The Effects of Resistance Exercise on the Cardiorespiratory Tissue of Rats with Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Animals
2.2. PD Induction
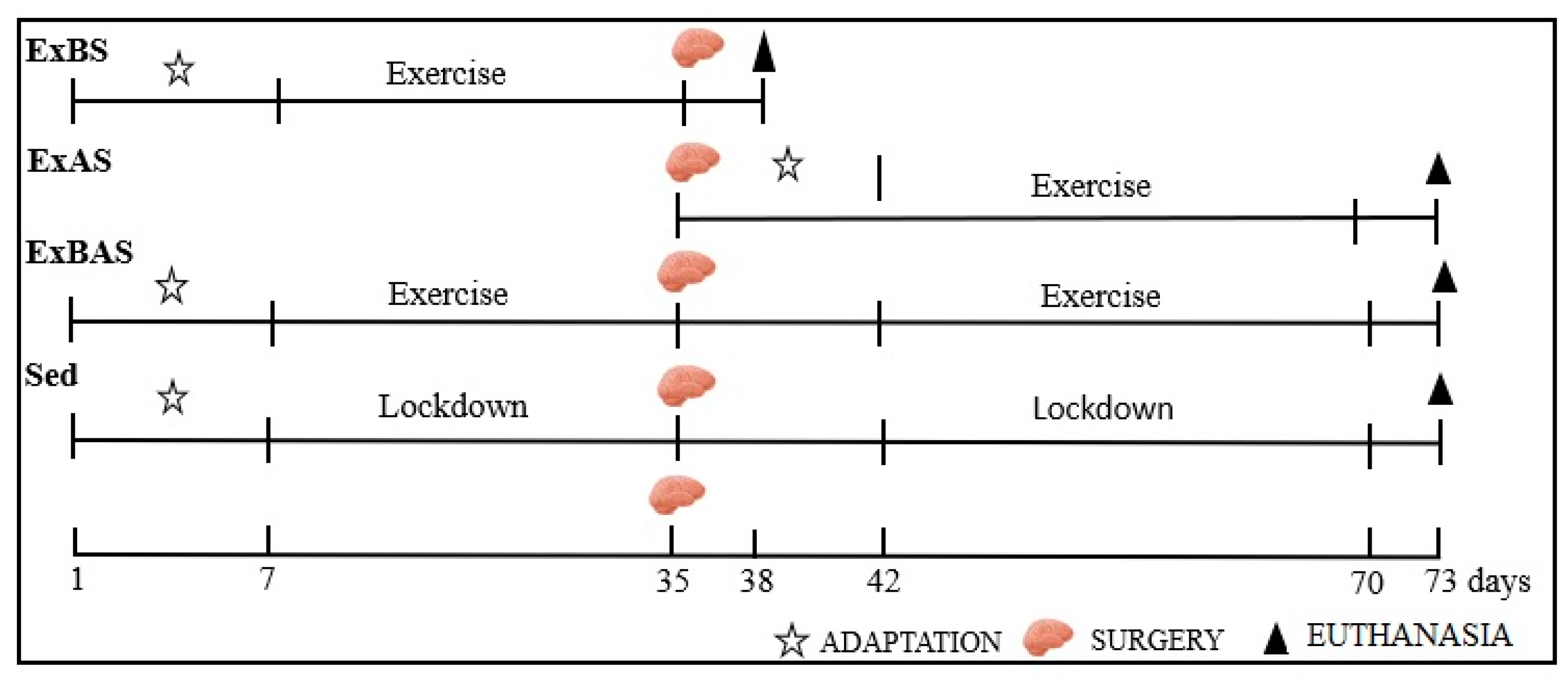
2.3. Physical Training
2.4. Histological Processing and Histomorphometric Analysis of the Substantia Nigra of the Rat Brain
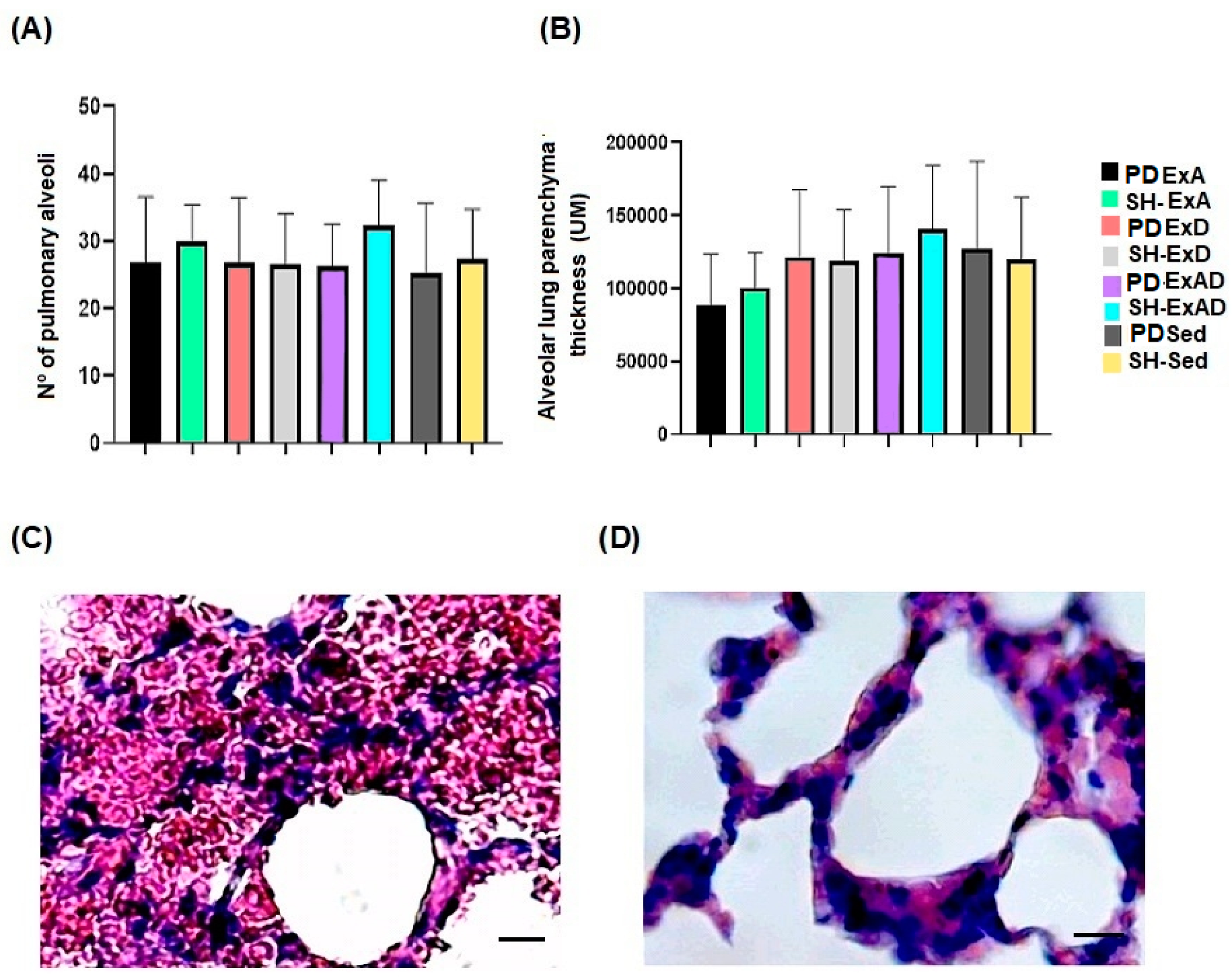
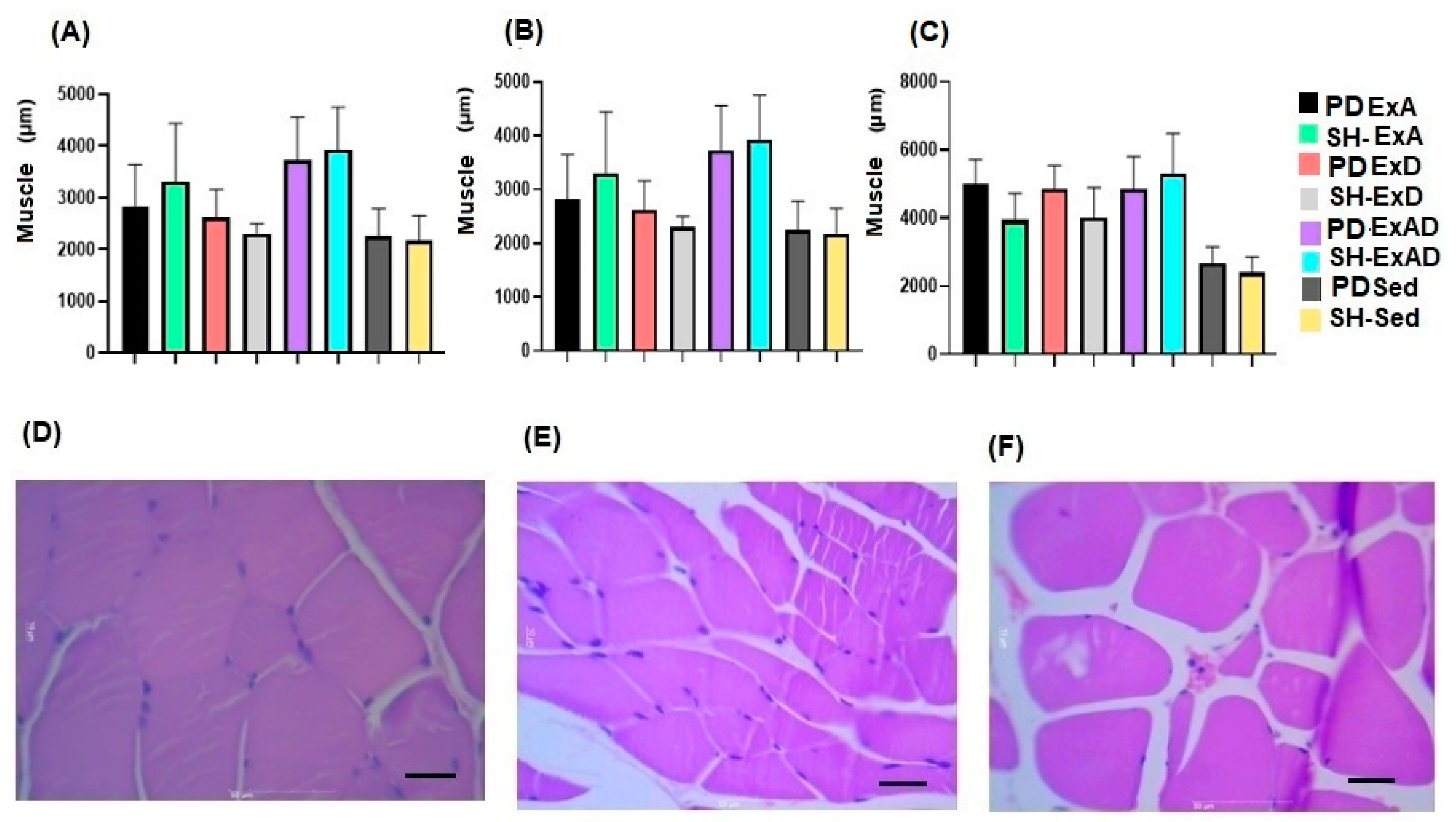
2.5. Histological Processing and Histomorphometric Analysis of the Parenchyma and Respiratory Muscle Tissue
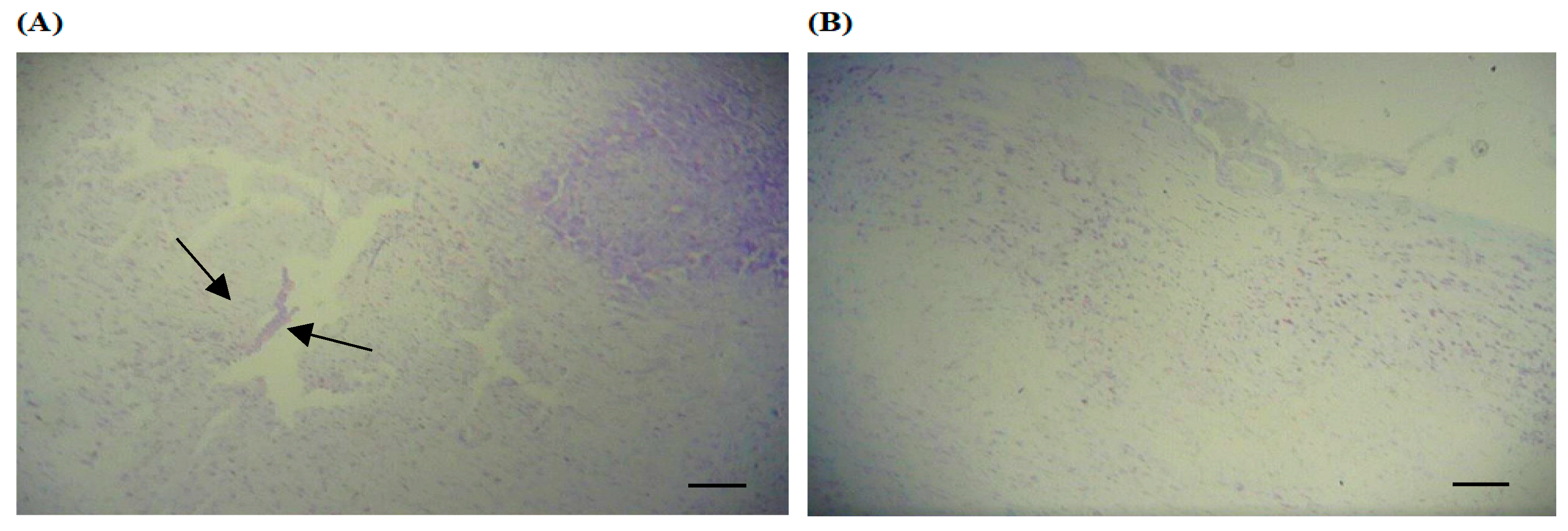
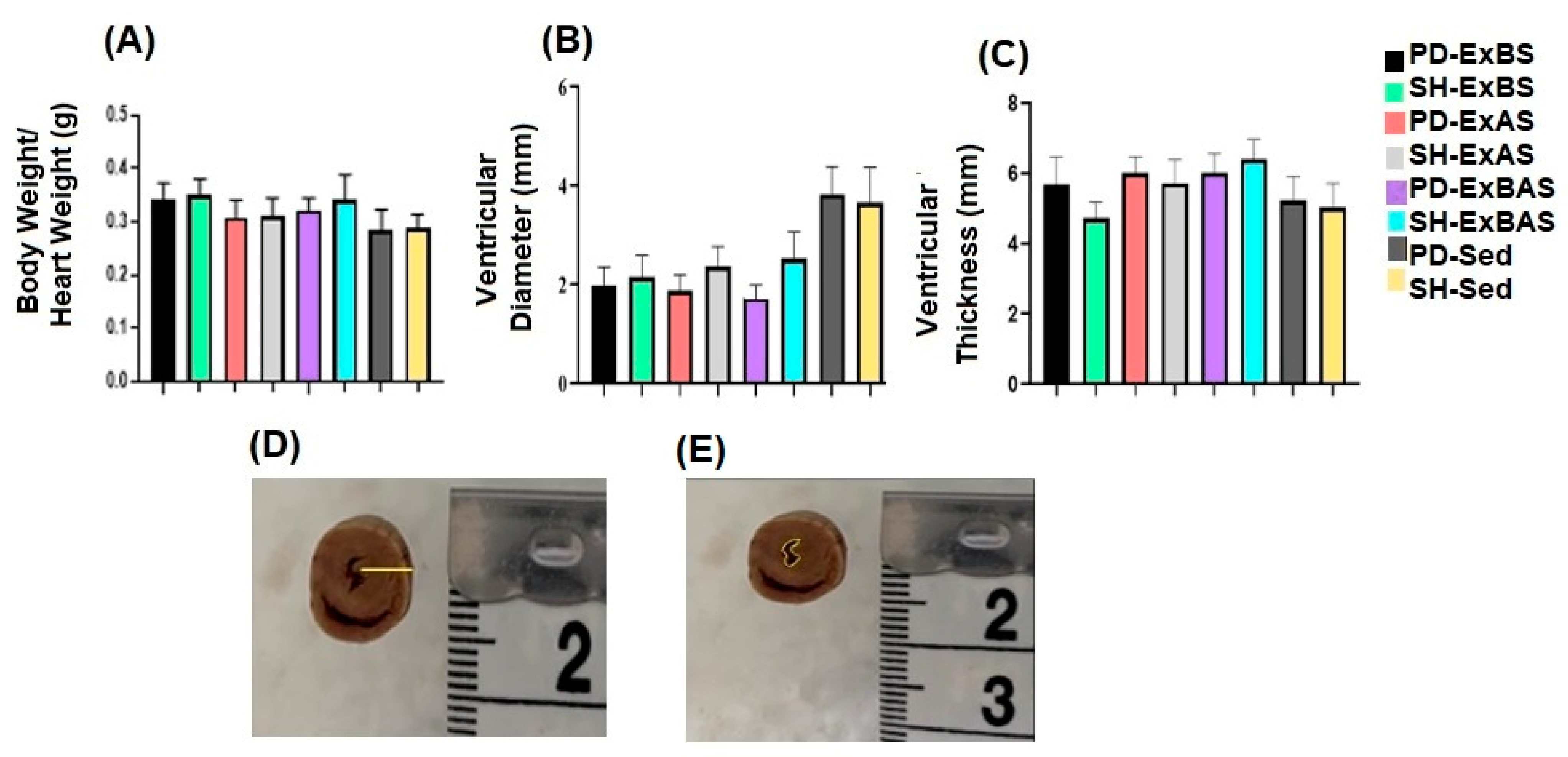
2.6. Analysis of the Heart
2.7. Heart Rate Analysis
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonçalves, L.H.T.; Alvarez, A.M.; Arruda, M.C. Patients’ experience with Parkinson’s disease. Acta Paul. Enferm. 2007, 20, 62–68. [Google Scholar] [CrossRef]
- Teive, H.G. Etiopathogenesis of Parkinson Disease. Rev. Neuroc. 2005, 13, 2001–2013. [Google Scholar]
- Oxtoby, M.; Williams, A. Tudo Sobre Doença de Parkinson: Respostas a Suas Dúvidas; Andrei Editora LTDA: São Paulo, Brazil, 2000. [Google Scholar]
- Fasano, A.; Canning, C.G.; Hausdorff, J.M.; Lord, S.; Rochester, L. Falls in Parkinson’s disease: A complex and evolving picture. Mov. Disord. 2017, 32, 1524–1536. [Google Scholar]
- Silva, T.; Carvalho, C. Parkinson’s Disease: The occupational therapeutic treatment in the perspective of professionals and elderly. Cad. Bras. Ter. Ocup. 2019, 27, 331–344. [Google Scholar]
- Santos, B.; Fraga, A.S.; Coriolano, M.D.G.W.D.S.; Tiburtino, B.F.; Lins, O.G.; Esteves, A.C.F.; Asano, N.M.J. Respiratory muscle strength and lung function in the stages of Parkinson’s disease. J. Bras. Pneumol. 2019, 6, 1–6. [Google Scholar]
- Titova, N.; Padmakumar, C.; Lewis, S.J.; Chaudhuri, K.R. Parkinson’s: A syndrome rather than a disease? J. Neural. Transm. 2017, 124, 907–914. [Google Scholar] [CrossRef]
- Flores, J.P.; López-García, A.; Moreno-Reig, Á.; González-Martínez, A.; Hernández-González, A.; Vaamonde-Gamo, J.; Jurado-Román, A. Structural and functional alterations of the heart in Parkinson’s disease. Neurol. Res. 2018, 40, 53–61. [Google Scholar]
- Lucas, S.J.W.; Cotte, J.D.; Brassard, P.; Bailey, D.M. High-intensity interval exercise and cerebrovascular health: Curiosity, cause, and consequence. J. Cereb. Blood Flow Metab. 2015, 6, 902–911. [Google Scholar] [CrossRef]
- Quindry, J.C.; Franklin, B.A.; Chapman, M.; Humphrey, R.; Mathis, S. Benefits and Risks of High-Intensity Interval Training in Patients With Coronary Artery Disease. Am. J. Cardiol. 2019, 123, 1370–1377. [Google Scholar]
- Kim, Y.; Lai, B.; Mehta, T.; Thirumalai, M.; Padalabalanarayanan, S.; Rimmer, J.H.; Motl, R.W. Exercise training guidelines for multiple sclerosis, stroke, and Parkinson’s disease: Rapid review and synthesis. Am. J. Phys. Med. Rehabil. 2019, 98, 613–621. [Google Scholar] [CrossRef]
- Lezcano, L.B.; Lorigados Pedre, L.D.C.; Fernandez Verdecia, C.I.; Serrano Sanchez, T.; Pavon Fuentes, N.; Francis Turner, L.I.L.I.A.N.A. Modify Beam Transversal Test to Evaluate Hemiparkinsonian Rats. Acta Biol. Colomb. 2010, 15, 189–201. [Google Scholar]
- Gomes, M.; Bel, D. Effects of electrolytic and 6-hydroxydopamine lesions of rat nigrostriatal pathway on nitric oxide synthase and nicotinamide adenine dinucleotide phosphate diaphorase. Brain Res. Bull. 2003, 62, 107–115. [Google Scholar]
- Hornberger, T.A.; Farrar, R.P. Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can. J. Appl. Physiol. Champaign 2004, 29, 16–31. [Google Scholar] [CrossRef]
- Peixinho-Pena, L.F.; Fernandes, J.; de Almeida, A.A.; Gomes, F.G.N.; Cassilhas, R.; Venancio, D.P.; de Mello, M.T.; Scorza, F.A.; Cavalheiro, E.A.; Arida, R.M. A strength exercise program in rats with epilepsy is protective against seizures. Epilepsy Behav. 2012, 25, 323–328. [Google Scholar] [CrossRef]
- Cassilhas, R.C.; Reis, I.T.; Venâncio, D.; Fernandes, J.; Tufik, S.; De Mello, M.T. Animal model for progressive resistance exercise: A detailed description of model and its implications for basic research in exercise. Motriz 2013, 19, 178–184. [Google Scholar] [CrossRef]
- Gonçalves, L.V.; Herlinger, A.L.; Ferreira, T.A.A.; Coitinho, J.B.; Pires, R.G.W.; Martins-Silva, C. Environmental enrichment cognitive neuroprotection in na experimental model of cerebral ischemia: Biochemical and molecular aspects. Behav. Brain Res. 2018, 348, 171–183. [Google Scholar] [CrossRef]
- Scorza, F.A.; Menezes-Rodrigues, F.S.; Olszewer, E.; Errante, P.R.; Tavares, J.G.P.; Scorza, C.A.; Ballalai Ferraz, H.; Finsterer, J.; Caricati-Neto, A. The mitochondrial calcium uniporter: A new therapeutic target for Parkinson’s disease-related cardiac dysfunctions? Clinics 2020, 70, 1–5. [Google Scholar]
- Damã¡zio, L.C.M.; Melo, R.T.R.; Lima, M.D.C.; Dos Santos, H.B.; Ribeiro, R.I.M.D.A.; Alves, N.R.; Monteiro, B.S.; Natali, A.J.; Del Carlo, R.J.; Maldonado, I.R.D.S.C. Effect of Exercise Prior or After Ischemia on the Density of Neurons and Astrocytes in the Brain of Rats. Am. J. Neurosc. 2014, 5, 18–25. [Google Scholar]
- Melo, R.; Damázio, L.; Lima, M.; Pereira, V.; Okano, B.; Monteiro, B.; Natali, A.; Del Carlo, R.; Maldonado, I. Effects of physical exercise on skeletal muscles of rats with cerebral ischemia. Braz. J. Med. Biol. Res. 2019, 52, e8576. [Google Scholar] [CrossRef]
- Vasconcelos, N.N.; Pereira, L.A.; Silva, R.S.R.; Dias, K.S.S.A.; Mourão, T.S.; Pereira, L.C.; Rosa Cota, V.; Pinto, F.C.H.; Damázio, L.C.M. High-intensity physical exercise promotes increased brain injury in rats with cerebral ischemia induced by bilateral common carotid artery occlusion. Braz. J. Med. Biol. Res. 2021, 30, 106148. [Google Scholar]
- Cardoso, S.R.X.; Pereira, J.S. Analysis of breathing function in Parkinson’s disease. Arq. Neurop. 2002, 60, 91–95. [Google Scholar]
- Monteiro, R.; Jatene, F.B.; Correia, A.T.; Manoel, L.A.; Bernardo, W.M.; Rivero, D.H.R.F. Evaluation of the cardiac morphological alterations secondary to the pulmonary emphysema: Experimental study in rats. Rev. Bras. Cir. Cardiov. 2004, 19, 341–347. [Google Scholar] [CrossRef]
- Sarmiento, A.R.; Orozco-Levi, M.; Guell, R.; Barreiro, E.; Hernandez, N.; Mota, S.; Sangenis, M.; Broquetas, J.M.; Casan, P.; Gea, J. Treinamento muscular inspiratório em pacientes com doença pulmonar obstrutiva crônica: Adaptação estrutural e resultados fisiológicos. Am. J. Respir. Crit. Care Med. 2002, 166, 1491–1497. [Google Scholar]
- Bisschop, A. Intermittent Inspiratory Muscle Training Induces Fiber Hypertrophy in Rat Diaphragm. Am. J. Respir. Crit. Care Med. 1997, 155, 1583–1589. [Google Scholar]
- Bowen, S.; Brauer, D.; Rolim, N.P.; Bækkerud, F.H.; Kricke, A.; Ormbostad Berre, A.M.; Fischer, T.; Linke, A.; da Silva, G.J.; Wisloff, U.; et al. Exercise Training Reveals Inflexibility of the Diaphragm in an Animal Model of Patients With Obesity-Driven Heart Failure With a Preserved Ejection Fraction. J. Am. Heart Assoc. 2017, 6, e006416. [Google Scholar] [CrossRef]
- Toti, L.; Bartalucci, A.; Ferrucci, M.; Fulceri, F.; Lazzeri, G.; Lenzi, P.; Soldani, P.; Gobbi, P.; La Torre, A.; Gesi, M. High-intensity exercise training induces morphologucal and biochemical changes in skeletal muscles. Biol. Sport 2013, 30, 301–309. [Google Scholar] [CrossRef]
- Melo, S.F.S. Activation of AKT-mTor signalling pathways by angiotensin II receptor type 1 after a session of strength exercise in cardiac muscle of rats. Rev. Bras. Educ. Fís. Esporte 2011, 25, 377–385. [Google Scholar]
- Nishida, N.; Yoshida, K.; Hata, Y. Sudden unexpected death in early Parkinson’s disease: Neurogenic or cardiac death? Cardiov. Pathol. 2017, 30, 19–22. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, G.M.S.; Damázio, L.C.M.; Silva, S.V.d.; Silveira, A.T.; Mesquita, I.G.; Silva, L.A.d.S.; Pereira, L.A.; Costa, A.C.S.; Santos, I.A.L.; Campos, M.E.P.; et al. The Effects of Resistance Exercise on the Cardiorespiratory Tissue of Rats with Parkinson’s Disease. Int. J. Environ. Res. Public Health 2023, 20, 2925. https://doi.org/10.3390/ijerph20042925
Moreira GMS, Damázio LCM, Silva SVd, Silveira AT, Mesquita IG, Silva LAdS, Pereira LA, Costa ACS, Santos IAL, Campos MEP, et al. The Effects of Resistance Exercise on the Cardiorespiratory Tissue of Rats with Parkinson’s Disease. International Journal of Environmental Research and Public Health. 2023; 20(4):2925. https://doi.org/10.3390/ijerph20042925
Chicago/Turabian StyleMoreira, Graziele Mayra Santos, Laila Cristina Moreira Damázio, Silvana Venâncio da Silva, Augusto Targino Silveira, Isabella Giordano Mesquita, Luana Aparecida de Sousa Silva, Luan Alves Pereira, Ana Clara Silva Costa, Ismael Augusto Lima Santos, Maria Eduarda Paiva Campos, and et al. 2023. "The Effects of Resistance Exercise on the Cardiorespiratory Tissue of Rats with Parkinson’s Disease" International Journal of Environmental Research and Public Health 20, no. 4: 2925. https://doi.org/10.3390/ijerph20042925
APA StyleMoreira, G. M. S., Damázio, L. C. M., Silva, S. V. d., Silveira, A. T., Mesquita, I. G., Silva, L. A. d. S., Pereira, L. A., Costa, A. C. S., Santos, I. A. L., Campos, M. E. P., Vaz, L. S. C., Cardoso, Z. A., Gomes, J. V. R. S., Júnior, P. H. A. C., & Ide, L. M. (2023). The Effects of Resistance Exercise on the Cardiorespiratory Tissue of Rats with Parkinson’s Disease. International Journal of Environmental Research and Public Health, 20(4), 2925. https://doi.org/10.3390/ijerph20042925







