Seasonal Oxy-Inflammation and Hydration Status in Non-Elite Freeskiing Racer: A Pilot Study by Non-Invasive Analytic Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
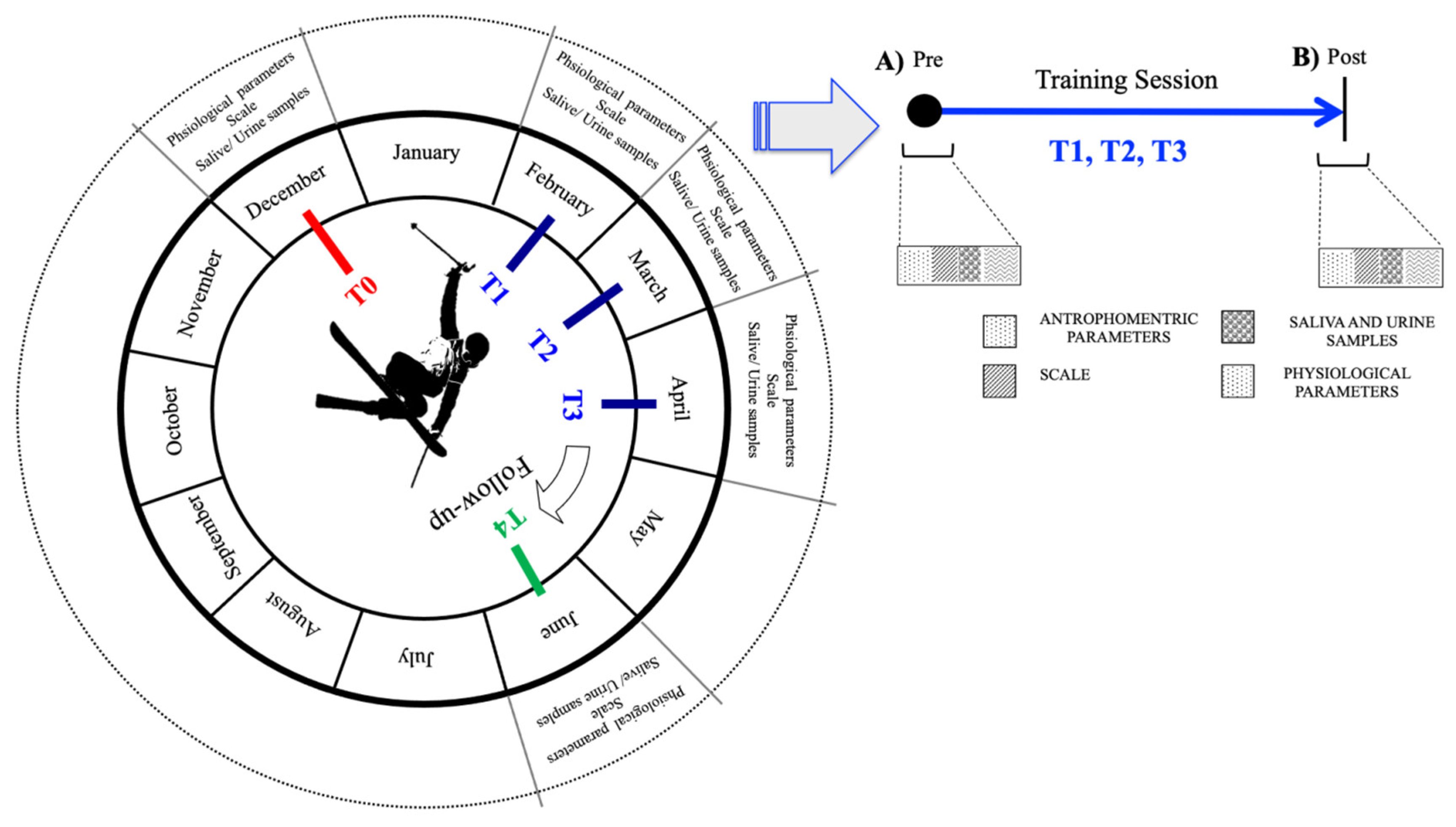
2.2. Experimental Design
- In December (T0), before the season beginning. These were basal measures;
- In February (T1), at the start of training session;
- In March (T2), during the middle period of the season;
- In April (T3), at the end of the season;
- In June (T4), two months after the end of the season.
Training Exercise Session
- 9.00–9.15 a.m.: dry land warm-up (stretching) before going to ski run;
- 9.15–10.00 a.m.: warm-up on skin run (flat tricks);
- 10.00–10.15 a.m.: speed and snow condition check;
- 10.15–11.30 a.m.: riding in snow park, Part 1 (jumps, rails, jibbing, and slang);
- 11.30–11.45 a.m.: break;
- 11.45 a.m.–1.00 p.m.: riding in snow park, Part 2 (jumps, rails, jibbing, and slang).
- Hand Drag 180, a 180° turn involving landing on the hands while the legs are up in the air; and Hand Drag 360, a full turn with the legs up in the air while landing on the hands.
- Nollie, a jump on a flat slope where skiers push from their noses; and Nollie 180, a jump similar to Nollie with a 180° turn.
- Nose Butter 360, a 360° rotation around the vertical axis with pressure being put on the noses; and Nose Butter 540, a 540° rotation around the vertical axis with pressure being put on the noses and back end.
- Switch Tail Butter 360, a 360° rotation around the vertical axis with pressure being put on the tails, performed from switch.
- -
- 1st 100 m: 2 Nose Butter 360 and 1 Nose Butter 540;
- -
- 2nd 100 m: 3 Switch Tail Butter 360;
- -
- 3th 100 m: 2 Nose Butter 360 and 2 Nose Butter 540;
- -
- 4th 100 m: 2 Nollie and 2 Nollie 180;
- -
- 5th 100 m: 2 Switch Tail Butter 360, 1 Hand Drag 180, and 1 Hand Drag 360.
2.3. Sample Collection
2.4. Biomarkers Assessment
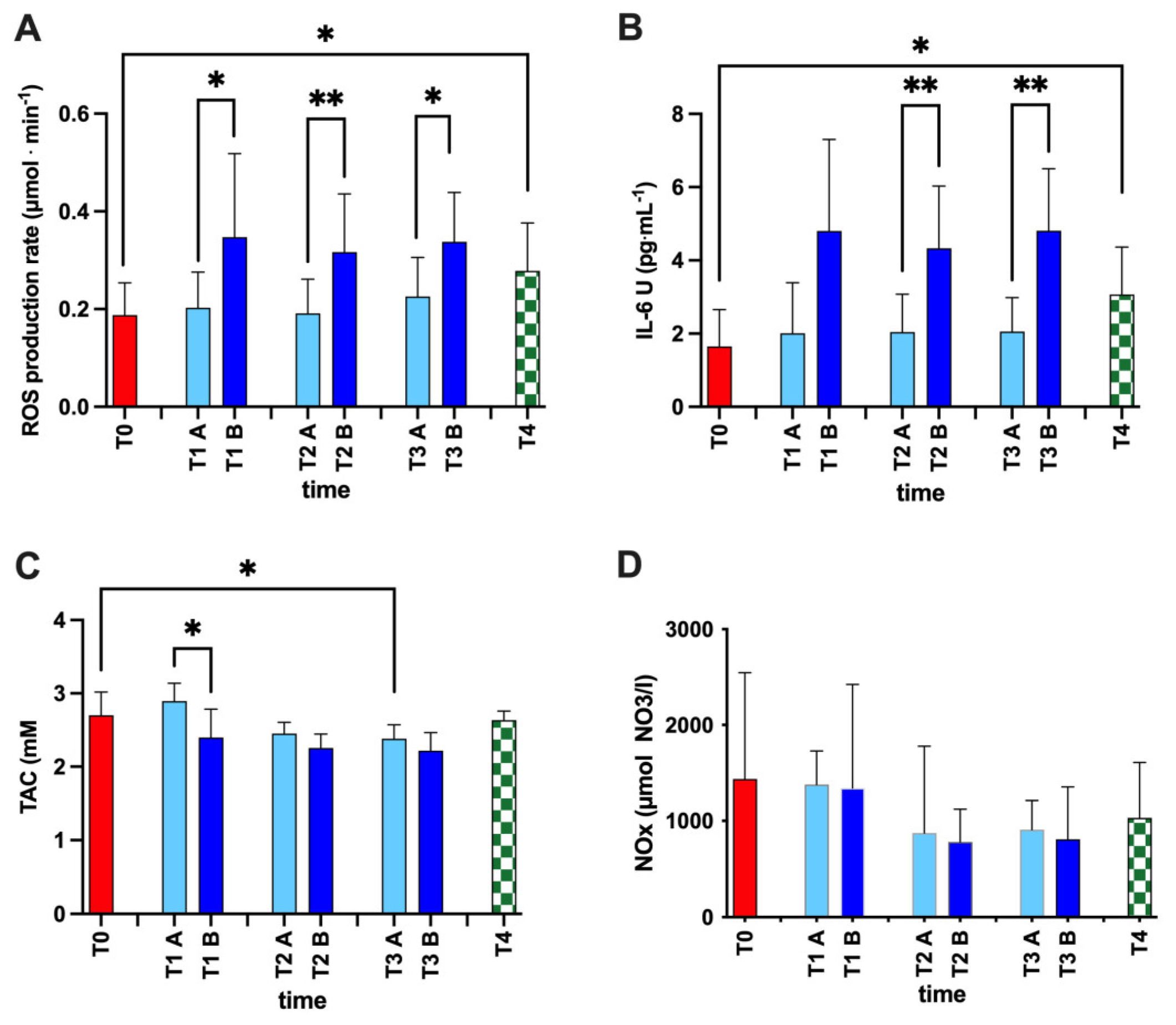
2.4.1. Reactive Oxygen Species (ROS) and Total Antioxidant Capacity (TAC)
2.4.2. Interleukin-6 (IL-6)
2.4.3. NO Metabolites
2.4.4. Creatinine, Uric Acid, and Electrolytes
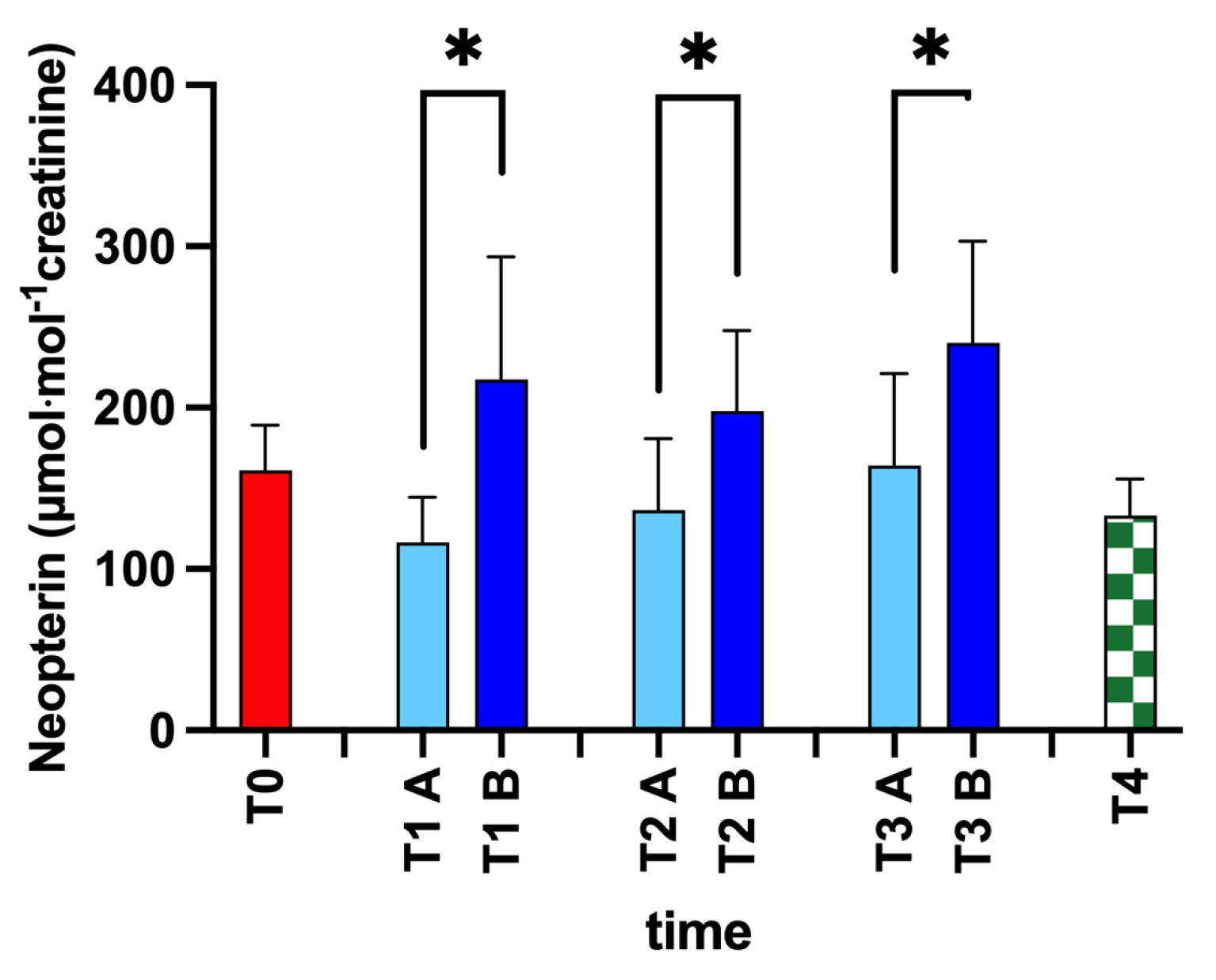
2.4.5. Neopterin
2.4.6. Urine Test Strip
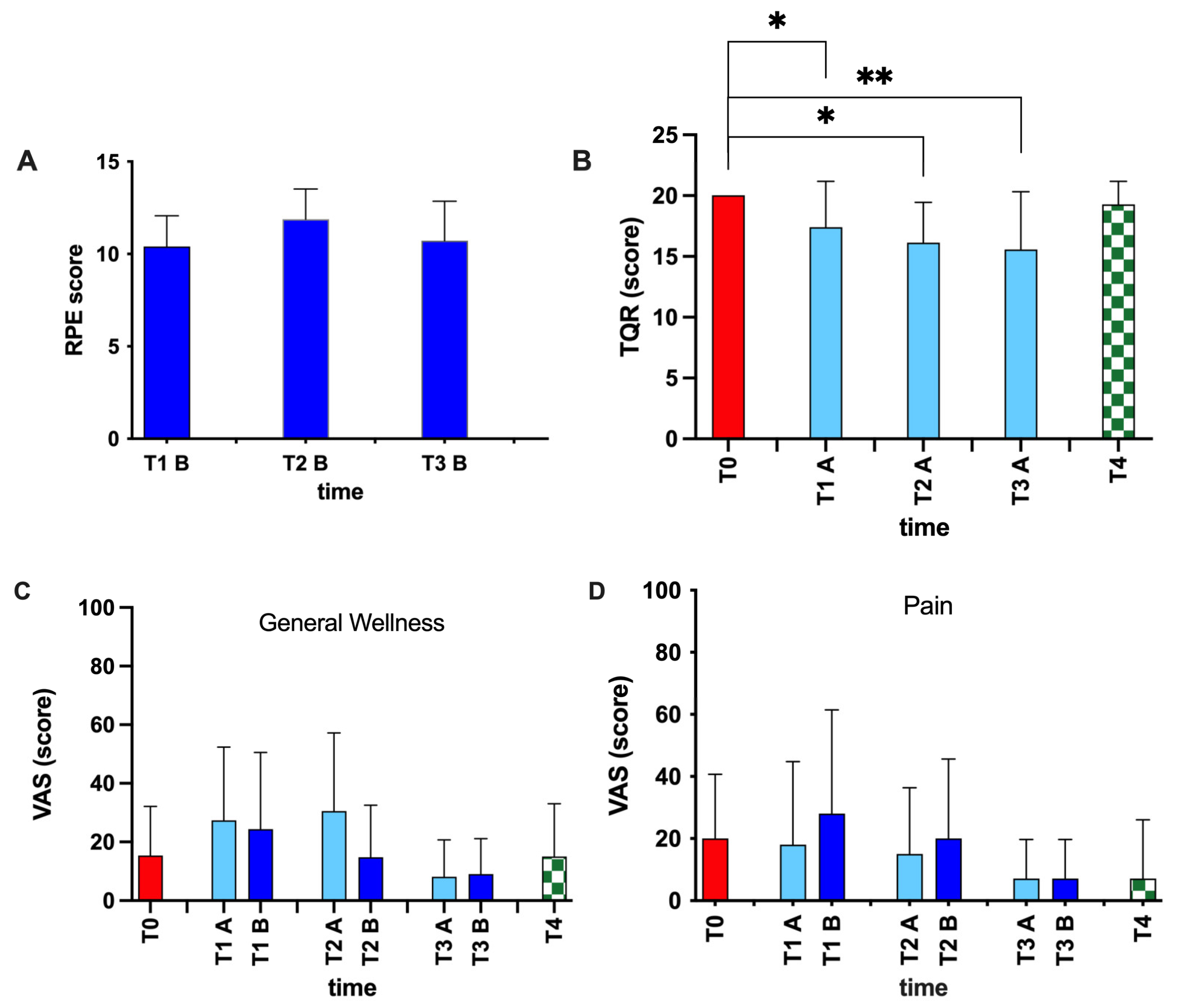
2.5. Scale for Assessment of Physical Fatigue and Recovery
2.6. Statistical Analysis
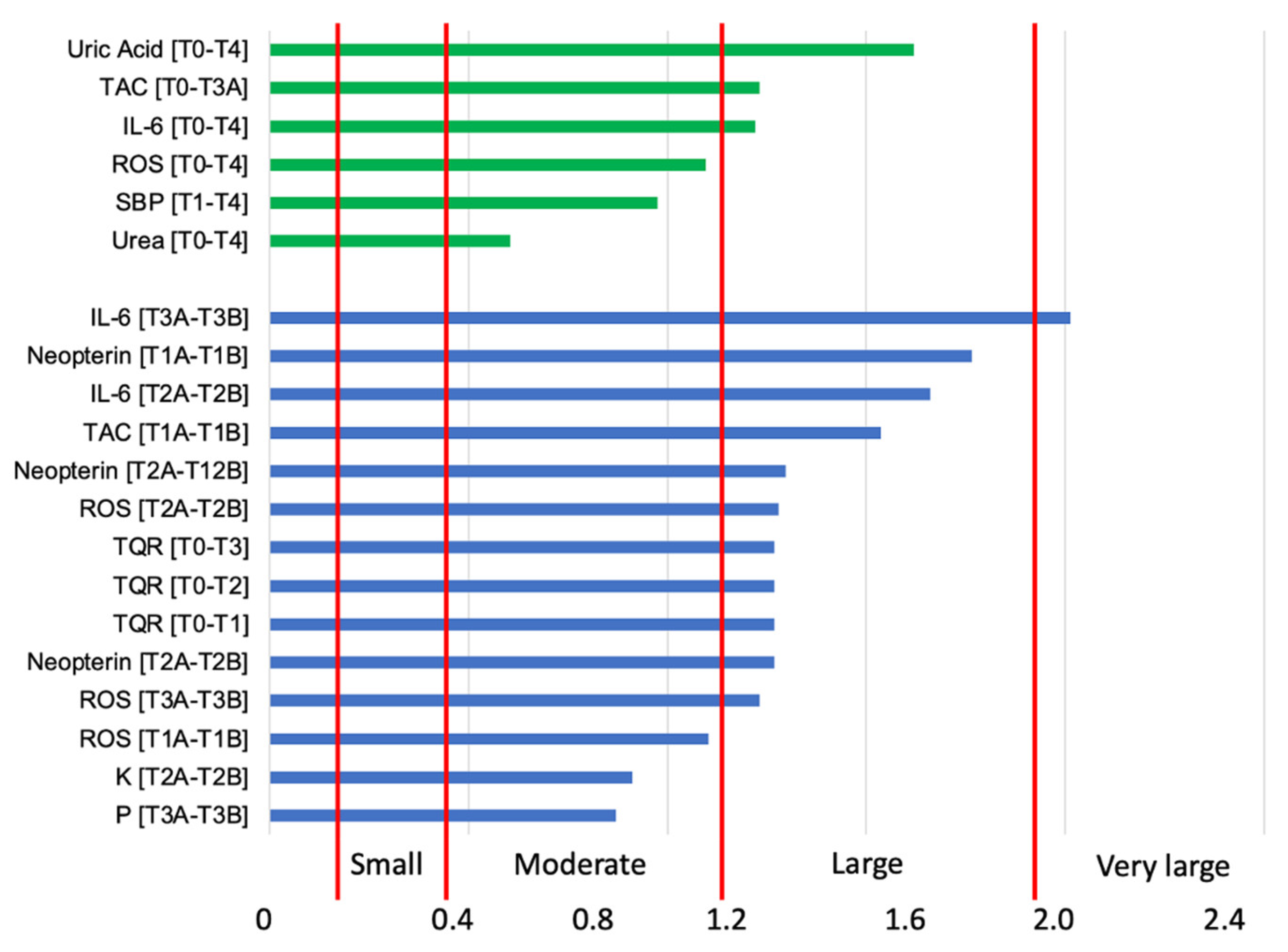
3. Results
4. Discussion
5. Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ca2+ | Calcium |
| Cl− | Chlorine |
| EPR | Electron paramagnetic resonance |
| HPLC | High-pressure liquid chromatography |
| IL-6 | Interleukin-6 |
| K+ | Potassium |
| Mg2+ | Magnesium |
| Na+ | Sodium |
| NO | Nitric oxide |
| NO2 | Nitrite |
| NO3 | Nitrate |
| NOx | NO metabolites |
| P | Phosphorus |
| POMS | Profile of Mood States |
| ROS | Reactive oxygen species |
| RPE | Rate of Perceived Exertion |
| SpO2 | Peripheral arterial oxygen saturation |
| TAC | Total antioxidant capacity |
| TQR | Total Quality of Recovery |
| VAS | Visual Analog Scale |
References
- Kurpiers, N.; McAlpine, P.R.; Kersting, U.G. Perspectives for comprehensive biomechanical analyses in Mogul skiing. Res. Sport. Med. 2009, 17, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, M.G.; Jamurtas, A.Z. Blood as a reactive species generator and redox status regulator during exercise. Arch. Biochem. Biophys. 2009, 490, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. CB 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- Mrakic-Sposta, S.; Gussoni, M.; Montorsi, M.; Porcelli, S.; Vezzoli, A. Assessment of a standardized ROS production profile in humans by electron paramagnetic resonance. Oxidative Med. Cell. Longev. 2012, 2012, 973927. [Google Scholar] [CrossRef]
- Vezzoli, A.; Dellanoce, C.; Mrakic-Sposta, S.; Montorsi, M.; Moretti, S.; Tonini, A.; Pratali, L.; Accinni, R. Oxidative Stress Assessment in Response to Ultraendurance Exercise: Thiols Redox Status and ROS Production according to Duration of a Competitive Race. Oxidative Med. Cell. Longev. 2016, 2016, 6439037. [Google Scholar] [CrossRef]
- Souissi, W.; Bouzid, M.A.; Farjallah, M.A.; Ben Mahmoud, L.; Boudaya, M.; Engel, F.A.; Sahnoun, Z. Effect of Different Running Exercise Modalities on Post-Exercise Oxidative Stress Markers in Trained Athletes. Int. J. Environ. Res. Public Health 2020, 17, 3729. [Google Scholar] [CrossRef]
- Done, A.J.; Traustadottir, T. Nrf2 mediates redox adaptations to exercise. Redox Biol. 2016, 10, 191–199. [Google Scholar] [CrossRef]
- Steller, H. Mechanisms and genes of cellular suicide. Science 1995, 267, 1445–1449. [Google Scholar] [CrossRef]
- Liu, J.F.; Chang, W.Y.; Chan, K.H.; Tsai, W.Y.; Lin, C.L.; Hsu, M.C. Blood lipid peroxides and muscle damage increased following intensive resistance training of female weightlifters. Ann. N. Y. Acad. Sci. 2005, 1042, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Araneda, O.F.; Garcia, C.; Lagos, N.; Quiroga, G.; Cajigal, J.; Salazar, M.P.; Behn, C. Lung oxidative stress as related to exercise and altitude. Lipid peroxidation evidence in exhaled breath condensate: A possible predictor of acute mountain sickness. Eur. J. Appl. Physiol. 2005, 95, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Pialoux, V.; Mounier, R.; Ponsot, E.; Rock, E.; Mazur, A.; Dufour, S.; Richard, R.; Richalet, J.P.; Coudert, J.; Fellmann, N. Effects of exercise and training in hypoxia on antioxidant/pro-oxidant balance. Eur. J. Clin. Nutr. 2006, 60, 1345–1354. [Google Scholar] [CrossRef]
- Schippinger, G.; Fankhauser, F.; Abuja, P.M.; Winklhofer-Roob, B.M.; Nadlinger, K.; Halwachs-Baumann, G.; Wonisch, W. Competitive and seasonal oxidative stress in elite alpine ski racers. Scand. J. Med. Sci. Sport. 2009, 19, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Wonisch, W.; Uhl, K.; Schimetta, W.; Rabl, H. Pre-operative delivery of a vitamin cocktail diminished oxidative stress after vascular surgery in PAD patients—A pilot investigation. Biofactors 2005, 24, 299–303. [Google Scholar] [CrossRef]
- Oral, O. Nitric oxide and its role in exercise physiology. J. Sport. Med. Phys. Fit. 2021, 61, 1208–1211. [Google Scholar] [CrossRef]
- Elliott, S.N.; Wallace, J.L. Nitric oxide: A regulator of mucosal defense and injury. J. Gastroenterol. 1998, 33, 792–803. [Google Scholar] [CrossRef]
- Munzel, T.; Gori, T.; Bruno, R.M.; Taddei, S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur. Heart J. 2010, 31, 2741–2748. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Porcelli, S.; Pugliese, L.; Pavei, G.; Bellistri, G.; Montorsi, M.; Tacchini, P.; Vezzoli, A. Training effects on ROS production determined by electron paramagnetic resonance in master swimmers. Oxidative Med. Cell. Longev. 2015, 2015, 804794. [Google Scholar] [CrossRef]
- Miyazaki, H.; Ohishi, S.; Ookawara, T.; Kizaki, T.; Toshinai, K.; Ha, S.; Haga, S.; Ji, L.L.; Ohno, H. Strenuous endurance training in humans reduces oxidative stress following exhausting exercise. Eur. J. Appl. Physiol. 2001, 84, 1–6. [Google Scholar] [CrossRef]
- McKenzie, D.C. Markers of excessive exercise. Can. J. Appl. Physiol. 1999, 24, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Tiidus, P.M. Radical species in inflammation and overtraining. Can. J. Physiol. Pharmacol. 1998, 76, 533–538. [Google Scholar] [CrossRef]
- Meyer, N.L.; Manore, M.M.; Helle, C. Nutrition for winter sports. J. Sport. Sci. 2011, 29 (Suppl. 1), S127–S136. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.; Young, A.J.; Sawka, M.N. Hypohydration and thermoregulation in cold air. J. Appl. Physiol. 1998, 84, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Radomski, M.W.; Boutelier, C. Hormone response of normal and intermittent cold-preadapted humans to continuous cold. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1982, 53, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Seifert, J.G.; Burke, E.R.; White, A.; Luetkemeier, M.J. The effects of ad libitum fluid ingestion on fluid balance during alpine skiing in recreational skiers. J. Sport. Sci. 2006, 24, 137–142. [Google Scholar] [CrossRef]
- Keller, U.; Szinnai, G.; Bilz, S.; Berneis, K. Effects of changes in hydration on protein, glucose and lipid metabolism in man: Impact on health. Eur. J. Clin. Nutr. 2003, 57 (Suppl. 2), S69–S74. [Google Scholar] [CrossRef]
- Murray, B.; Rosenbloom, C. Fundamentals of glycogen metabolism for coaches and athletes. Nutr. Rev. 2018, 76, 243–259. [Google Scholar] [CrossRef]
- Giacon, T.A.; Bosco, G.; Vezzoli, A.; Dellanoce, C.; Cialoni, D.; Paganini, M.; Mrakic-Sposta, S. Oxidative stress and motion sickness in one crew during competitive offshore sailing. Sci. Rep. 2022, 12, 1142. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Vezzoli, A.; D’Alessandro, F.; Paganini, M.; Dellanoce, C.; Cialoni, D.; Bosco, G. Change in Oxidative Stress Biomarkers During 30 Days in Saturation Dive: A Pilot Study. Int. J. Environ. Res. Public Health 2020, 17, 7118. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Montorsi, M.; Porcelli, S.; Vezzoli, A. A quantitative method to monitor reactive oxygen species production by electron paramagnetic resonance in physiological and pathological conditions. Oxidative Med. Cell. Longev. 2014, 2014, 306179. [Google Scholar] [CrossRef] [PubMed]
- Mrakic-Sposta, S.; Vezzoli, A.; Rizzato, A.; Della Noce, C.; Malacrida, S.; Montorsi, M.; Paganini, M.; Cancellara, P.; Bosco, G. Oxidative stress assessment in breath-hold diving. Eur. J. Appl. Physiol. 2019, 119, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Mrakic-Sposta, S.; Gussoni, M.; Vezzoli, A.; Dellanoce, C.; Comassi, M.; Giardini, G.; Bruno, R.M.; Montorsi, M.; Corciu, A.; Greco, F.; et al. Acute Effects of Triathlon Race on Oxidative Stress Biomarkers. Oxidative Med. Cell. Longev. 2020, 2020, 3062807. [Google Scholar] [CrossRef] [PubMed]
- Bosco, G.; Giacon, T.A.; Paolocci, N.; Vezzoli, A.; Noce, C.D.; Paganini, M.; Agrimi, J.; Garetto, G.; Cialoni, D.; D’Alessandro, N.; et al. Dopamine/BDNF loss underscores narcosis cognitive impairment in divers: A proof of concept in a dry condition. Eur. J. Appl. Physiol. 2022, 123, 143–158. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Moretti, S.; Pratali, L.; Giardini, G.; Tacchini, P.; Dellanoce, C.; Tonacci, A.; Mastorci, F.; Borghini, A.; et al. Effects of Mountain Ultra-Marathon Running on ROS Production and Oxidative Damage by Micro-Invasive Analytic Techniques. PLoS ONE 2015, 10, e0141780. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sport. Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Kentta, G.; Hassmen, P. Overtraining and recovery. A conceptual model. Sport. Med. 1998, 26, 1–16. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Vezzoli, A.; Maderna, L.; Gregorini, F.; Montorsi, M.; Moretti, S.; Greco, F.; Cova, E.; Gussoni, M. R(+)-Thioctic Acid Effects on Oxidative Stress and Peripheral Neuropathy in Type II Diabetic Patients: Preliminary Results by Electron Paramagnetic Resonance and Electroneurography. Oxidative Med. Cell. Longev. 2018, 2018, 1767265. [Google Scholar] [CrossRef]
- Morfeld, M.; Petersen, C.; Kruger-Bodeker, A.; von Mackensen, S.; Bullinger, M. The assessment of mood at workplace—Psychometric analyses of the revised Profile of Mood States (POMS) questionnaire. Psychosoc. Med. 2007, 4, Doc06. [Google Scholar]
- McNair, D.; Lorr, M.; Droppleman, L.F. Manual for the Profile of Mood States; Educational and Industrial Testing Service: San Diego, CA, USA, 1971. [Google Scholar]
- Meeusen, R.; Duclos, M.; Foster, C.; Fry, A.; Gleeson, M.; Nieman, D.; Raglin, J.; Rietjens, G.; Steinacker, J.; Urhausen, A.; et al. Prevention, diagnosis, and treatment of the overtraining syndrome: Joint consensus statement of the European College of Sport Science and the American College of Sports Medicine. Med. Sci. Sport. Exerc. 2013, 45, 186–205. [Google Scholar] [CrossRef]
- Knez, W.L.; Jenkins, D.G.; Coombes, J.S. Oxidative stress in half and full Ironman triathletes. Med. Sci. Sport. Exerc. 2007, 39, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Palazzetti, S.; Richard, M.J.; Favier, A.; Margaritis, I. Overloaded training increases exercise-induced oxidative stress and damage. Can. J. Appl. Physiol. Rev. Can. De Physiol. Appl. 2003, 28, 588–604. [Google Scholar] [CrossRef] [PubMed]
- Schippinger, G.; Wonisch, W.; Abuja, P.M.; Fankhauser, F.; Winklhofer-Roob, B.M.; Halwachs, G. Lipid peroxidation and antioxidant status in professional American football players during competition. Eur. J. Clin. Investig. 2002, 32, 686–692. [Google Scholar] [CrossRef]
- Reid, M.B. Invited Review: Redox modulation of skeletal muscle contraction: What we know and what we don’t. J. Appl. Physiol. 2001, 90, 724–731. [Google Scholar] [CrossRef]
- Powers, S.K.; Ji, L.L.; Kavazis, A.N.; Jackson, M.J. Reactive oxygen species: Impact on skeletal muscle. Compr. Physiol. 2011, 1, 941–969. [Google Scholar]
- Powers, S.K.; Duarte, J.; Kavazis, A.N.; Talbert, E.E. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp. Physiol. 2010, 95, 1–9. [Google Scholar] [CrossRef]
- Cavarretta, E.; Peruzzi, M.; Frati, G.; Sciarretta, S. When enough is more than enough: The hidden side of the cardiac effects of intense physical exercise. Int. J. Cardiol. 2018, 258, 224–225. [Google Scholar] [CrossRef]
- Finaud, J.; Scislowski, V.; Lac, G.; Durand, D.; Vidalin, H.; Robert, A.; Filaire, E. Antioxidant status and oxidative stress in professional rugby players: Evolution throughout a season. Int. J. Sport. Med. 2006, 27, 87–93. [Google Scholar] [CrossRef]
- Cialoni, D.; Brizzolari, A.; Samaja, M.; Bosco, G.; Paganini, M.; Pieri, M.; Lancellotti, V.; Marroni, A. Nitric Oxide and Oxidative Stress Changes at Depth in Breath-Hold Diving. Front. Physiol. 2020, 11, 609642. [Google Scholar] [CrossRef]
- Seifi-Skishahr, F.; Damirchi, A.; Farjaminezhad, M.; Babaei, P. Physical Training Status Determines Oxidative Stress and Redox Changes in Response to an Acute Aerobic Exercise. Biochem. Res. Int. 2016, 2016, 3757623. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.P.; Bolin, A.P.; Campoio, T.R.; Guerra, B.A.; Otton, R. Oxidative stress and antioxidant status response of handball athletes: Implications for sport training monitoring. Int. Immunopharmacol. 2013, 17, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Subudhi, A.W.; Davis, S.L.; Kipp, R.W.; Askew, E.W. Antioxidant status and oxidative stress in elite alpine ski racers. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Vezzoli, A.; Pugliese, L.; Marzorati, M.; Serpiello, F.R.; La Torre, A.; Porcelli, S. Time-course changes of oxidative stress response to high-intensity discontinuous training versus moderate-intensity continuous training in masters runners. PLoS ONE 2014, 9, e87506. [Google Scholar] [CrossRef] [PubMed]
- Jonsdottir, I.H.; Schjerling, P.; Ostrowski, K.; Asp, S.; Richter, E.A.; Pedersen, B.K. Muscle contractions induce interleukin-6 mRNA production in rat skeletal muscles. J. Physiol. 2000, 528 Pt 1, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Steensberg, A.; Schjerling, P. Muscle-derived interleukin-6: Possible biological effects. J. Physiol. 2001, 536 Pt 2, 329–337. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Pedersen, B.K. Muscle-derived interleukin-6: Mechanisms for activation and possible biological roles. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2002, 16, 1335–1347. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Steensberg, A.; Fischer, C.; Keller, C.; Keller, P.; Plomgaard, P.; Wolsk-Petersen, E.; Febbraio, M.A. The metabolic role of IL-6 produced during exercise: Is IL-6 an exercise factor? Proc. Nutr. Soc. 2004, 63, 263–267. [Google Scholar] [CrossRef]
- Bruunsgaard, H.; Galbo, H.; Halkjaer-Kristensen, J.; Johansen, T.L.; MacLean, D.A.; Pedersen, B.K. Exercise-induced increase in serum interleukin-6 in humans is related to muscle damage. J. Physiol. 1997, 499 Pt 3, 833–841. [Google Scholar] [CrossRef]
- Bailey, S.J.; Fulford, J.; Vanhatalo, A.; Winyard, P.G.; Blackwell, J.R.; DiMenna, F.J.; Wilkerson, D.P.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J. Appl. Physiol. 2010, 109, 135–148. [Google Scholar] [CrossRef]
- Larsen, F.J.; Schiffer, T.A.; Borniquel, S.; Sahlin, K.; Ekblom, B.; Lundberg, J.O.; Weitzberg, E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011, 13, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J.S.; Meissner, G. Physiology of nitric oxide in skeletal muscle. Physiol. Rev. 2001, 81, 209–237. [Google Scholar] [CrossRef]
- Owen-Smith, B.; Quiney, J.; Read, J. Salivary urate in gout, exercise, and diurnal variation. Lancet 1998, 351, 1932. [Google Scholar] [CrossRef] [PubMed]
- Sutton, J.R.; Toews, C.J.; Ward, G.R.; Fox, I.H. Purine metabolism during strenuous muscular exercise in man. Metab. Clin. Exp. 1980, 29, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.L.; Huang, C.T.; Chen, M.L.; Mao, I.F. Effects of profuse sweating induced by exercise on urinary uric acid excretion in a hot environment. Chin. J. Physiol. 2010, 53, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Poortmans, J.R. Exercise and renal function. Sport. Med. 1984, 1, 125–153. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Moriwaki, Y.; Takahashi, S.; Nasako, Y.; Higashino, K. Effect of lactate infusion on renal transport of purine bases and oxypurinol. Nephron 1993, 65, 73–76. [Google Scholar] [CrossRef]
- Yamamoto, T.; Moriwaki, Y.; Takahashi, S.; Tsutsumi, Z.; Hada, T. Effect of norepinephrine on the urinary excretion of purine bases and oxypurinol. Metab. Clin. Exp. 2001, 50, 1230–1233. [Google Scholar] [CrossRef]
- Stathis, C.G.; Carey, M.F.; Snow, R.J. The influence of allopurinol on urinary purine loss after repeated sprint exercise in man. Metab. Clin. Exp. 2005, 54, 1269–1275. [Google Scholar] [CrossRef]
- Knochel, J.P.; Dotin, L.N.; Hamburger, R.J. Heat stress, exercise, and muscle injury: Effects on urate metabolism and renal function. Ann. Intern. Med. 1974, 81, 321–328. [Google Scholar] [CrossRef]
- Hellsten, Y.; Sjodin, B.; Richter, E.A.; Bangsbo, J. Urate uptake and lowered ATP levels in human muscle after high-intensity intermittent exercise. Am. J. Physiol. 1998, 274, E600–E606. [Google Scholar] [CrossRef] [PubMed]
- Weiner, I.D.; Mitch, W.E.; Sands, J.M. Urea and Ammonia Metabolism and the Control of Renal Nitrogen Excretion. Clin. J. Am. Soc. Nephrol. CJASN 2015, 10, 1444–1458. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Guttman, H.N. Trace Minerals and Exercise. In Exercise, Nutrition, and Energy Metabolism; Macmillan: New York, NY, USA, 1988. [Google Scholar]
- Ashizawa, N.; Fujimura, R.; Tokuyama, K.; Suzuki, M. A bout of resistance exercise increases urinary calcium independently of osteoclastic activation in men. J. Appl. Physiol. 1997, 83, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Noakes, T.D.; Sharwood, K.; Speedy, D.; Hew, T.; Reid, S.; Dugas, J.; Almond, C.; Wharam, P.; Weschler, L. Three independent biological mechanisms cause exercise-associated hyponatremia: Evidence from 2135 weighed competitive athletic performances. Proc. Natl. Acad. Sci. USA 2005, 102, 18550–18555. [Google Scholar] [CrossRef] [PubMed]
- de Lucas, R.D.; Caputo, F.; Mendes de Souza, K.; Sigwalt, A.R.; Ghisoni, K.; Lock Silveira, P.C.; Remor, A.P.; da Luz Scheffer, D.; Guglielmo, L.G.; Latini, A. Increased platelet oxidative metabolism, blood oxidative stress and neopterin levels after ultra-endurance exercise. J. Sport. Sci. 2014, 32, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Strasser, B.; Geiger, D.; Schauer, M.; Gatterer, H.; Burtscher, M.; Fuchs, D. Effects of Exhaustive Aerobic Exercise on Tryptophan-Kynurenine Metabolism in Trained Athletes. PLoS ONE 2016, 11, e0153617. [Google Scholar] [CrossRef]
- White, R.L.; Babic, M.J.; Parker, P.D.; Lubans, D.R.; Astell-Burt, T.; Lonsdale, C. Domain-Specific Physical Activity and Mental Health: A Meta-analysis. Am. J. Prev. Med. 2017, 52, 653–666. [Google Scholar] [CrossRef]
- Stubbs, B.; Koyanagi, A.; Hallgren, M.; Firth, J.; Richards, J.; Schuch, F.; Rosenbaum, S.; Mugisha, J.; Veronese, N.; Lahti, J.; et al. Physical activity and anxiety: A perspective from the World Health Survey. J. Affect. Disord. 2017, 208, 545–552. [Google Scholar] [CrossRef]
- Stubbs, B.; Koyanagi, A.; Schuch, F.; Firth, J.; Rosenbaum, S.; Gaughran, F.; Mugisha, J.; Vancampfort, D. Physical Activity Levels and Psychosis: A Mediation Analysis of Factors Influencing Physical Activity Target Achievement Among 204 186 People Across 46 Low- and Middle-Income Countries. Schizophr. Bull. 2017, 43, 536–545. [Google Scholar] [CrossRef]
- Nixdorf, I.; Frank, R.; Beckmann, J. Comparison of Athletes’ Proneness to Depressive Symptoms in Individual and Team Sports: Research on Psychological Mediators in Junior Elite Athletes. Front. Psychol. 2016, 7, 893. [Google Scholar] [CrossRef]
- Callaghan, P. Exercise: A neglected intervention in mental health care? J. Psychiatr. Ment. Health Nurs. 2004, 11, 476–483. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Time Point | ||||
|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | |
| SpO2(%) | 97.0 ± 0.9 | 97.4± 1.1 | 97.3± 1.1 | 97.1± 1.0 | 97.5± 1.3 |
| SBP(mmHg) | 135.6 ± 24.9 | 120.0 ± 7.3 | 116.9 ± 16.4 | 117.9 ± 5.8 | 116.5 ± 12.1 * |
| DBP(mmHg) | 79.5 ± 11.3 | 76.8 ± 5.4 | 65.2± 5.9 | 67.6± 8.7 | 74.0 ± 7.5 |
| Time Point | ||||||||
|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | ||||
| A | B | A | B | A | B | |||
| Uric Acid (mg/dL) | 9.3 ± 4.6 | 16.4 ± 18.5 | 32.1 ± 26.1 | 21.8 ± 25.0 | 30.4 ± 19.5 | 25.6 ± 15.1 | 23.4 ± 14.3 | 41.9 ± 28.1 * |
| Urea (mg/dL) | 984.0 ± 346.3 | 1555.0 ± 362.7 | 1225.0 ± 184.0 | 1058.0 ± 255.4 | 1040 ± 373.4 | 1426.0 ± 386.3 | 1028.0 ± 546.1 | 736.9 ± 351.6 * |
| Na (mmol/L) | 106.6 ± 39.7 | 144.8 ± 17.7 | 191.6 ± 59.7 | 153.6 ± 60.5 | 198.6 ± 50.4 | 148.3 ± 64.1 | 139.9 ± 95.7 | 131.7 ± 68.2 |
| K (mmol/L) | 61.5 ± 30.6 | 62.9 ± 22.7 | 95.7 ± 36.9 | 60.5 ± 35.1 | 92.0 ± 34.0 †† | 40.0 ± 9.0 | 43.7 ± 20.6 | 62.7 ± 38.1 |
| Cl (mmol/L) | 124.8 ± 43.5 | 122.0 ± 44.1 | 204.0 ± 73.9 | 157.0 ± 78.7 | 224.1 ± 50.1 | 125.4 ± 53.1 | 138.0 ± 81.6 | 149.0 ± 58.3 |
| P (mg/dL) | 106.3 ± 58.6 | 142.3 ± 71.3 | 66.6 ± 41.2 | 81.1± 36.6 | 50.8 ± 20.4 | 105.7 ± 51.6 | 54.2 ± 65.6 † | 53.2 ± 35.1 |
| Mg (mg/dL) | 5.0 ± 5.1 | 15.2 ± 8.7 | 6.7 ± 4.2 | 8.4 ± 3.2 | 6.3 ± 3.1 | 11.9 ± 5.1 | 7.9 ± 7.4 | 5.8 ± 3.0 |
| Ca (mg/dL) | 7.9 ± 8.0 | 16.0 ± 5.8 | 10.2 ± 7.9 | 13.3 ± 5.0 | 11.6 ± 6.0 | 15.4 ± 6.6 | 10.7 ± 7.1 | 9.8 ± 5.1 |
| Total proteins (mg/dL) | 51.3 ± 3.5 | 50.0 ± 1.0 | 81.2 ± 69.7 | 50.0 ± 1.0 | 50.0 ± 1.0 | 50.0 ± 1.0 | 70.6 ± 30.8 | 66.7 ± 19.4 |
| Time Point | |||||
|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | |
| Bilirubin (μmol·L−1) | 4.3 ± 12.37 | 3.1 ± 7.8 | 3.9 ± 7.5 | 4.1 ± 6.6 | 4.9 ± 8.7 |
| Urobilinogen (μmol·L−1) | 0.1 ± 0.07 | 0.2 ± 0.2 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.8 |
| Ketones (mmol·L−1) | 1.0 ± 5.3 | 1.3 ± 4.4 | 2.5 ± 2.5 | 2.9 ± 3.6 | 2.6 ± 3.3 |
| pH | 5.9 ± 0.6 | 6.3 ± 0.5 | 6.3 ± 0.5 | 6.2 ± 0.5 | 6.2 ± 0.5 |
| Leucocytes (Leuko·μL−1) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Specific gravity | 1.02 ± 0.0 | 1.02 ± 0.0 | 1.02 ± 0.0 | 1.02 ± 0.0 | 1.02 ± 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brizzolari, A.; Bosco, G.; Vezzoli, A.; Dellanoce, C.; Barassi, A.; Paganini, M.; Cialoni, D.; Mrakic-Sposta, S. Seasonal Oxy-Inflammation and Hydration Status in Non-Elite Freeskiing Racer: A Pilot Study by Non-Invasive Analytic Method. Int. J. Environ. Res. Public Health 2023, 20, 3157. https://doi.org/10.3390/ijerph20043157
Brizzolari A, Bosco G, Vezzoli A, Dellanoce C, Barassi A, Paganini M, Cialoni D, Mrakic-Sposta S. Seasonal Oxy-Inflammation and Hydration Status in Non-Elite Freeskiing Racer: A Pilot Study by Non-Invasive Analytic Method. International Journal of Environmental Research and Public Health. 2023; 20(4):3157. https://doi.org/10.3390/ijerph20043157
Chicago/Turabian StyleBrizzolari, Andrea, Gerardo Bosco, Alessandra Vezzoli, Cinzia Dellanoce, Alessandra Barassi, Matteo Paganini, Danilo Cialoni, and Simona Mrakic-Sposta. 2023. "Seasonal Oxy-Inflammation and Hydration Status in Non-Elite Freeskiing Racer: A Pilot Study by Non-Invasive Analytic Method" International Journal of Environmental Research and Public Health 20, no. 4: 3157. https://doi.org/10.3390/ijerph20043157
APA StyleBrizzolari, A., Bosco, G., Vezzoli, A., Dellanoce, C., Barassi, A., Paganini, M., Cialoni, D., & Mrakic-Sposta, S. (2023). Seasonal Oxy-Inflammation and Hydration Status in Non-Elite Freeskiing Racer: A Pilot Study by Non-Invasive Analytic Method. International Journal of Environmental Research and Public Health, 20(4), 3157. https://doi.org/10.3390/ijerph20043157










