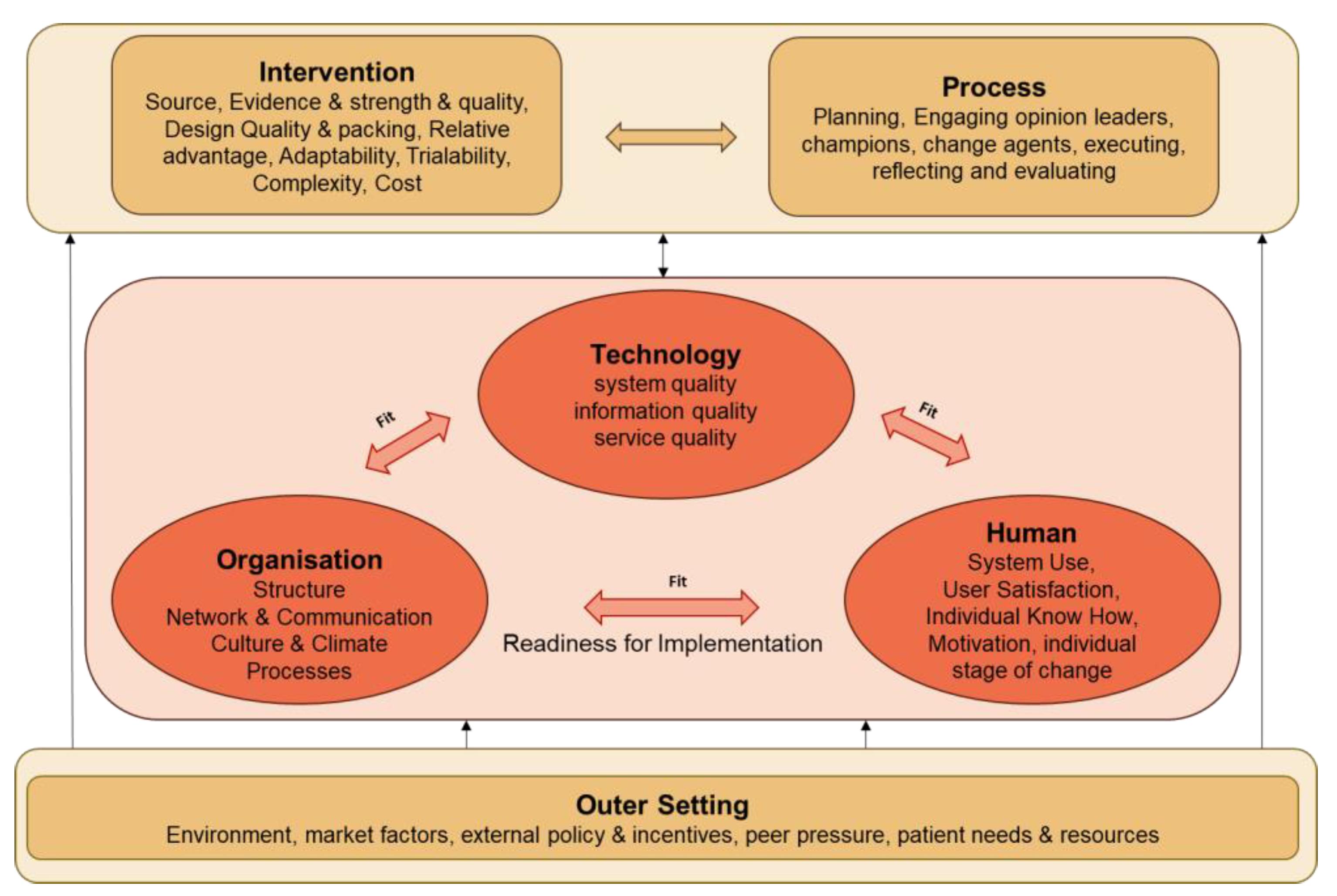
2.9.2. Implementation Assessment
2.9.3. Socio-Economic Assessment
3. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lunney, J.R.; Lynn, J.; Foley, D.J.; Lipson, S.; Guralnik, J.M. Patterns of functional decline at the end of life. JAMA 2003, 289, 2387–2392. [Google Scholar] [CrossRef] [PubMed]
- Tanuseputro, P.; Wodchis, W.P.; Fowler, R.; Walker, P.; Bai, Y.Q.; Bronskill, S.E.; Manuel, D. The Health Care Cost of Dying: A Population-Based Retrospective Cohort Study of the Last Year of Life in Ontario, Canada. PLoS ONE 2015, 10, e0121759. [Google Scholar] [CrossRef] [PubMed]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef]
- Blanco, I.; Diego, I.; Bueno, P.; Casas-Maldonado, F.; Miravitlles, M. Geographic distribution of COPD prevalence in the world displayed by Geographic Information System maps. Eur. Respir. J. 2019, 54, 1900610. [Google Scholar] [CrossRef] [PubMed]
- Quaderi, S.A.; Hurst, J.R. The unmet global burden of COPD. Glob. Health Epidemiol. Genom. 2018, 3, e4. [Google Scholar] [CrossRef]
- Iheanacho, I.; Zhang, S.; King, D.; Rizzo, M.; Ismaila, A.S. Economic Burden of Chronic Obstructive Pulmonary Disease (COPD): A Systematic Literature Review. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 439–460. [Google Scholar] [CrossRef] [PubMed]
- Shafie, A.A.; Tan, Y.P.; Ng, C.H. Systematic review of economic burden of heart failure. Heart Fail. Rev. 2018, 23, 131–145. [Google Scholar] [CrossRef]
- Gray, C.S. Integrated Care’s New Protagonist: The Expanding Role of Digital Health. Int. J. Integr. Care 2021, 21, 1. [Google Scholar] [CrossRef]
- Yadav, L.; Gill, T.K.; Taylor, A.; Jasper, U.; De Young, J.; Visvanathan, R.; Chehade, M.J. Cocreation of a digital patient health hub to enhance education and person-centred integrated care post hip fracture: A mixed-methods study protocol. BMJ Open 2019, 9, e033128. [Google Scholar] [CrossRef]
- Baxter, S.; Johnson, M.; Chambers, D.; Sutton, A.; Goyder, E.; Booth, A. The effects of integrated care: A systematic review of UK and international evidence. BMC Health Serv. Res. 2018, 18, 350. [Google Scholar] [CrossRef]
- Allen, D.; Rixson, L. How has the impact of ‘care pathway technologies’ on service integration in stroke care been measured and what is the strength of the evidence to support their effectiveness in this respect? Int. J. Evid. Based Healthc. 2008, 6, 78–110. [Google Scholar] [PubMed]
- Brännström, M.; Boman, K. Effects of person-centred and integrated chronic heart failure and palliative home care. PREFER: A randomized controlled study. Eur. J. Heart Fail. 2014, 16, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Dorr, D.A.; Wilcox, A.B.; Brunker, C.P.; Burdon, R.E.; Donnelly, S.M. The Effect of Technology-Supported, Multidisease Care Management on the Mortality and Hospitalization of Seniors. J. Am. Geriatr. Soc. 2008, 56, 2195–2202. [Google Scholar] [CrossRef] [PubMed]
- Hogg, W.; Lemelin, J.; Dahrouge, S.; Liddy, C.; Armstrong, C.D.; Legault, F.; Dalziel, B.; Zhang, W. Randomized controlled trial of anticipatory and preventive multidisciplinary team care: For complex patients in a community-based primary care setting. Can. Fam. Physician 2009, 55, e76–e85. [Google Scholar] [PubMed]
- Janse, B.; Huijsman, R.; De Kuyper, R.D.M.; Fabbricotti, I.N. The effects of an integrated care intervention for the frail elderly on informal caregivers: A quasi-experimental study. BMC Geriatr. 2014, 14, 58. [Google Scholar] [CrossRef]
- Boult, C.; Leff, B.; Boyd, C.M.; Wolff, J.L.; Marsteller, J.A.; Frick, K.D.; Wegener, S.; Reider, L.; Frey, K.; Mroz, T.M.; et al. A Matched-Pair Cluster-Randomized Trial of Guided Care for High-Risk Older Patients. J. Gen. Intern. Med. 2013, 28, 612–621. [Google Scholar] [CrossRef]
- Colla, C.H.; Lewis, V.A.; Kao, L.S.; O’Malley, A.J.; Chang, C.H.; Fisher, E.S. Association between Medicare Accountable Care Organization Implementation and Spending among Clinically Vulnerable Beneficiaries. JAMA Intern. Med. 2016, 176, 1167–1175. [Google Scholar] [CrossRef]
- Counsell, S.R.; Callahan, C.M.; Clark, D.O.; Tu, W.; Buttar, A.B.; Stump, T.E.; Ricketts, G.D. Geriatric Care Management for Low-Income Seniors. A Randomized Controlled Trial. JAMA 2007, 298, 2623–2633. [Google Scholar] [CrossRef]
- Fagan, P.J.; Schuster, A.B.; Boyd, C.; Marsteller, J.A.; Griswold, M.; Murphy, S.M.E.; Dunbar, L.; Forrest, C.B. Chronic Care Improvement in Primary Care: Evaluation of an Integrated Pay-for-Performance and Practice-Based Care Coordination Program among Elderly Patients with Diabetes. Health Serv. Res. 2010, 45, 1763–1782. [Google Scholar] [CrossRef]
- Gray, D.; Armstrong, C.D.; Dahrouge, S.; Hogg, W.; Zhang, W. Cost-effectiveness of Anticipatory and Preventive multidisciplinary Team Care for complex patients: Evidence from a randomized controlled trial. Can. Fam. Physician 2010, 56, e20–e29. [Google Scholar]
- ADLIFE. Recommendations for Change Management in Integrated Personalized Care Delivery. 2022. CORDIS Reports. Available online: https://cordis.europa.eu/project/id/875209/results (accessed on 5 July 2022).
- Diamond, A.; Sekhon, J.S. Genetic Matching for Estimating Causal Effects: A General Multivariate Matching Method for Achieving Balance in Observational Studies. Rev. Econ. Stat. 2013, 95, 932–945. [Google Scholar] [CrossRef]
- Akpan, A.; Roberts, C.; Bandeen-Roche, K.; Batty, B.; Bausewein, C.; Bell, D.; Bramley, D.; Bynum, J.; Cameron, I.D.; Chen, L.-K.; et al. Standard set of health outcome measures for older persons. BMC Geriatr. 2018, 18, 36. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.J.; Arora, J.; Okunade, O.; Beltrame, J.F.; Bernardez-Pereira, S.; Crespo-Leiro, M.G.; Filippatos, G.S.; Hardman, S.; Hoes, A.W.; Hutchison, S.; et al. International Consortium for Health Outcomes Measurement (ICHOM): Standardized Patient-Centered Outcomes Measurement Set for Heart Failure Patients. JACC Heart Fail. 2020, 8, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Yusof, M.M.; Kuljis, J.; Papazafeiropoulou, A.; Stergioulas, L.K. An evaluation framework for Health Information Systems: Human, organization and technology-fit factors (HOT-fit). Int. J. Med. Inform. 2008, 77, 386–398. [Google Scholar] [CrossRef]
- Damschroder, L.J.; Aron, D.C.; Keith, R.E.; Kirsh, S.R.; Alexander, J.A.; Lowery, J.C. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implement. Sci. 2009, 4, 50. [Google Scholar] [CrossRef]
- Coleman, E.A.; Eilertsen, T.B.; Kramer, A.M.; Magid, D.J.; Beck, A.; Conner, D. Reducing emergency visits in older adults with chronic illness. A randomized, controlled trial of group visits. Eff. Clin. Pract. ECP 2001, 4, 49–57. [Google Scholar]
- Moe, J.; Kirkland, S.W.; Rawe, E.; Ospina, M.B.; Vandermeer, B.; Campbell, S.; Rowe, B.H. Effectiveness of Interventions to Decrease Emergency Department Visits by Adult Frequent Users: A Systematic Review. Acad. Emerg. Med. 2017, 24, 40–52. [Google Scholar] [CrossRef]
- Mateo-Abad, M.; Fullaondo, A.; Merino, M.; Gris, S.; Marchet, F.; Avolio, F.; Graps, E.; Ravic, M.; Kovac, M.; Benkovic, V.; et al. Impact Assessment of an Innovative Integrated Care Model for Older Complex Patients with Multimorbidity: The CareWell Project. Int. J. Integr. Care 2020, 20, 8. [Google Scholar] [CrossRef]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Graf, C. The Lawton Instrumental Activities of Daily Living Scale. AJN Am. J. Nurs. 2008, 108, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Spertus, J.A.; Jones, P.G.; Sandhu, A.T.; Arnold, S.V. Interpreting the Kansas City Cardiomyopathy Questionnaire in Clinical Trials and Clinical Care. J. Am. Coll. Cardiol. 2020, 76, 2379–2390. [Google Scholar] [CrossRef]
- Jones, P.W.; Harding, G.; Berry, P.; Wiklund, I.; Chen, W.H.; Kline Leidy, N. Development and first validation of the COPD Assessment Test. Eur. Respir. J. 2009, 34, 648–654. [Google Scholar] [CrossRef]
- Mahler, D.A.; Wells, C.K. Evaluation of Clinical Methods for Rating Dyspnea. Chest 1988, 93, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, M.; Davis, D. User Acceptance of Information Technology: Toward a Unified View. MIS Q. 2003, 27, 425. [Google Scholar] [CrossRef]
- Drummond, M.F.; Sculpher, M.J.; Claxton, K.; Stoddart, G.L.; Torrance, G.W. Methods for the Economic Evaluation of Health Care Programmes; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Bédard, M.; Molloy, D.W.; Squire, L.; Dubois, S.; Lever, J.A.; O’Donnell, M. The Zarit Burden Interview. Gerontologist 2001, 41, 652–657. [Google Scholar] [CrossRef]
- Tennant, R.; Hiller, L.; Fishwick, R.; Platt, S.; Joseph, S.; Weich, S.; Parkinson, J.; Secker, J.; Stewart-Brown, S. The Warwick-Edinburgh mental well-being scale (WEMWBS): Development and UK validation. Health Qual. Life Outcomes 2007, 5, 63. [Google Scholar] [CrossRef]
- Bloemeke, J.; Moll, C.; Arndt, F.; González, N.; Ortega, A.; Verdoy, D.; Groene, O. Qualitative Research Protocol as Part of the ADLIFE International Large-Scale Pilot Project. Available online: https://osf.io/stx68 (accessed on 13 July 2022).
- onFHIR Repository. Available online: https://github.com/srdc/onfhir (accessed on 1 October 2022).
- ADLIFE. D1.1 Data Management Plan. Available online: https://ec.europa.eu/research/participants/documents/downloadPublic?documentIds=080166e5d30d0923&appId=PPGMS (accessed on 2 March 2022).
- Vaismoradi, M.; Turunen, H.; Bondas, T. Content analysis and thematic analysis: Implications for conducting a qualitative descriptive study. Nurs. Health Sci. 2013, 15, 398–405. [Google Scholar] [CrossRef]
- Tong, A.; Sainsbury, P.; Craig, J. Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups. Int. J. Qual. Health Care 2007, 19, 349–357. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 16; StataCorp. LLC.: College Station, TX, USA, 2019. [Google Scholar]
- Karnon, J.; Stahl, J.; Brennan, A.; Caro, J.J.; Mar, J.; Möller, J. Modeling using Discrete Event Simulation: A Report of the ISPOR-SMDM Modeling Good Research Practices Task Force-4. Value Health 2012, 15, 821–827. [Google Scholar] [CrossRef] [PubMed]
- ADLIFE. Communication and Dissemination Plan and Communication Material (CORDIS Reports). Available online: https://static1.squarespace.com/static/5e6fed256e0cba537c99c528/t/632d755b1d510e171d53d552/1663923549037/D2.1+Communication+and+dissemination+plan+and+communication+material.pdf (accessed on 31 October 2022).
- Tunis, S.R.; Stryer, D.B.; Clancy, C.M. Practical clinical trials. JAMA 2003, 290, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Skivington, K.; Matthews, L.; Simpson, S.A.; Craig, P.; Baird, J.; Blazeby, J.M.; Boyd, K.A.; Craig, N.; French, D.P.; McIntosh, E.; et al. A new framework for developing and evaluating complex interventions: Update of Medical Research Council guidance. BMJ 2021, 374, n2061. [Google Scholar] [CrossRef]
- Hawe, P.; Shiell, A.; Riley, T. Complex interventions: How “out of control” can a randomised controlled trial be? BMJ 2004, 328, 1561–1563. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Lorenzo, B.; Gorostiza, A.; González, N.; Larrañaga, I.; Mateo-Abad, M.; Ortega-Gil, A.; Bloemeke, J.; Groene, O.; Vergara, I.; Mar, J.; et al. Assessment of the Effectiveness, Socio-Economic Impact and Implementation of a Digital Solution for Patients with Advanced Chronic Diseases: The ADLIFE Study Protocol. Int. J. Environ. Res. Public Health 2023, 20, 3152. https://doi.org/10.3390/ijerph20043152
García-Lorenzo B, Gorostiza A, González N, Larrañaga I, Mateo-Abad M, Ortega-Gil A, Bloemeke J, Groene O, Vergara I, Mar J, et al. Assessment of the Effectiveness, Socio-Economic Impact and Implementation of a Digital Solution for Patients with Advanced Chronic Diseases: The ADLIFE Study Protocol. International Journal of Environmental Research and Public Health. 2023; 20(4):3152. https://doi.org/10.3390/ijerph20043152
Chicago/Turabian StyleGarcía-Lorenzo, Borja, Ania Gorostiza, Nerea González, Igor Larrañaga, Maider Mateo-Abad, Ana Ortega-Gil, Janika Bloemeke, Oliver Groene, Itziar Vergara, Javier Mar, and et al. 2023. "Assessment of the Effectiveness, Socio-Economic Impact and Implementation of a Digital Solution for Patients with Advanced Chronic Diseases: The ADLIFE Study Protocol" International Journal of Environmental Research and Public Health 20, no. 4: 3152. https://doi.org/10.3390/ijerph20043152
APA StyleGarcía-Lorenzo, B., Gorostiza, A., González, N., Larrañaga, I., Mateo-Abad, M., Ortega-Gil, A., Bloemeke, J., Groene, O., Vergara, I., Mar, J., Lim Choi Keung, S. N., Arvanitis, T. N., Kaye, R., Dahary Halevy, E., Nahir, B., Arndt, F., Dichmann Sorknæs, A., Juul, N. K., Lilja, M., ... de Manuel Keenoy, E., on behalf of the ADLIFE Study Group. (2023). Assessment of the Effectiveness, Socio-Economic Impact and Implementation of a Digital Solution for Patients with Advanced Chronic Diseases: The ADLIFE Study Protocol. International Journal of Environmental Research and Public Health, 20(4), 3152. https://doi.org/10.3390/ijerph20043152






