Freshwater Water-Quality Criteria for Chloride and Guidance for the Revision of the Water-Quality Standard in China
Abstract
:1. Introduction
2. Sources, Pollution Status, and Hazards of Chloride in Water Bodies
2.1. Sources of Chloride
2.2. Concentration Distribution of Chloride
2.3. Hazards of Chlorides
3. Domestic Chloride Water-Quality Standard Limit Value, Development Basis, and Comparative Analysis
4. Global Water-Quality Criteria and Standards for Chloride Levels
4.1. Water-Quality Criteria and Water-Quality Standard for Chloride Levels in the United States
4.1.1. Chloride Water-Quality Criteria Studies in the United States
- The chloride of K+, Ca2+, and Mg2+ were generally considered to be more acutely toxic to aquatic animals than NaCl, and chloride in aqueous environments was generally considered to be primarily associated with Na;
- Only the NaCl had sufficient data to be used in the derivation of WQC;
- No significant relationships were found between the acute toxicity of chloride to freshwater animals and the hardness, alkalinity, or pH levels;
- The exposure times of 24 h and 48 h were mainly chosen, with very little change observed in the acute values from 24 h to 48 h and 96 h.
4.1.2. Chloride Water-Quality Standards Studies in the United States
4.2. Development of the Water-Quality Criteria and Standards for Chloride in Canada
5. The Derivation of the Water-Quality Criteria for Chloride in China
5.1. Toxicity Data Collection and Selection
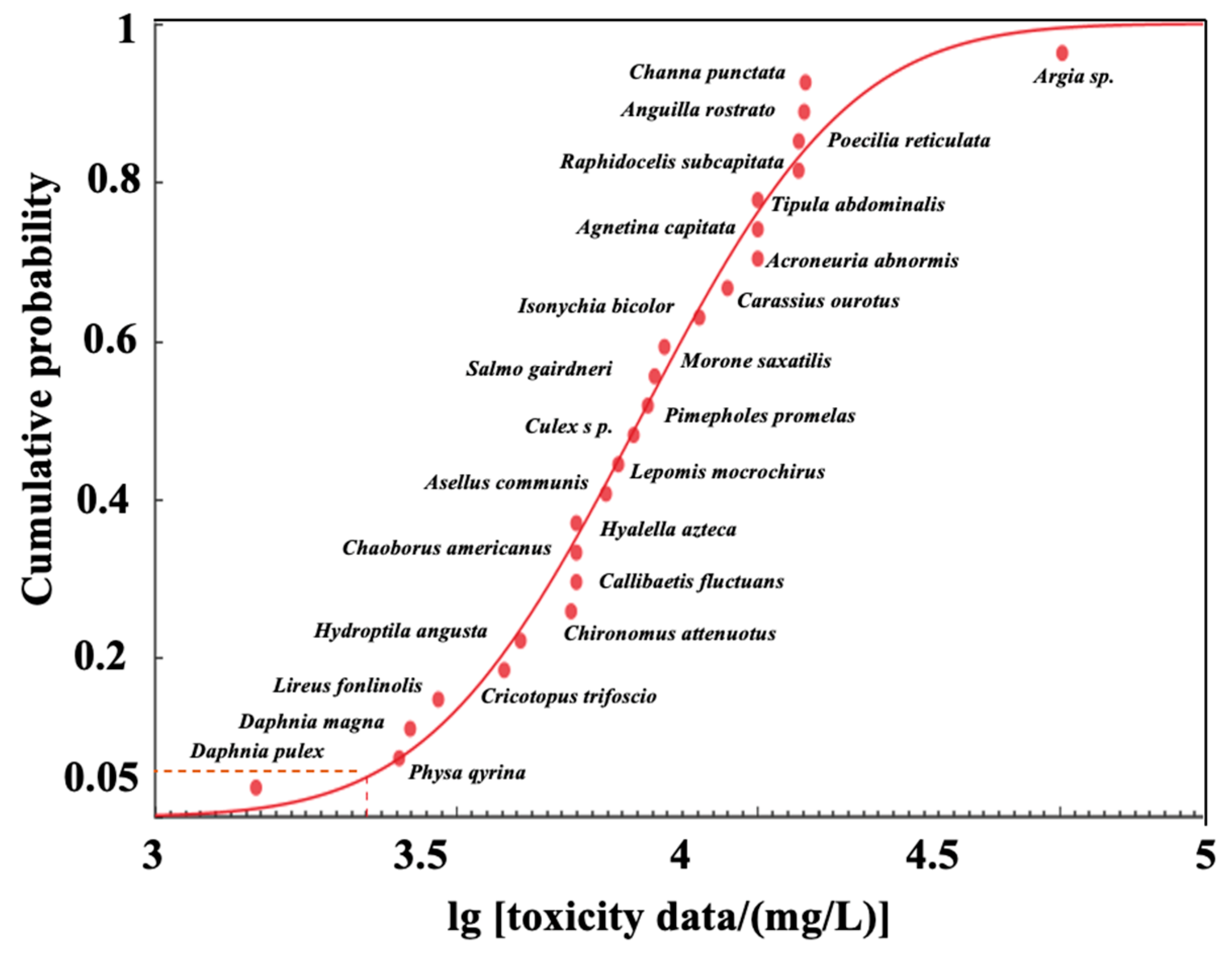
5.2. Derivation of the Water-Quality Criteria Value for Chloride
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, Y.; Guo, L.; Chen, B.; Wu, J. Thermodynamic modelling and experimental investigation on chloride binding in cement exposed to chloride and chloride-sulfate solution. Constr. Build. Mater. 2020, 246, 118398. [Google Scholar] [CrossRef]
- He, F.; Wang, R.; Shi, C.; Zhang, R.; Shi, Z.; Zhang, D. Effect of bound chloride on extraction of water soluble chloride in cement-based materials exposed to a chloride salt solution. Constr. Build. Mater. 2018, 160, 223–232. [Google Scholar] [CrossRef]
- Yuan, Q.; Shi, C.; De Schutter, G.; Audenaert, K.; Deng, D. Chloride binding of cement-based materials subjected to external chloride environment—A review. Constr. Build. Mater. 2009, 23, 1–13. [Google Scholar] [CrossRef]
- Alhaidary, A.; Mohamed, H.; Beynen, A. Nephrocalcinosis and urinary mineral concentrations in rats fed diets containing supplemental chloride. J. Anim. Veter- Adv. 2010, 9, 2409–2411. [Google Scholar] [CrossRef]
- Zajac, M.; Chakraborty, K.; Saha, S.; Mahadevan, V.; Infield, D.T.; Accardi, A.; Qiu, Z.; Krishnan, Y. What biologists want from their chloride reporters—A conversation between chemists and biologists. J. Cell Sci. 2020, 133, jcs240390. [Google Scholar] [CrossRef]
- Feng, C.L.; Wu, F.C.; Mu, Y.S.; Dyer, S.D.; Fan, M.; Raimondo, S.; Barron, M.G. Interspecies correlation estimation-applications in water quality criteria and ecological risk assessment. Environ. Sci. Technol. 2013, 47, 11382–11383. [Google Scholar] [CrossRef]
- Feng, C.; Wu, F.; Zhao, X.; Li, H.; Chang, H. Water quality criteria research and progress. Sci. China Earth Sci. 2012, 55, 882–891. [Google Scholar] [CrossRef]
- Wu, F.; Meng, W.; Zhao, X.; Li, H.; Zhang, R.; Cao, Y.; Liao, H. China embarking on development of its own national water quality criteria system. Environ. Sci. Technol. 2010, 44, 7992–7993. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Mu, Y.; Chang, H.; Zhao, X.; Giesy, J.P.; Wu, K.B. Predicting water quality criteria for protecting aquatic life from physicochemical properties of metals or metalloids. Environ. Sci. Technol. 2012, 47, 446–453. [Google Scholar] [CrossRef]
- Wu, F.; Fang, Y.; Li, Y.; Cui, X.; Zhang, R.; Guo, G.; Giesy, J.P. Predicted no-effect concentration and risk assessment for 17-[beta]-estradiol in waters of China. Rev. Environ. Contam. Toxicol. 2013, 228, 31–56. [Google Scholar] [CrossRef]
- Hong, Y.; Feng, C.; Yan, Z.; Wang, Y.; Liu, D.; Liao, W.; Bai, Y. Nonylphenol occurrence, distribution, toxicity and analytical methods in freshwater. Environ. Chem. Lett. 2020, 18, 2095–2106. [Google Scholar] [CrossRef]
- Hong, Y.J.; Li, H.; Feng, C.L.; Liu, D.; Yan, Z.F.; Qiao, Y.; Bai, Y.C.; Wu, F.C. A review on the water quality criteria of nonylphenol and the methodological construction for reproduction toxicity endocrine disrupting chemicals. Rev. Environ. Contam. Toxicol. 2022, 260, 4–18. [Google Scholar] [CrossRef]
- Hong, Y.; Feng, C.; Jin, X.; Xie, H.; Liu, N.; Bai, Y.; Wu, F.; Raimondo, S. A QSAR–ICE–SSD model prediction of the PNECs for alkylphenol substances and application in ecological risk assessment for rivers of a megacity. Environ. Int. 2022, 167, 107367. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.C. Introduction to Water Quality Benchmarking Theory and Methodology, 2nd ed.; Science Press: Beijing, China, 2020. (In Chinese) [Google Scholar]
- Zhang, Y.; Lin, J.N.; Wang, H.; Guo, C.S.; Ding, S.; Jia, X.B.; Huo, S.L.; Xu, J.; Liu, Y.; Wang, H.Y.; et al. Research on environmental quality standard for surface water. Res. Environ. Sci. 2020, 33, 2523–2528. (In Chinese) [Google Scholar]
- Fu, Z.Y.; Zhang, Y.S.; Feng, C.L.; Guo, C.S.; You, J.; Sun, Y.W.; Liu, X.M.; Wu, F.C. Progress, challenge and strategic countermeasures of Chinese pollution risk management of water environment. Res. Environ. Sci. 2021, 34, 1532–1541. (In Chinese) [Google Scholar]
- Ellis, M.M. Detection and measurement of stream pollution. Bull. Bur. Fish. 1937, 48, 365–437. [Google Scholar]
- USEPA. Ambient Water Quality Criteria for Cadmium; Office of Water Regulations and Standards: Washington, DC, USA, 1980.
- USEPA. Guidelines for Deriving Numerical National Water Quality Criteria for the Protection of Aquatic Organisms and Their Uses; Office of Research and Development: Washington, DC, USA, 1985.
- CCME. Canadian Water Quality Guidelines for the Protection of Aquatic Life-Chloride; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 2011. [Google Scholar]
- Caldwell, D.J.; Mastrocco, F.; Anderson, P.D.; Lnge, R.; Sumpter, J.P. Predicted-no-effect concentrations for the steroid estrogens estrone, 17β-estradiol, estriol, and 17α-ethinylestradiol. Environ. Toxicol. Chem. 2012, 31, 1396–1406. [Google Scholar] [CrossRef]
- Feng, C.; Li, H.; Yan, Z.; Wang, Y.; Wang, C.; Fu, Z.; Liao, W.; Giesy, J.P.; Bai, Y. Technical study on national mandatory guideline for deriving water quality criteria for the protection of freshwater aquatic organisms in China. J. Environ. Manag. 2019, 250, 109539. [Google Scholar] [CrossRef]
- Liu, N.; Jin, X.; Feng, C.; Wang, Z.; Wu, F.; Johnson, A.C.; Xiao, H.; Hollert, H.; Giesy, J.P. Ecological risk assessment of fifty pharmaceuticals and personal care products (PPCPs) in Chinese surface waters: A proposed multiple-level system. Environ. Int. 2020, 136, 105454. [Google Scholar] [CrossRef]
- Ministry of Environmental Protection of the People’s Republic of China. Technical Guideline for Deriving Water Quality Criteria for Freshwater Organisms; Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2022. (In Chinese)
- ECB. European Union Risk Assessment Report 4-Nonylphenol (Branched) and Nonylphenol; European Chemicals Bureau: Helsinki, Finland, 2002. [Google Scholar]
- Simmons, J.A. Toxicity of major cations and anions (Na+, K+, Ca2+, Cl−, and SO42−) to a macrophyte and an alga. Environ. Toxicol. Chem. 2012, 31, 1370–1374. [Google Scholar] [CrossRef]
- Wurtz, C.B.; Bridges, C.H. Preliminary results from macro-invertebrate bioassays. Proc. Pa. Acad. Sci. 1961, 35, 51–56. [Google Scholar]
- Anderson, B.G. The toxicity thresholds of various sodium salts determined by the use of Daphnia magna. Sew. Work. J. 1946, 18, 82–87. [Google Scholar]
- Benbow, M.E.; Merritt, R.W. Road-salt toxicity of select Michigan wetland macroinvertebrates under different testing conditions. Wetlands 2004, 24, 68–76. [Google Scholar] [CrossRef]
- Dheer, J.M.S.; Dheer, T.R.; Mahajan, C.L. Haematological and haematopoietic response to sodium chloride stress in a freshwater air-breathing fish channa punctatus bloch. J. Fish Biol. 1986, 28, 119–128. [Google Scholar] [CrossRef]
- Hughes, J.S. Acute Toxicity of Thirty Chemicals to Striped Bass (Morone saxatilis); Louisiana Department of Wildlife and Fish Report 318-343-2417; Louisiana Department of Wildlife and Fish: Baton Rouge, LA, USA, 1973.
- Environ, I.C. Chloride Toxicity Test Results, Project No.20-22235A, Report Prepared for Lowa Water Pollut. Control Assoc., ENVIRON Intl. Corp, Nashville, TN:56 p. Available online: https://cfpub.epa.gov/ecotox/ (accessed on 1 December 2022).
- Blasius, B.J.; Merritt, R.W. Field and laboratory investigations on the effects of road salt (NaCl) on stream macroinvertebrate communities. Environ. Pollut. 2002, 120, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Echols, B.S.; Currie, R.J.; Cherry, D.S. Preliminary results of laboratory toxicity tests with the mayfly, isonychia bicolor (ephemeroptera: Isonychiidae) for development as a standard test organism for evaluating streams in the appalachian coalfields of virg. Environ. Monit. Assess. 2010, 169, 487–500. [Google Scholar] [CrossRef]
- Jin, X.; Wang, Y.; Jin, W.; Rao, K.; Giesy, J.P.; Hollert, H.; Richardson, K.L.; Wang, Z. Ecological risk of nonylphenol in China surface waters based on reproductive fitness. Environ. Sci. Technol. 2013, 48, 1256–1262. [Google Scholar] [CrossRef]
- Lockhart, E.E.; Tucker, C.L.; Merritt, M.C. The effect of water impurities on the flavor of brewed coffee ab. J. Food Sci. 1955, 20, 598–605. [Google Scholar] [CrossRef]
- Richter, C.P.; Mclean, A. Salt taste threshold of humans. Am. J. Physiol. 1939, 126, 1–6. [Google Scholar] [CrossRef]
| Industries | Sources of Wastewater | Chloride Content (Cl−, mg/L) | Median (Cl−, mg/L) |
|---|---|---|---|
| Metallurgical factory | Iron smelters wash sewage | 100.0–600.0 | 350.0 |
| Metallurgical factory | Nylon production sewage | 475.0–3340.0 | 1907.5 |
| Petrochemical industry | Synthetic rubber sewage | 2670.0–2800.0 | 2735.0 |
| Petrochemical industry | Butadiene sewage | 1277.0–1350.0 | 1313.5 |
| Petrochemical industry | Ethylene propylene rubber sewage | 361.0–602.0 | 481.5 |
| Printing and dyeing mill | Steam sewage | 103.3–168.1 | 135.7 |
| Printing and dyeing mill | Rinse the sewage | 234.4–296.0 | 265.2 |
| Tannery | Wastewater for ash removal | 1700.0 | - |
| Tannery | Chromite tanning wastewater | 215,000.0 | - |
| Area | Type of Water | Chloride Content (Cl−, mg/L) | Median (Cl−, mg/L) |
|---|---|---|---|
| Luoyang City | Tap water | 24.01–42.97 | 33.49 |
| Recycling water | 22.37–47.55 | 34.96 | |
| Underground water | 52.97–59.49 | 56.23 | |
| Surface water | 12.07–52.97 | 32.52 | |
| Yangtze estuary water | Surface water | 45.16–178.11 | 111.42 |
| Qiantang Estuary | Surface water | 12.70–48.50 | 30.60 |
| Minjiang River Estuary | Water plant intake | 132.00–977.00 | 435.50 |
| Nandu River | Surface water | 17.44–9564.80 | 487.12 |
| Kanazawa Reservoir | Surface water | 42.00–52.42 | 47.21 |
| Hun River | Surface water | 76.77–94.21 | 85.49 |
| Liao River | Surface water | 47.09–64.77 | 55.93 |
| Standards | Category | Limit mg/L |
|---|---|---|
| GB 3838-2002 | Standard limit of supplementary projects of centralized domestic water source of surface water | 250 |
| GB 5749-2006 | Water-quality routine indexes and limits/sensory traits and general chemical indexes | 250 |
| Partial water-quality indicators and limits/sensory traits and general chemical indicators for small centralized and decentralized water supplies | 300 | |
| GB/T 14848-2017 | Groundwater quality classification index class I | ≤50 |
| Groundwater quality classification index class II | ≤150 | |
| Groundwater quality classification index class III | ≤250 | |
| Groundwater quality classification index class IV | ≤350 | |
| Groundwater quality classification index class V | >350 | |
| GB 5084-2021 | Basic control project standard value of irrigation water quality/water farming | 350 |
| Basic control project standard value of irrigation water quality/dry farming | 350 | |
| Basic control project standard value of irrigation water quality/vegetable | 350 | |
| CJ 94-2005 | Drinking water-quality standards/general chemical index limits | 100 |
| CJ/T206-2005 | Routine inspection items of water quality of urban water supply and limited/sensory characters and general chemical indexes | 250 |
| Genus | Species Name | Species Latin Name | Toxic Effect | Endpoint | Exposure (h) | SGMV (mg/L) | References |
|---|---|---|---|---|---|---|---|
| Algae | Green Algae | Raphidocelis subcapitata | Physiology | EC50 | 96 | 11,688.56 | [26] |
| Crustaceans | Aquatic Sowbug | Asellus communis | Mortality | LC50 | 24 | 5600 | [27] |
| Crustaceans | Water Flea | Daphnia magna | Intoxication | Immobile | 48 | 4200 | [28] |
| Crustaceans | Scud | Hyalella azteca | Mortality | LC50 | 96 | 5000 | [29] |
| Fish | Snake-Head Catfish | Channa punctata | Mortality | LC50 | 96 | 12,000 | [30] |
| Fish | Striped Bass | Morone saxatilis | Mortality | LC50 | 24 | 7000 | [31] |
| Fish | Guppy | Poecilia reticulata | Mortality | LC50 | 96 | 11,700 | [32] |
| Insects/Spiders | Common Stonefly | Acroneuria abnormis | Mortality | LC50 | 96 | 10,000 | [33] |
| Insects/Spiders | Stonefly | Agnetina capitata | Mortality | LC50 | 96 | 10,000 | [33] |
| Insects/Spiders | Damselfly | Argia sp. | Mortality | LC50 | 24 | 32,000 | [27] |
| Insects/Spiders | Mayfly | Callibaetis fluctuans | Mortality | LC50 | 96 | 5000 | [29] |
| Insects/Spiders | Midge | Chaoborus americanus | Mortality | LC50 | 96 | 5000 | [29] |
| Insects/Spiders | Mayfly | Isonychia bicolor | Mortality | LC50 | 24~72 | 8000 | [34] |
| Insects/Spiders | Crane Fly | Tipula abdominalis | Mortality | LC50 | 96 | 10,000 | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.; Zhu, Z.; Liao, W.; Yan, Z.; Feng, C.; Xu, D. Freshwater Water-Quality Criteria for Chloride and Guidance for the Revision of the Water-Quality Standard in China. Int. J. Environ. Res. Public Health 2023, 20, 2875. https://doi.org/10.3390/ijerph20042875
Hong Y, Zhu Z, Liao W, Yan Z, Feng C, Xu D. Freshwater Water-Quality Criteria for Chloride and Guidance for the Revision of the Water-Quality Standard in China. International Journal of Environmental Research and Public Health. 2023; 20(4):2875. https://doi.org/10.3390/ijerph20042875
Chicago/Turabian StyleHong, Yajun, Ziwei Zhu, Wei Liao, Zhenfei Yan, Chenglian Feng, and Dayong Xu. 2023. "Freshwater Water-Quality Criteria for Chloride and Guidance for the Revision of the Water-Quality Standard in China" International Journal of Environmental Research and Public Health 20, no. 4: 2875. https://doi.org/10.3390/ijerph20042875
APA StyleHong, Y., Zhu, Z., Liao, W., Yan, Z., Feng, C., & Xu, D. (2023). Freshwater Water-Quality Criteria for Chloride and Guidance for the Revision of the Water-Quality Standard in China. International Journal of Environmental Research and Public Health, 20(4), 2875. https://doi.org/10.3390/ijerph20042875







