Chemical Composition of Earthworm (Dendrobaena veneta Rosa) Biomass Is Suitable as an Alternative Protein Source
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
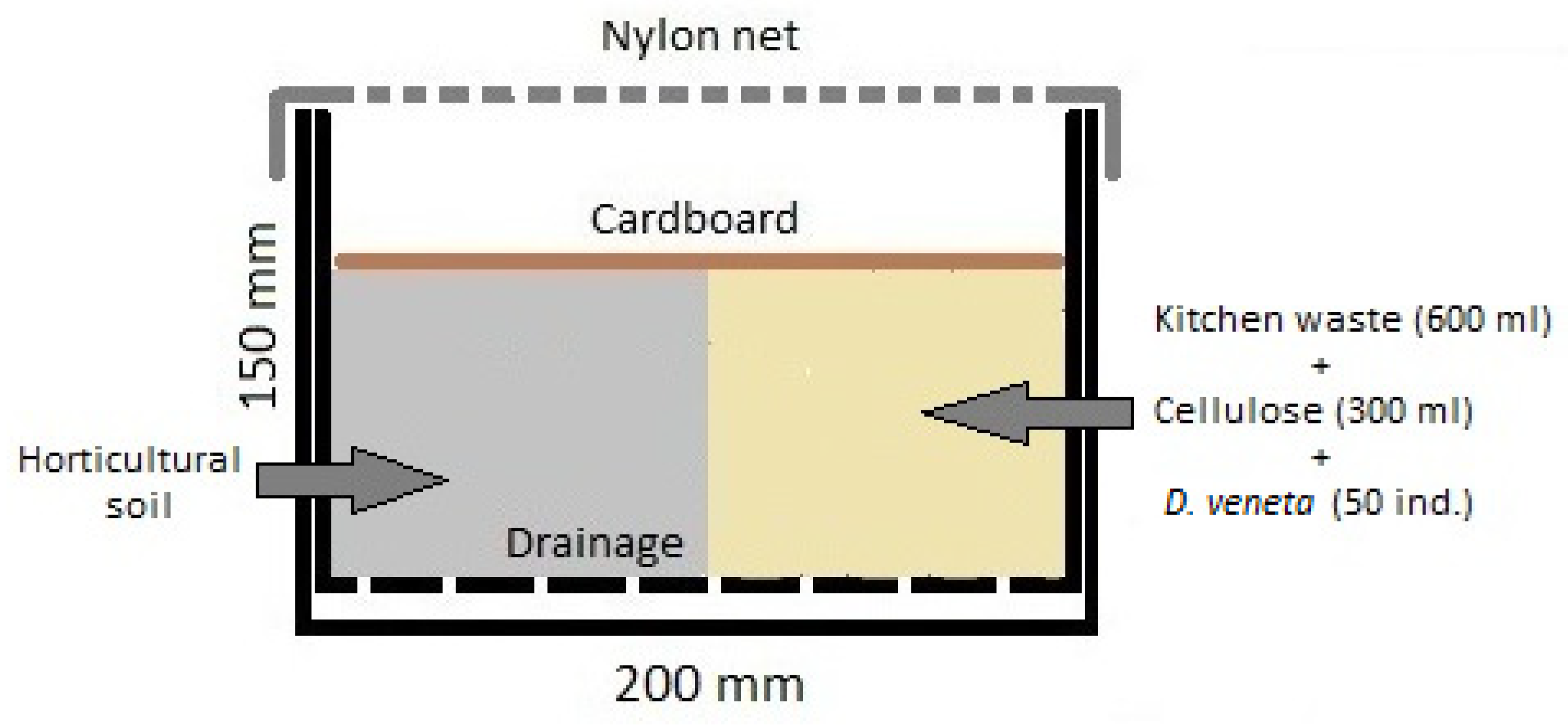
2.2. Laboratory-Scale D. veneta Biomass Production
2.3. Determination of Earthworm Chemical Composition
- –
- water content (according to PN-ISO1442, which involves drying the sample in an Ecocell laboratory dryer from BMT at a temperature of 103 ± 2 °C to obtain dry matter);
- –
- total ash (according to a method complying with PN-ISO936, which involves drying the analyzed sample, to be subsequently incinerated in a Snol muffle furnace at a temperature of (550 ± 25) °C, and after cooling down the mass of the residue is determined;
- –
- total nitrogen (with the Kjeldahl method in compliance with PN-75 A-04018, with conversion to protein);
- –
- fat (with the Soxhlet method in a Kjeltec 2200 apparatus manufactured by Boss. Before this, the samples were subjected to hydrolysis with hydrochloric acid);
- –
- contents of amino acids by Zeng et al. [41] (by hydrolyzing the sample with 6M HCl for 24 h at a temperature of 110 °C and rinsing with 0.1 molar solution of HCl and distilled water; the hydrolysate was then evaporated, and the residue was dissolved in a buffer of pH 2.2; the contents of amino acids were determined with the use of AAA-400 amino acid analyzer, which performs an assay based on liquid chromatography—following separation in the column, amino acids react with ninhydrin. The sulfur-containing amino acids were subjected to oxidizing hydrolysis with formic acid and hydrogen peroxide and then examined with AAA-400);
- -
- profile of fatty acids by Zhang et al. [42] (the samples were prepared following the Folch method (extraction with chloroform-methanol (2:1) mixture, methylation BF3/methanol). The profile was examined with a Varian 3400CX gas chromatograph, equipped with a flame ionization detector (FID), with the use of a CP-WAX column (length 50 m, diameter 0.53 mm); conditions of chromatograph operation: carrier gas—argon, the temperature of the dispenser −200 °C, temperature of the detector −240 °C, temperature of the column −60–220 °C). The analytical results of the fatty acid profiles were used to calculate the sum of saturated and unsaturated acids in fat. The content of amino acids and the profile of fatty acids in the fat of earthworms were determined in the material previously subjected to the freeze-drying process. Due to a lack of specific information on the chemical composition of D. veneta, research was undertaken to determine the content of protein and amino acids and the profile of fatty acids in the integumentary-muscular sacs as a possible raw material for use in product compositions for human and livestock nutrition. Thereafter, the composition of selected indicators of nutritional quality of integumentary-muscle sacs of D. veneta were compared with E. fetida, an earthworm for which much more information is available.
3. Results
Chemical Composition of the D. veneta Earthworm
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint WHO/FAO/UNU Expert Consultation; WHO Technical Report Series; WHO: Geneva, Switzerland, 2007; pp. 1–265. Available online: https://apps.who.int/iris/handle/10665/43411 (accessed on 10 November 2022).
- Singh, R.; Srivastava, P.; Singh, P.; Upadhyay, S.; Raghubanshi, A.S. Human Overpopulation and Food Security: Challenges for the Agriculture Sustainability. In Urban Agriculture and Food Systems: Breakthroughs in Research and Practice; Information Resources Management Association: Hershey, PA, USA, 2019; pp. 439–467. [Google Scholar] [CrossRef]
- McGuire, S. FAO, IFAD, and WFP. The state of food insecurity in the world 2015: Meeting the 2015 international hunger targets: Taking stock of uneven progress. Rome: FAO, 2015. Adv. Nutr. 2015, 6, 623–624. [Google Scholar] [CrossRef] [PubMed]
- Bueschke, M.; Kulczyński, B.; Gramza-Michałowska, A.; Kubiak, T. Alternative Sources of Protein in Human Nutrition. Sci. J. Wars. Univ. Life Sci. Probl. World Agric. 2017, 17, 49–59. [Google Scholar] [CrossRef]
- Bonnet, C.; Bouamra-Mechemache, Z.; Réquillart, V.; Treich, N. Viewpoint: Regulating meat consumption to improve health, the environment and animal welfare. Food Policy 2020, 97, 101847. [Google Scholar] [CrossRef]
- Gedrovica, I. Assessment of earthworm (Lumbricidae) species suitably for processing into powder. Am. J. Entomol. 2020, 4, 45–50. [Google Scholar] [CrossRef]
- Antonova, E.; Titov, I.; Pashkova, I.; Stom, D. Vermiculture as a source of animal protein. E3S Web Conf. 2021, 254, 08006. [Google Scholar] [CrossRef]
- Mariutti, L.R.B.; Rebelo, S.R.; Bisconsin-Junior, A.; Santos de Morais, J.; Magnani, M.; Maldonade, I.R.; Madeira, N.R.; Tiengo, A.; Maróstica, M.R.J.; Cazarin, C.B.B. The use of alternative food sources to improve health and guarantee access and food intake. Food Res. Int. 2021, 13, 3982–3992. [Google Scholar] [CrossRef]
- Vargas, A.M.; de Moura, A.P.; Deliza, R.; Cunha, L.M. The Role of Local seasonal foods in enhancing sustainable food consumption: A Systematic Literature Review. Foods 2021, 10, 2206. [Google Scholar] [CrossRef]
- Lowe, C.N.; Butt, K.R.; Sherman, R.L. Current and Potential Benefits of Mass Earthworm Culture. In Mass Production of Beneficial Organisms. Invertebrates and Entomopathogens; Morales-Ramos, J., Rojas, G., Shapiro-Ilan, D.I., Eds.; Academic Press of Elsevier: Cambridge, MA, USA, 2014; pp. 683–709. [Google Scholar]
- Kostecka, J.; Konieczna, K.; Cunha, L.M. Evaluation of insect-based food acceptance by representatives of Polish consumers in the context of natural resources processing retardation. J. Ecol. Eng. 2017, 18, 166–174. [Google Scholar] [CrossRef]
- Gaddie, R.; Douglas, D. Earthworm for Ecology and Profit; Bookworm Publishing Company: Ontario, CA, USA, 1977. [Google Scholar]
- Conti, C.; Costa, A.; Balzaretti, C.M.; Russo, V.; Tedesco, D.E.A. Survey on food preferences of university students: From tradition to new food customs? Agriculture 2018, 8, 155. [Google Scholar] [CrossRef]
- Verneau, F.; La Barbera, F.; Kolle, S.; Amato, M.; Del Giudice, T.; Grunert, K. The effect of communication and implicit associations on consuming insects: An experiment in Denmark and Italy. Appetite 2016, 106, 30–36. [Google Scholar] [CrossRef]
- Tedesco, D.E.A.; Castrica, M.; Tava, A.; Panseri, S.; Balzaretti, C.M. From a Food Safety Prospective: The Role of Earthworms as Food and Feed in Assuring Food Security and in Valuing Food Waste. Insects 2020, 11, 293. [Google Scholar] [CrossRef] [PubMed]
- Conti, C.; Castrica, M.; Balzaretti, C.M.; Tedesco, D.E.A. Edible earthworms in a food safety perspective: Preliminary data. Ital. J. Food Saf. 2019, 8, 7695. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Regulation of 15 November 2005 on Microbiological Criteria for Foodstuffs, 2073/2005/EC. In Official Journal, L 338, 22 December 2005. 2005. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02005R2073-20140601&rid=1 (accessed on 5 February 2023).
- Sims, R.W.; Gerard, B.M. Earthworms. Notes for Identification of British Species; Linnean Society of London and the Estuarine and Coastal Sciences Association: London, UK, 1999; Volume 13, 169p. [Google Scholar]
- Sinha, R.K.; Agarwal, S.; Chauhan, K.; Chandran, V.; Soni, B.K. Vermiculture Technology: Reviving the Dreams of Sir Charles Darwin for Scientific Use of Earthworms in Sustainable Development Programs. Technol. Invest. 2010, 1, 155–172. [Google Scholar] [CrossRef]
- Grdiša, M.; Gršić, K.; Grdiša, M.D. Earthworms—Role in soil fertility to the use in medicine and as a food. Invertebr. Surviv. J. 2013, 10, 38–45. [Google Scholar]
- Dominguez, J.; Edwards, C.A. Vermicomposting organic wastes: A review. In Soil Zoology for Sustain Development in the 21st Century; Hanna, S.H.S., Mikhall, W.Z.A., Eds.; S. H. Shakir Hanna: Cairo, Egypt, 2004; pp. 369–395. [Google Scholar]
- Kostecka, J.; Garczyńska, M.; Pączka, G. Food waste in the organic recycling system and a sustainable development. Probl. Sustain. Dev. 2018, 13, 157–164. [Google Scholar]
- Garczyńska, M.; Pączka, G.; Podolak, A.; Mazur-Pączka, A.; Szura, R.; Butt, K.R.; Kostecka, J. Effects of Owinema biopreparation on vermicomposting in earthworm ecological boxes. Appl. Sci. 2020, 10, 456. [Google Scholar] [CrossRef]
- Kangmin, L. Vermiculture in Circular Economy. Chinese Academy of Fishery. Sciences Freshwater Research Center Asian Pacific Regional Research and Training Center for Integrated Fish Farming. 2005. Available online: https://bestgro.com/Vermiculture%20Industry%20in%20Circular%20Economy.pdf (accessed on 12 November 2022).
- Russo, V.; Songa, G.; Marin, L.E.M.; Balzaretti, C.M.; Tedesco, D.E.A. Novel Food-Based Product Communication: A Neurophysiological Study. Nutrients 2020, 12, 2092. [Google Scholar] [CrossRef]
- Kostecka, J.; Pączka, G. Possible use of earthworm Eisenia fetida (Sav.) biomass for breeding aquarium fish. Eur. J. Soil Biol. 2006, 42, 231–233. [Google Scholar] [CrossRef]
- Monhata, K.N.; Subramanian, S.; Korikanthimath, V.S. Potential of earthworm (Eisenia foetida) as dietary protein source for rohu (Labeo rohita) advanced fry. Coget Food Agric. 2016, 2, 1138594. [Google Scholar] [CrossRef]
- Ngoc, T.N.; Pucher, J.; Becker, K.; Focken, U. Earthworm powder as an alternative protein source in diets for common carp (Cyprinus carpio L.). Aquac. Res. 2015, 47, 2917–2927. [Google Scholar] [CrossRef]
- Loh, T.C.; Fong, L.Y.; Foo, H.L.; Thanh, N.T.; Sheikh-Omar, A.R. Utilisation of earthworm meal in partial replacement of soybean and fish meals in diets of broilers. J. Appl. Anim. Res. 2009, 36, 29–32. [Google Scholar] [CrossRef]
- Rezeaeipour, V.; Nejad, O.A.; Miri, H.Y. Growth performance, blood metabolites and jejunum morphology of broiler chickens fed diets containing earthworm (Eisenia foetida) meal as a source of protein. Int. J. Adv. Biol. Biom. Res. 2014, 2, 2483–2494. [Google Scholar]
- Sabine, J.R. Earthworms as a source of food and drugs. In Earthworm Ecology from Darvin to Vermiculture; Satchell, J.E., Ed.; Chapman & Halls: London, UK; New York, NY, USA, 1983; pp. 285–296. [Google Scholar]
- Edwards, C.A.; Arancon, N.Q. The use of earthworms in the breakdown of organic waste to produce vermicomposts and animals feed protein. In Earthworm Ecology; Edwards, C.A., Ed.; C.R.C. Press: Boca Raton, FL, USA, 2004; pp. 345–379. [Google Scholar]
- MacDonald, D.W. Predation on earthworms by terrestrial vertebrates. In Earthworm Ecology: From Darwin to Vermiculture; Satchell, J.E., Ed.; Chapman and Hall: London, UK, 1983; pp. 393–414. [Google Scholar]
- Edwards, C.A.; Bohlen, P.J. Biology and Ecology of Earthworms; Chapman & Hall: London, UK, 1996; 426p. [Google Scholar]
- Kasprzak, K. Soil oligochaetes, III. Family: Earthworms (Lumbricidae). In The Key to the Determination of Invertebrates in Poland; PWN: Warszawa, Poland, 1986; Volume 6, pp. 1–88. [Google Scholar]
- Dominguez, J.; Edwards, C.A. Biology and Ecology of Earthworm Species Used for Vermicomposting; Taylor & Francis Group, LLC: Abingdon, UK, 2011; pp. 28–38. [Google Scholar]
- Pączka, G.; Mazur-Pączka, A.; Garczyńska, M.; Kostecka, J.; Butt, K.R. Effects of Vermireactor Modifications on the Welfare of Earthworms Eisenia fetida (Sav.) and Properties of Vermicomposts. Agriculture 2020, 10, 481. [Google Scholar] [CrossRef]
- Floro-Hum. Available online: https://www.floro.webd.pl/podloza.html (accessed on 31 January 2023).
- Kostecka, J. Investigation into vermicomposting of organic wastes. Sci. Pap. Agric. Univ. Crac. 2000, 268, 88. (In Polish) [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC International, 17th ed.; Association of Analytical Communities: Gaithersburg, MD, USA, 2003. [Google Scholar]
- Zeng, Y.; Cai, W.; Shao, X. Quantitative analysis of 17 amino acids in tobacco leaves using an amino acid analyzer and chemometric resolution. J. Sep. Sci. 2015, 38, 2053–2058. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Liu, O. Development and validation of a GC–FID method for quantitative analysis of oleic acid and related fatty acid. J. Pharm. Anal. 2015, 5, 223–230. [Google Scholar] [CrossRef]
- Kostecka, J.; Garczyńska, M.; Pączka, G.; Mazur-Pączka, A. Chemical composition of earthworm (Eisenia fetida Sav.) biomass and selected determinants for its production. J. Ecol. Eng. 2022, 23, 169–179. [Google Scholar] [CrossRef]
- Ding, S.; Lin, X.; He, S. Earthworms: A Source of Protein. J. Food Sci. Eng. 2019, 9, 159–170. [Google Scholar] [CrossRef]
- Borowiec, F.; Rościszewska, M.; Popek, W.; Łapiński, S. The chemical composition of the Eisenia fetida (Sav.). Rocz. Nauk. Zoot. Supl. 2001, 12, 357–363. [Google Scholar]
- Koreleski, J.; Ryś, R.; Kubicz, M.; Górska-Matusiak, Z.; Gawlik, Z. Nutritional value of California earthworm meal depending on the type of substrate and drying temperature. Rocz. Nauk. Zoot. 1994, 21, 205–214. [Google Scholar]
- Taboga, L. The nutritional value of earthworms for chickens. Brit. Poult. Sci. 1980, 21, 405–410. [Google Scholar] [CrossRef]
- Bartnikowska, E.; Kulasek, G. The importance of unsaturated fatty acids in human and animal nutrition. Deficiencies and nutritional treatment of deficiencies. Vet. Mag. 1994, 4, 34–38. [Google Scholar]
- Baltić, B.; Starčević, M.; Đorđević, J.; Mrdović, B.; Marković, R. Importance of medium chain fatty acids in animal nutrition. IOP Conf. Ser. Earth Environ. Sci. 2017, 85, 012048. [Google Scholar] [CrossRef]
- Isea-Leon, F.; Acosta-Balbias, V.; Rial-Betancoutd, L.B.; Medina-Gallardo, A.L.; Celestin, B.M. Evaluation of the fatty acid composition of earthworm Eisenia andrei meal as an alternative lipid source for fish feed. J. food Nutr. Res. 2019, 7, 696–700. [Google Scholar] [CrossRef]
- Aluko, R.E. Functional Foods and Nutraceuticals; Springer: New York, NY, USA, 2012; 22p. [Google Scholar] [CrossRef]
- Gunya, B.; Masika, P.J.; Hugo, A.; Muchenje, V. Nutrient composition and fatty acid profiles of oven dried and freeze-dried earthworm Eisenia foetida. J. Food Nutr. Res. 2016, 4, 343–348. [Google Scholar] [CrossRef]
- Paoletti, M.G.; Dufour, D.L.; Cerda, H.; Torres, F.; Pizzoferrato, L.; Pimental, D. The importance of leaf and litter feeding invertebrates as sources of animal protein for the Amazonian Amerindians. Proc. R. Soc. B Biol. Sci. 2000, 267, 2247–2252. [Google Scholar] [CrossRef]
- Paoletti, M.G.; Buscardo, E.; VanderJagt, D.J.; Pastuszyn, A.; Pizzoferrato, L.; Huang, Y.S.; Chuang, L.T.; Millson, M.; Cerda, H.; Torres, F.; et al. Nutrient content of earthworms consumed by Ye’Kuana Amerindians of the Alto Orinoco of Venezuela. Proc. R. Soc. B Biol. Sci. 2003, 270, 249–257. [Google Scholar] [CrossRef]
- Medina, A.L.; Cova, J.A.; Vielma, R.A.; Pujic, P.; Carlos, M.P.; Torres, J.V. Immunological and chemical analysis of proteins from Eisenia foetida earthworm. Food Agric. Immunol. 2003, 15, 255–263. [Google Scholar] [CrossRef]
- Sun, Z.; Jiang, H. Nutritive Evaluation of Earthworms as Human Food. In Future Foods; Mikkola, H., Ed.; IntechOpen: London, UK, 2017; pp. 128–141. [Google Scholar] [CrossRef]
- Moreno, A.G.; Paoletti, M.G. Andiorrhinus (Amazonidrilus) kuru sp. nov. (Oliogocheta: Glassoscolecidae), a giant earthworm as food resource for Makiritate Indians of the Alto RioPadamo, Amazonas, Venezuela. Can. J. Zool. 2004, 82, 1000–1004. [Google Scholar] [CrossRef]
- Petrescu-Mag, R.M.; Kopaei, H.R.; Petrescu, D.C. Consumers’ acceptance of the first novel insect food approved in the European Union: Predictors of yellow mealworm chips consumption. Food Sci. Nutr. 2022, 10, 846–862. [Google Scholar] [CrossRef]

| Traits | D. veneta Content |
|---|---|
| in Dry Matter | |
| dry mass | 100.0 ± 0.02 |
| crude ash | 5.17 ± 0.01 |
| total protein | 76.82 ± 0.38 |
| crude fat | 12.21 ± 0.57 |
| Amino Acids | D. veneta | E. fetida * | Beef ** | Pork ** | Pattern Protein Egg ** |
|---|---|---|---|---|---|
| Endogenous amino acids | |||||
| Aspartic acid (Asp) | 8.22 ± 0.52 | 5.75 ± 2.03 | |||
| Serine (Ser) | 3.83 ± 0.39 | 2.89 ± 0.85 | |||
| Glutamic acid (Glu) | 8.94 ± 1.13 | 7.12 ± 1.72 | |||
| Proline (Pro) | 2.55 ± 0.21 | 1.94 ± 0.15 | |||
| Glycine (Gly) | 3.50 ± 0.69 | 2.59 ± 0.69 | |||
| Alanine (Ala) | 3.48 ± 0.69 | 2.62 ± 0.69 | |||
| Cysteine (Cys) | 0.84 ± 0.10 | 0.64 ± 0.18 | |||
| Tyrosine (Tyr) | 2.16 ± 0.38 | 1.43 ± 0.39 | |||
| Essential and semi-essential amino acids (EAA) | |||||
| Phenylalanine (Phe) | 2.91 ± 0.40 | 2.3 ± 0.25 | 1.8 | 1.7 | 2.6 |
| Histidine (His) | 1.62 ± 0.29 | 2.1 ± 0.28 | 1.5 | 1.7 | 1.2 |
| Lysine (Lys) | 4.74 ± 0.51 | 4.9 ± 0.38 | 3.7 | 3.9 | 3.4 |
| Methionine (Met) | 0.80 ± 0.08 | 1.1 ± 0.20 | 1.2 | 1.1 | 1.5 |
| Threonine (Thr) | 2.34 ± 0.20 | 2.8 ± 0.43 | 1.7 | 2.0 | 2.2 |
| Valine (Val) | 3.68 ± 0.44 | 3.8 ± 0.53 | 2.2 | 2.4 | 3.1 |
| Tryptophan (Trp) | — | — | 0.2 | 0.5 | 0.8 |
| Isoleucine (Ile) | 3.05 ± 0.52 | 2.9 ± 0.41 | 2.0 | 2.0 | 2.5 |
| Leucine (Leu) | 3.66 ± 0.45 | 6.2 ± 0.39 | 3.5 | 3.5 | 4.3 |
| Arginine (Arg) | 5.16 ± 0.45 | 6.0 ± 0.33 | 2.9 | 2.7 | 3.2 |
| Total EAA | 27.96 | 32.1 | 20.7 | 21.5 | 24.8 |
| CPSP (%) | 1.1 | 1.3 | 0.8 | 0.9 | 1 |
| Fatty Acids Profile (% in Total Acids) | ||
|---|---|---|
| Fatty Acids | D. veneta | E. fetida * |
| saturated fatty acids | ||
| Lauric acid C12 | 2.67 ± 0.16 | 3.60 ± 0.18 |
| Tridecanic acid C13 | 0.16 ± 0.02 | 0.27 ± 0.04 |
| Myristic acid C14 | 5.41 ± 0.15 | 5.64 ± 0.13 |
| Pentadecanoic acid C15 | 0.29 ± 0.03 | 0.25 ± 0.03 |
| Palmitic acid C16 | 16.19 ± 0.2 | 17.06 ± 0.12 |
| Heptadecanoic acid C17 | 0.51 ± 0.04 | 0.50 ± 0.02 |
| Stearic acid C18 | 6.74 ± 0.22 | 6.45 ± 0.61 |
| Kwas arachidowy C20 | 0.16 ± 0.01 | 0.12 ± 0.01 |
| Behenic acid C22 | 1.09 ± 0.04 | 0.96 ± 0.06 |
| Lignoceric acid C24 | 0.13 ± 0.01 | 0.12 ± 0.01 |
| Total saturated acids | 33.38 | 35.31 ± 0.64 |
| monounsaturated fatty acids | ||
| Myristoleic acid C14:1 | 0.26 ± 0.02 | 0.33 ± 0.05 |
| C15:1 | 0.15 ± 0.01 | 0.13 ± 0.01 |
| Palmitoleic acid C16:1 | 4.77 ± 0.87 | 5.60 ± 0.76 |
| C17:1 | 0.68 ± 0.12 | 0.79 ± 0.05 |
| Oleic acid C18:1 | 30.68 ± 1.14 | 31.05 ± 0.48 |
| Eicosenoic acid C20:1 | 1.97 ± 0.28 | 1.88 ± 0.32 |
| Euric acid C22:1 | 1.15 ± 0.16 | 0.56 ± 0.33 |
| Total monounsaturated acids | 39.67 | 40.860 ± 0.678 |
| polyunsaturated fatty acids | ||
| C14:2 | 1.03 ± 0.07 | 1.24 ± 0.13 |
| Linoleic acid C18:2 | 9.51 ± 0.17 | 9.77 ± 0. 42 |
| Linolenic acid C18:3 | 1.21 ± 0.16 | 1.12 ± 0.17 |
| Eicosadienic acid C20:2 | 0.70 ± 0.04 | 0.77 ± 0.08 |
| Arachidonic acid C20:4 | 1.17 ± 0.7 | 1.24 ± 0.09 |
| Docosapentaenoic acid C22:5 | 0.246 ± 0.03 | 0.13 ± 0.01 |
| Clupandonic acid C22:6 | 0.730 ± 0.0 | 0.20 ± 0.04 |
| Total polyunsaturated acids | 14.62 | 14.487 ± 0.834 |
| Unmarked | 10.02 | 9.645 ± 1.025 |
| Fatty Acid | Function | D. veneta | E. fetida |
|---|---|---|---|
| palmitic acid | activator of many enzymes | 16.19 ± 0.2 | 17.06 ± 0.12 |
| oleic acid | increases the immunity of animals and prevents cardiovascular diseases | 30.68 ± 1.14 | 31.05 ± 0.48 |
| linoleic acid | participates in the formation of prostaglandin PGE1 and prostacyclin, which regulate the tone of the walls of blood vessels | 9.51 ± 0.17 | 9.77 ± 0.42 |
| eicosadienoic acid: | is part of omega-3 fatty acids, contributing to the increase in immunity | 0.70 ± 0.04 | 0.77 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garczyńska, M.; Kostecka, J.; Pączka, G.; Mazur-Pączka, A.; Cebulak, T.; Butt, K.R. Chemical Composition of Earthworm (Dendrobaena veneta Rosa) Biomass Is Suitable as an Alternative Protein Source. Int. J. Environ. Res. Public Health 2023, 20, 3108. https://doi.org/10.3390/ijerph20043108
Garczyńska M, Kostecka J, Pączka G, Mazur-Pączka A, Cebulak T, Butt KR. Chemical Composition of Earthworm (Dendrobaena veneta Rosa) Biomass Is Suitable as an Alternative Protein Source. International Journal of Environmental Research and Public Health. 2023; 20(4):3108. https://doi.org/10.3390/ijerph20043108
Chicago/Turabian StyleGarczyńska, Mariola, Joanna Kostecka, Grzegorz Pączka, Anna Mazur-Pączka, Tomasz Cebulak, and Kevin R. Butt. 2023. "Chemical Composition of Earthworm (Dendrobaena veneta Rosa) Biomass Is Suitable as an Alternative Protein Source" International Journal of Environmental Research and Public Health 20, no. 4: 3108. https://doi.org/10.3390/ijerph20043108
APA StyleGarczyńska, M., Kostecka, J., Pączka, G., Mazur-Pączka, A., Cebulak, T., & Butt, K. R. (2023). Chemical Composition of Earthworm (Dendrobaena veneta Rosa) Biomass Is Suitable as an Alternative Protein Source. International Journal of Environmental Research and Public Health, 20(4), 3108. https://doi.org/10.3390/ijerph20043108







