Abstract
Immersive experiences in green areas, particularly in forests, have long been known to produce beneficial effects for human health. However, the exact determinants and mechanisms leading to healthy outcomes remain to be elucidated. The purpose of this observational cohort study was to investigate whether inhaling plant-emitted biogenic volatile compounds, namely monoterpenes (MTs), can produce specific effects on anxiety symptoms. Data from 505 subjects participating in 39 structured forest therapy sessions at different Italian sites were collected. The air concentration of monoterpenes was measured at each site. STAI state questionnaires were administered before and after the sessions as a measure of anxiety. A propensity score matching analysis was then performed, considering an above-average exposure to inhalable air MTs as the treatment. The estimated effect was −1.28 STAI-S points (95% C.I. −2.51 to −0.06, p = 0.04), indicating that the average effect of exposure to high MT air concentrations during forest therapy sessions was to decrease anxiety symptoms.
1. Introduction
The preventive and healing effects of green environments on psychophysical well-being have been documented extensively in scientific literature and numerous studies suggest that forest exposure is associated with a wide range of benefits, covering both psychological [1,2,3,4,5], and physiological aspects [6,7,8,9]. In particular, a few experimental studies have demonstrated the effects of forest immersion in stress mitigation and the induction of physiological relaxation, especially with regard to anxiety levels [10,11,12,13,14], a topic analyzed in recent literature reviews [5,15,16].
The beneficial effects of visiting a forest derive from the combined stimulation of all senses by specific features of the natural environment [7] whose impact and quantitative assessment have not yet been fully elucidated. Biogenic volatile organic compounds (BVOCs) emitted by plants and soil in the forest atmosphere, and in particular monoterpenes (MTs), as key constituents of BVOCs, have often been suggested as one of the determinants of the interaction between forest ecosystems and human health [17,18,19,20], especially those related to long-lasting effects on the immune system after forest exposure [18,21,22,23,24,25].
All plant organs, from flowers to roots, can generate and release MTs [26], with leaves generally responsible for the highest emission rates [27]. MT emission rates and chemical profiles vary widely among different species of forest trees [28,29] and they depend on physiological (plant age and developmental stage) and physicochemical factors, i.e., related to leaf structure (presence or absence of storage structures and stomatal openness) [30]. The individual perception of benefits induced by plant-emitted MTs has stimulated research efforts to expedite the modeling and prediction of forest emissions [31,32], along with preliminary studies aimed at predicting nose-level concentrations of BVOCs [33]. Important efforts have also been made to design forest therapy programs and trails based on potential emissions of BVOCs released by dominant plant species [34].
Recently, several studies aiming at assessing and quantifying BVOCs in forest sites have been published [20,31,32,35,36], owing to the need to gain further insights into forest characteristics potentially related to specific effects on health outcomes [34,37,38,39,40,41,42].
Overall, the evidence about MT activity on human health mostly derives from in vitro, animal, or indoor/laboratory experiments, usually with a small number of study participants [43], and very few studies take into consideration MT concentrations along with the measurement of health outcomes [23,44]. Only recently, a few studies investigated the most important psychophysiological short-term effects of individual exposure to plant-emitted MTs. However, such studies were limited to specific and/or numerically scarce target groups, for example, maladjusted soldiers [45], or to specific plant species, such as Cinnamomum burmannii, growing in partially controlled environments [46]. In general, even though the results of these studies are encouraging, quantitative experiments in natural environments are still lacking.
There is also a dearth of studies investigating any specific health effects of terpenes inhaled during forest exposure and their mechanism of action from a pharmacological point of view [47]. Only two studies measured the serum concentration of monoterpenes after forest exposure, reporting a sixfold increase of α-pinene levels after walking in a conifer forest for one hour [48] and a higher absorption of α-pinene after walking in an evergreen broadleaf forest for two hours, but only in participants with lower baseline blood concentrations of monoterpenes [35]. Although relevant, the impact of the latter two studies was affected by small sample sizes. Moreover, some preclinical evidence exists from animal studies of the possible anxiolytic activity of α-pinene [7,49,50,51].
Anxiety is a highly prevalent nonpsychotic mental disorder, and one of the most increasing psychiatric disorders of the last decades. Anxiety disorders have a worldwide prevalence of up to 15% in the general population and are more common in women [52,53]. The global burden of hypertension is extensively impacted by anxiety, both as a risk factor and as an exacerbating factor [54]. Anxiety is also a known risk factor for major adverse outcomes in heart diseases, especially in heart failure, chronic and acute coronary syndromes, and cardiomyopathies [55,56,57,58]. Finding new sustainable strategies to decrease the burden of anxiety symptoms is an important goal for researchers and would have a significant impact on health policies.
The aim of this study was to assess whether exposure to inhalable MTs during 3-h long forest therapy sessions can produce specific effects on anxiety levels. This study follows a previous pilot study, where the total concentration of BVOCs was preliminarily suggested as a contributing factor associated with short-term improvements in mood states, in particular with anxiety reduction in subjects involved in forest therapy sessions organized in remote green areas [14]. To the authors’ best knowledge, this is the first attempt, grounded on a large number of participants, to clarify the extent of the effect on anxiety levels of MTs inhaled in a natural setting.
2. Materials and Methods
2.1. Design and Participants
This research was originally designed as an observational cohort study. Experimental sessions were conducted between September 2021 and October 2022 at 39 sites, including 27 remote mountain forest sites, 3 hill forest sites, 1 remote coastal pinewood forest site, 7 urban parks in Italy, and 1 remote mountain forest site in Carinthia, Austria, located at altitudes between 5 m a.s.l. and 1800 m a.s.l. Figure 1 shows the location of these sites. During each session, one or more groups of participants, each made up of about 15–20 people, were guided along identified paths by trained operators, for a total duration of 3.0 ± 0.5 h. Data were collected immediately before and after each forest therapy session, as in pre–post studies without a follow-up period.
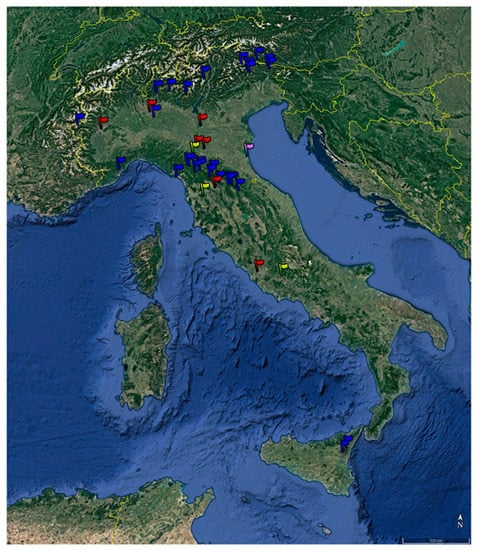
Figure 1.
Location of the experimental sites. Blue: remote mountain; yellow: hill; violet: remote coastal pinewood; red: urban park.
Only adults being able to autonomously fill in a questionnaire written in Italian and were willing to participate in forest therapy sessions were included in this study. Exclusion criteria were minor age (<18 years), any acute illnesses requiring medications other than those usually taken on a daily basis for chronic diseases, and any major physical or mental health problems incompatible with outdoor walking for a few hours. Participants were allowed to attend only one session, to prevent any possible carry-over effect.
Only participants who gave their informed consent were included in the data collection. All participants were treated in accordance with the ethical guidelines for research provided by the Declaration of Helsinki and its revisions, and the study was approved by the local ethics committee (CNR Ethics Committee n. 0069654/2021).
2.2. Outcome Measures
All participants autonomously reached each forest therapy site, and upon arrival, they were required to fill in the informed consent form. Then, every participant was presented with an anonymous questionnaire (with a unique ID code), which was aimed at collecting information about gender, age, place of residence, job, height, weight, chronic diseases, allergies, medications used, smoking habit, sports activities, and any previous experience in meditation practices. Moreover, the participants were asked to rate their subjective satisfaction in their relationships with friends and relatives with a 10-point Likert scale. Then, they were asked to fill in two psychometric questionnaires, the State-Trait Anxiety Inventory (STAI) and the Profile of Mood States (POMS) questionnaires.
The STAI questionnaire is widely used to measure state and trait nondisorder-specific anxiety, in both healthy and clinical populations [59]. It consists of a 40-item self-report measure of anxiety, using a 4-point Likert-type scale for each item. It has two scales: state anxiety, i.e., how one feels at a given moment (STAI-S), and trait anxiety, i.e., how one generally feels (STAI-T), both consisting of 20 items. Total scores were calculated by summing up the items in each respective form of anxiety (ranging from 20 to 80), where higher scores reflect greater levels of anxiety. The POMS questionnaire consists of 40 items, each measured with a 5-point Likert scale, related to domains such as tension, depression, anger, vigor, fatigue, confusion, and self-esteem [60]. Although both the STAI-S and the POMS questionnaires are widely used to explore different domains, the STAI-S was chosen as the outcome measure of state anxiety, i.e., the outcome measure of this study. Unlike the POMS, the STAI-S questionnaire is often used with clinical and subclinical study populations, and it can detect subtle changes in anxiety levels, even when there are large differences in baseline scores, which occur in groups differing in their vulnerability to anxiety, as is the case with highly heterogeneous participants to forest therapy sessions [61].
The STAI-S questionnaire was administered immediately before and after each forest therapy session in order to intercept any short-term effects (changes from baseline) elicited during the sessions. Change-from-baseline STAI-S scores reported by the study participants were considered the main outcome measure.
The STAI-T questionnaire was administered only at the baseline to better characterize the participants on the basis of their trait anxiety, which is a stable long-term tendency to anxiety. For a more comprehensive evaluation, the POMS questionnaires were also collected at baseline in order to characterize the participants in terms of other baseline mood states, i.e., nonanxiety-related POMS domains.
2.3. Exposure
The study participants were involved in forest therapy sessions aimed at maximizing their exposure to air MTs for an amount of time considered sufficient to be absorbed systemically and possibly exert a specific pharmacodynamic effect.
A professional psychologist or psychotherapist took part in each of the forest therapy sessions. The conduction protocol consisted of simple instructions to the participants to focus one’s attention on the external environment. The protocol was chosen to give reproducible instructions for all the psychologists or psychotherapists involved, in order to minimize any interferences related to the therapists’ personal styles and to achieve the most homogeneous experimental conditions possible.
The overall duration of each session was approximately 3-h, including short, slow walks, interspersed with five stops, and a final walk, before filling out the STAI-S questionnaires after the forest therapy experience. The physical intensity of the sessions was kept low to avoid possible biases due to adrenergic hyperactivation. Each session was performed in the morning, starting around 10:00 a.m. and ending around 1:00 p.m., local time (corresponding for all sessions to GMT+2). Forest therapy sessions were performed only if the weather was at least fair, without rain, and with comfortable temperatures within the range of 12 to 26 °C, collected by hand-held thermometers at the BVOCs sampling sites. The standardization of the method of conducting all forest therapy sessions, along with the same timing, was aimed at reducing possible biases.
Measurement of Biogenic Volatile Organic Compounds
The total and individual concentrations of different BVOCs in each forest site were measured through 1-h air samplings performed at 9.00 a.m., 11.30 a.m. and 2.00 p.m. Air was passed through the adsorption traps by using portable pumps (Pocket389Pump, SKC Inc., Washington County, PA, USA), that provided a constant flow of air of 200 mL/min, yielding sampling volumes of 12 l per hour.
Traps were made of inert metal tubes (8 cm × 0.3 cm i.d.) filled with Tenax TA and Carbograph 1TD (350 mg; 35/60 and 40/60 mesh, respectively), provided by Markes International, Ltd. (Llantrisant, UK), and they were stored at −20 °C until the analysis. BVOCs, released from traps using a thermal-desorption unity series 2, (Markes International, Sacramento, CA, USA), were injected into a 60 m capillary column (HP-1, 0.25 mm I.D.) internally coated with a 0.25 μm film of polymethylsiloxane, provided by J&W Scientific USA, Agilent Technologies (Palo Alto, CA, USA). BVOC separation was performed on a 7890A gas chromatograph and eluted compounds were detected with a 5975C mass spectrometer (GC–MS, Agilent Technologies, Wilmington, DE, USA).
For BVOC identification, retention times and mass spectra were collected and compared with the NIST 11 library, using the Agilent MassHunter Qualitative Analysis software. After identification, the compounds were quantified with an external standard calibration approach, using a gas cylinder with predefined concentrations of different BVOCs (isoprene and α-pinene; manufacturer: Apel-Riemer Environmental Inc., Broomfield, CO, USA). The precision of BVOC analysis varied among different chemical species and was generally characterized by a limited percentage range (2–5%). Blank emissions and artifact formation, which can affect the sensitivity and overall performance of this method, were determined with clean tubes. The detection limit of BVOCs was commonly achieved at the 0.1 ng level. Relative response factors were determined for all components, and for a minority of them, interpolation techniques were used. The concentration of each compound sampled in the experimental sites was calculated in µgm−3 as the mean and standard error of the three replicates [62,63,64].
Calibration curves were obtained with standard compounds (Sigma–Aldrich Chemical Co. St Louis, MO, USA). The concentration of each compound was calculated in µgm−3 as the mean and standard error of the three replicates. Figure 2 shows the calibration curve for α-pinene as one of the most representative MTs.
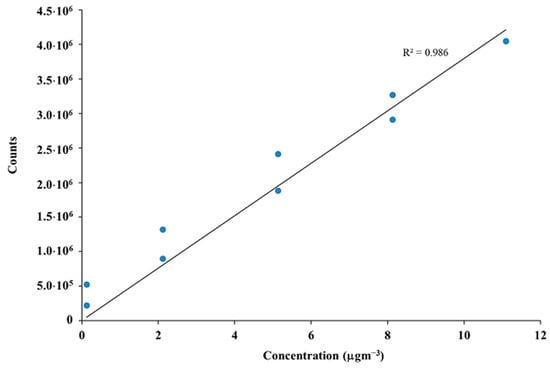
Figure 2.
Calibration curve for α-pinene.
Among BVOCs, the air concentrations of MTs α-pinene, camphene, o-cymene, and sabinene were examined. For the purposes of the study, the concentration of MTs was measured at 11.30 a.m. at each site, because that timing was the most representative of the sessions. The total air concentration of MTs was selected as the principal exposure factor.
2.4. Predictors and Confounders
Possible predictors and confounders for a specific effect of MT exposure on anxiety symptoms were chosen among the different demographic, physiologic, psychologic, and sociologic domains in order to take advantage of the large sample size recruited for this study, to choose a good comparison between groups and, therefore, to intercept any possible specific effect of the exposure to inhaled MTs. The peculiarity of the propensity-score matching is indeed the chance to group individuals exposed and unexposed to a specific intervention by forming “couples” of subjects who were as similar as possible for all the covariates included in the propensity-matching model. Therefore, this technique allows for the correction of possible selection biases determined by a nonrandomized controlled trial design. In fact, when a good covariate balance between intervention and control groups was obtained, an estimate of the average treatment effect on the treated individual (ATT) was possible and accountable as a specific effect.
The demographic variables included in this study were age, sex, body height, and weight. Physiologic variables were major health risk factors for the general population, namely the body mass index (BMI), hypertension, diabetes mellitus, dyslipidemia, smoking habit, and chronic disorders such as asthma or allergies that may be triggered by environmental stimuli during nature experiences. Other physiologic variables considered as possible confounders of anxiety symptoms in the experimental setting were the physical exercise habit, any experience in meditation practices, and the session site type. The sociological variables considered were having children and the degree of satisfaction from the relationship with friends and relatives. Psychological covariates considered as possible confounders and modifiers were baseline long-term tendency to anxiety (STAI-T scores) and, due to the short-term nature of anxiety symptoms (measured with the STAI-S questionnaire as above described), other psychological covariates, such as baseline POMS depression, vigor, fatigue, confusion, anger, and self-esteem domain scores. Missing data in one or more of the aforementioned key covariates implied the exclusion of the participant from the final analysis.
2.5. Data Analysis
Microsoft Excel was used to organize the data collected. The software used for statistical analyses was “R” [65], using RStudio ver. 2022.07.2 [66], and the packages “matchit” [67], “marginaleffects” and “stats” [65]. The threshold for statistical significance of the overall effect size was set at p < 0.05.
Total MT air concentrations at any considered site were analyzed, and the average concentration value, equal to 0.20 µgm−3 (range 0.01 to 0.99 µgm−3), was established as a cut-off value between low and high MT exposure.
Choosing the average concentration of the volatile substances analyzed allowed the partitioning of the whole sample of participants into two subgroups of comparable numerosity and, therefore, maximized the effectiveness of the propensity score matching. High MTs exposure was considered as the treatment of interest.
The Mann–Whitney nonparametric test was used to test whether differences in MTs and α-pinene concentrations measured in different landscapes were statistically significant [68].
One-way ANOVA tests were performed to compare the effect of different MT concentrations on the anxiety outcome and test for a statistically significant difference in anxiety levels between the exposed and unexposed groups.
Generalized linear model regression was performed to test for any correlation between the anxiety level outcome as the dependent variable and other continuous independent variables.
Then, the propensity score matching, which is a well-known statistical method especially used in medicine, epidemiology, and behavioral research [69,70,71,72,73], was performed to estimate the average marginal effect of the exposure to high MT air concentrations during forest therapy sessions on change-from-baseline STAI-S scores reported by the study participants and accounting for confounders and effect modifiers, as described above. A rule-of-thumb of at least 20 participants per included covariate was applied.
It was first decided to try with a 1:1 nearest neighbor propensity score matching without replacement and a propensity score estimated using logistic regression of the intervention on the covariates. This matching specification yielded an acceptable, yet not fully satisfactory, balance (standardized mean differences for the covariates <0.15), so it was instead decided to opt for an optimal full matching on the propensity score, which yielded a satisfactory balance. The propensity score was estimated using a logistic regression of the intervention on the covariates. Baseline covariates were considered balanced across the high and low MT exposure groups in the matched sample if the absolute difference of the standardized difference was ≤0.1.
The efficacy endpoint, which was a significant short-term reduction in STAI-S scores after a forest therapy session, was compared across the propensity-matched groups fitting a linear regression model with the change-from-baseline (post-test values minus pre-test values) STAI-S score as the outcome of interest and the intervention, covariates, and their interactions as predictors. This analysis also included the full matching weights in order to estimate the effect of the intervention and its confidence interval. A g-computation was then performed in the matched sample to estimate the average effect of the intervention on those who received it (ATT). A cluster-robust variance was used to estimate its confidence interval with matching stratum membership as the clustering variable.
An additional analysis was performed, using the propensity score matching to estimate the average effect of the exposure to above average α-pinene air concentrations (0.10 μgm−3) on change-from-baseline anxiety symptom scores reported by the study participants, accounting for confounding by the aforementioned covariates. The same procedure and matching methods, as described above, were applied with similar results, with the only difference being that the propensity score was estimated using a probit regression of the intervention on the covariates, which in this case yielded better balance than logistic regression (covariates standardized mean difference <0.1 vs. <0.15).
Other additional analyses were performed considering the 3rd quartile of total MT and α-pinene concentrations as the cutoff values for treatment exposure, in order to check for possible dose-dependent effects. Therefore, the propensity score matching was applied to estimate the average effect of the exposure to MTs (0.28 μgm−3) and α-pinene air concentrations (0.16 μgm−3) above the 3rd quartile on change-from-baseline anxiety symptom scores reported by the study participants, accounting for the aforementioned covariates. As with the previous analyses, for the propensity score of the above-the-3rd quartile MT concentration, the best achievable covariate balance was estimated using a logit regression of the intervention on the covariates, which included three covariates with a standardized mean difference of <0.12 instead of <0.1. This was considered acceptable, especially considering the high number of covariates involved. For the propensity score of the above-the-3rd quartile α-pinene concentration, the best achievable covariate balance was estimated using a probit regression of the intervention on the covariates, yielding a good balance with a standardized mean difference of <0.1 for all covariates. Finally, further similar analyses were conducted for above-average sabinene, o-cymene, and camphene concentrations exposures, yielding a good balance (standardized mean difference <0.1) for each test.
In the above-described cases, the efficacy endpoint was compared across the propensity-matched groups fitting a linear regression model with the change-from-baseline STAI-S score as the outcome of interest and the intervention, the above-mentioned covariates, and their interactions as predictors, including the full matching weights, in order to estimate the effect of the intervention. A g-computation was then performed in the matched sample to estimate the ATT of those who received the intervention, and a cluster-robust variance was used to estimate its confidence interval with matching stratum membership as the clustering variable.
3. Results
3.1. Preliminary Analyses
Overall, 655 participants were recruited, and 150 of them were excluded from the final analysis due to missing data in one or more key variables (i.e., demographic data and psychometric tests). The final sample consisted of 505 participants, with a higher share of females (65%). The distribution of participants in different age groups was as follows: 18–29 years (10%), 30–44 years (19%), 45–54 years (19%), 55–69 years (42%), and over 70 (10%).
Figure 3 shows the average and standard deviation of MT and α-pinene concentration levels in mountain, hill, pinewood, and urban park sites. A clear tendency toward lower concentration levels of both MTs and α-pinene in urban parks can be noted. However, due to the high variability and relatively low numerosity of hill, pinewood, and urban parks, no significant difference based on the Mann–Whitney test was found. Apparently, α-pinene accounted on average for nearly half of the total MT concentrations in each landscape.
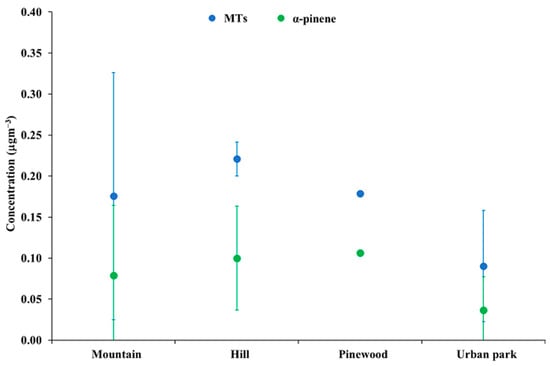
Figure 3.
Average and standard deviation of MT and α-pinene concentration levels in mountain, hill, pinewood, and urban park sites.
A linear regression considering STAI-S scores observed after any forest therapy sessions as the dependent variable and total MT concentration as the independent variable, which resulted in an estimated effect of −3.24 STAI-S points (F = 4.497, p = 0.03), thus showing that on average any increase in MTs concentration was associated with a decrease in STAI-S scores. However, in consideration of the many possible confounding variables, such a result should not be interpreted as causation.
Table 1 shows the one-way ANOVA results for change-from-baseline STAI-S scores after exposure to high or low MTs and α-pinene air concentrations, with thresholds chosen as the average and the 3rd quartile concentrations, as well as the gender-based subgroup analysis. The tendency for all groups was towards anxiety symptom reduction after exposure to higher total MT and α-pinene air concentrations. The one-way ANOVA for different landscapes (mountain, hill, and urban parks) indicated a tendency towards anxiety symptoms reduction after exposure to higher total MT and α-pinene air concentrations. However, no such analyses could achieve statistical significance.

Table 1.
Results of the one-way ANOVA for exposure of the whole group and the subgroups of females and males to MT and α-pinene air concentrations above the average and above the 3rd quartile on change-from-baseline STAI-S scores. Significant results are indicated by *.
Since the one-way ANOVA could not account for confounding variables, the propensity-matching methodology was used to test for possible specific effects of MT exposure on anxiety levels.
Figure 4 shows the balance of covariates before and after propensity matching for exposure to high total monoterpenes air concentration. After matching, all standardized mean differences for the covariates were below 0.1, indicating adequate balance. Since full matching normally uses all units allocated to the intervention and control, no units were discarded.
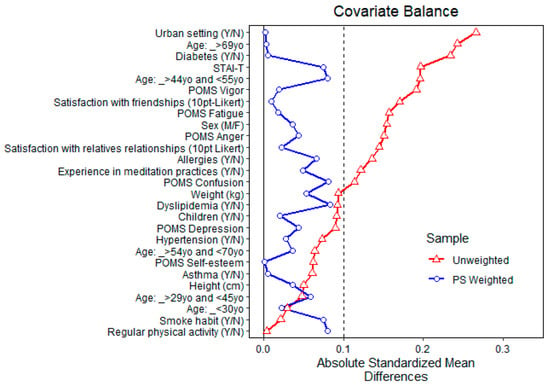
Figure 4.
Balance of covariates before and after propensity matching (PS weighted) for exposure to high (above the average) total monoterpenes air concentration.
Figure 5 shows the balance of covariates before and after propensity matching for exposure to high total α-pinene air concentration. After matching, all standardized mean differences for the covariates were below 0.1, indicating adequate balance. Since full matching was used as well, no units were discarded.
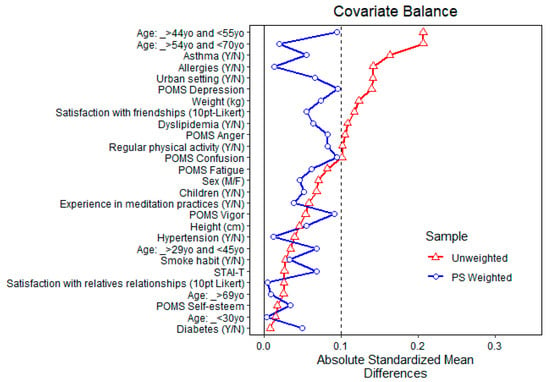
Figure 5.
Balance of covariates before and after propensity matching (PS weighted) for exposure to high (above the average) α-pinene air concentration.
Figure 6 and Figure 7 show the balance of covariates before and after propensity matching for exposure to above-the-3rd quartile total MTs and α-pinene air concentrations, respectively [69,70,71,72,73].
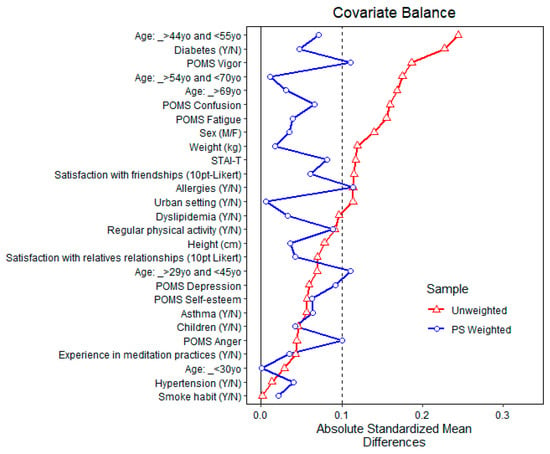
Figure 6.
Balance of covariates before and after propensity matching (PS weighted) for exposure to above-the-3rd quartile total monoterpenes air concentration.
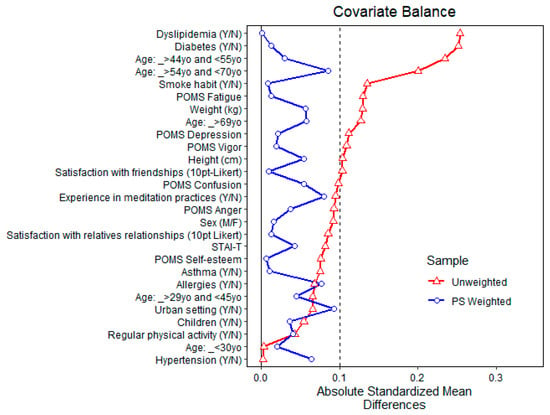
Figure 7.
Balance of covariates before and after propensity matching (PS weighted) for exposure to above-the-3rd quartile α-pinene air concentration.
3.2. Anxiolytic Effect of the Exposure to Monoterpenes and α-Pinene
Based on the propensity score matching analysis described in Section 3.1, the estimated effect on anxiety symptom levels, following the exposure to above average total concentration of MTs, was −1.28 STAI-S points (95% C.I. −2.51 to −0.06, p = 0.04), indicating that the average effect of the exposure to high MT air concentrations during forest therapy sessions was to decrease anxiety symptoms.
In the second analysis, focused on α-pinene, the estimated effect was −1.31 STAI-S points (95% C.I. −2.15 to −0.12, p = 0.03), indicating that the average effect of exposure to high α-pinene air concentrations was to decrease anxiety symptoms, confirming the previous result and suggesting a pivotal role of α-pinene in the anxiety-reducing effect.
In the third analysis, focused on above-the-3rd quartile MT concentrations, the estimated effect was −1.61 STAI-S points (95% C.I. −2.84 to −0.4, p = 0.01), indicating that the average effect of exposure to above-the-3rd quartile MTs concentrations in forests was to decrease anxiety symptoms.
In the fourth analysis, focused on above-the-3rd quartile α-pinene concentrations, the estimated effect was −1.68 STAI-S points (95% C.I. −3.2 to −0.16, p = 0.03), indicating that the average effect of exposure to above-the-3rd quartile α-pinene air concentrations was to decrease anxiety symptoms, suggesting that α-pinene may have a major role in the anxiolytic effects of inhaled MTs.
Finally, analyses conducted for investigating the effects of the exposure to above-the-average air concentrations of sabinene, o-cymene, and camphene on anxiety levels did not produce any statistically significant result, even after good covariate balance was obtained. However, it is interesting to note a trend toward statistical significance for above-average sabinene exposure (ATT = −1.226, p = 0.08).
Table 2 summarizes the above-reported results of the propensity score-matched analysis estimating the average effect on state anxiety (STAI-S) in the individuals exposed.

Table 2.
Results of the propensity score-matched analysis estimating the average effect on state anxiety (STAI-S) in the individuals exposed (ATT). Significant results are indicated by *.
4. Discussion
This study showed for the first time a specific effect of the total concentration of inhaled MTs and, in particular, of α-pinene, on anxiety symptom levels of participants in forest therapy sessions at various sites. Although this was not a randomized controlled trial, the propensity matching methodology, applied to a quite large cohort of subjects involved in several identically structured forest therapy sessions in different sites, allowed an accurate balance for all participants’ variables, including their psychological baseline characteristics and involvement in experiences in urban parks or remote forests. This way, the participants could be partitioned into an intervention group, exposed to MT concentrations above a cut-off level derived from the available data, and a control group, exposed to lower concentrations.
The quite comparable effect sizes of STAI-S reduction associated with above-average total MTs and high α-pinene air concentrations accounted for around 28% of the average decrease in STAI-S observed across the entire sample (−4.6 points), which provided an estimate of the specific anxiolytic contribution of these volatile compounds. Moreover, the increased anxiolytic effect of MTs and α-pinene above the 3rd quartile of the respective air concentration may suggest a certain degree of dose dependency.
These results confirm the hypothesis formulated in the previous pilot study, where short-term anxiety improvements observed after forest exposure were likely associated with high levels of BVOCs in the air [14].
Inhalation of natural essential oils (EOs), of which MTs (including α-pinene) are important constituents, has long been the subject of clinical studies that showed significant effects on depression and anxiety disorders in humans, as well as of preclinical examinations, which confirmed significant effects on depression and anxiety-like symptoms in animal models [74,75].
On the one hand, the role of the cardiorespiratory system, responsible for the systemic absorption of volatiles and the subsequent delivery to organs, including the central nervous system, and, on the other hand, the direct stimulation of the brain through olfactory organs, were hypothesized as fundamental for explaining the therapeutic efficacy of inhaled EO molecules on mood disturbances. Olfactory stimuli can directly affect mood, through direct anatomical and functional links of the olfactory system with the limbic system. However, more precise mechanisms of action can be revealed only by experiments with isolated EO constituents, including individual MTs such as α-pinene [74].
The results of this study allow the identification of a ubiquitous class of components of the atmosphere in green areas, namely MTs, as specific therapeutic agents with regard to the levels of anxiety symptoms. Moreover, our results show that α-pinene may play a major role in the anxiolytic effects of inhaling MT-rich air, leaving open questions about possible specific effects of other monoterpenes. These findings substantiate the emerging concept of the environment as a “healing environment”, a source of valuable elements actively promoting human health and well being, and not only as a resource to be protected and preserved from pollution [76].
The elucidation and quantification of a specific healing mechanism of forest environments and green areas can offer a powerful tool to direct the widespread efforts to plan and optimize forest therapy trails, often relying on the prediction of MT concentrations based on plant species and meteorological variables [31,32,34,77].
Studies documenting the effects of forest immersion experiences on anxiety symptoms and stress, such as those analyzed in recent comprehensive reviews [78,79], could be partially reinterpreted in light of our study findings, and future experiments should take these results into adequate account. As well, further studies might consider investigating the effects on anxiety symptoms of MT or α-pinene inhalation duration, the respective efficacy thresholds, and possibly the effects of MTs (also other than α-pinene) on physiological aspects.
Finally, this growing body of evidence indicates that green environments have an active and specific effect in reducing the burden of anxiety symptoms in the general population, thus impacting not only mental but also, indirectly, cardiovascular health, as shown in recent large cohort studies [80], which implies great potential in terms of saving public health expenditure. Therefore, advocacy for the encouragement of protecting and creating green areas should be addressed.
The main limitations of this study were the lack of a randomized controlled trial design and a significant drop-out rate from the final analysis due to suboptimal control over the participants’ compliance with filling out the self-report questionnaires. Another minor limitation consisted of the gender imbalance, with the dominance of female participants over males. These limitations should be considered for future studies on the topic.
5. Conclusions
The results of this study showed for the first time that inhalation of plant-emitted MTs, and in particular α-pinene, can produce a specific anxiolytic effect. On the basis of this evidence, as a practical recommendation to help manage anxiety symptoms, it can be suggested to regularly visit sites whose air is rich in MTs such as α-pinene. In this regard, in order to identify proper sites, it would be important to measure these BVOCs in the air, or at least to identify areas where there are tree species that are likely to release large quantities of these substances in the atmosphere. Inhaling these natural compounds during a 3-h outdoor relaxing session can improve anxiety levels and positively contribute to the individual’s well-being.
Our findings highlight the importance of focusing on the environment as a source of health-promoting factors, with the necessity to promote appropriate policies and raise awareness about environmental issues. Further perspectives are to investigate the possible effects of inhalable forest MTs on physiological outcome measures.
Author Contributions
Conceptualization, D.D., F.M. and F.Z.; Data curation, D.D., F.M., G.G. and F.Z.; Funding acquisition, F.M. and F.Z.; Investigation, D.D., F.M., R.B., L.N. and F.Z.; Methodology, D.D. and F.Z.; Project administration, F.Z.; Resources, F.M. and F.Z.; Software, D.D., R.B. and L.N.; Supervision, F.M. and F.Z.; Validation, D.D., F.M. and G.G.; Visualization, D.D. and F.M.; Writing—original draft, D.D., F.M., R.B., L.N. and F.Z.; Writing—review & editing, D.D., M.A., D.A. and G.N. All authors have read and agreed to the published version of the manuscript.
Funding
Some field trials were partially supported by the following funders: L’Ovile Cooperativa di Solidarietà Sociale SCRL, grant number 2957 of 06.14.2021 (Project RE.in.VENTA, funded by Fondazione Pietro Manodori); Acqua & Terme Fiuggi S.p.A., grant number 1990 of 04.25.2021; Consorzio Melinda S.c.a., grant number 5610 of 11.23.2021; Comune di Vaglia (FI), grant number 142 of 12.09.2021; University of Udine, grant number DG1079776 of 03.21.2022 (project FORTER, funded by INTERREG V-A IT-A CLLD-HEurOpen).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the National Research Council (CNR) Ethics Committee n. 0069654/2021 (20/10/2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data are available by contacting the corresponding author.
Acknowledgments
Giovanni Margheritini (Central Scientific Committee of the Italian Alpine Club) is gratefully acknowledged for his tireless support in all the field trials, and for his precious advice. Franco Finelli and Francesco Riccardo Becheri (president and head of the psychologist group of the Central Medical Committee of the Italian Alpine Club, respectively) are gratefully acknowledged, along with all the psychologists and psychotherapists who conducted the forest therapy sessions under their supervision: Ilaria Butti, Pasquale Costigliola, Anna Maria Debolini, Mila Ferrari, Patrizia Garberi, Vivian Pellegrinelli, Valentina Penati, Anna Roncoroni, Emanuela Venturelli. Chiara Castano and Marta Tondelli are gratefully acknowledged for digitizing part of the original data within their dissertation work for the Postgraduate Master Course in Clinical Phytotherapy, which was managed by CERFIT (AOU Careggi, Florence, Italy) and supervised by Fabio Firenzuoli, Eugenia Gallo and Valentina Maggini, to whom the authors are also very grateful. Francesco Centritto and Anna Corli (CNR-IBE), who digitized part of the data and analyzed part of the BVOC data, respectively, are gratefully acknowledged. The study could not have been performed without the material support of several Sections of the Italian Alpine Club. Carlo Natali and Giancarlo Tellini, as the president of the Tuscany Scientific Committee “Fiorenzo Gei” and president of the Tuscany Regional Group of the Italian Alpine Club, respectively, are gratefully acknowledged for their continuous support and advice.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hansen, M.M.; Jones, R.; Tocchini, K. Shinrin-Yoku (Forest Bathing) and Nature Therapy: A State-of-the-Art Review. Int. J. Environ. Res. Public Health 2017, 14, 851. [Google Scholar] [CrossRef]
- Li, Q. Effect of Forest Bathing (Shinrin-Yoku) on Human Health: A Review of the Literature. Santé Publique 2019, 1, 135–143. [Google Scholar] [CrossRef]
- Twohig-Bennett, C.; Jones, A. The health benefits of the great outdoors: A systematic review and meta-analysis of greenspace exposure and health outcomes. Environ. Res. 2018, 166, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Corazon, S.S.; Sidenius, U.; Poulsen, D.V.; Gramkow, M.C.; Stigsdotter, U.K. Psycho-Physiological Stress Recovery in Outdoor Nature-Based Interventions: A Systematic Review of the Past Eight Years of Research. Int. J. Environ. Res. Public Health 2019, 16, 1711. [Google Scholar] [CrossRef] [PubMed]
- Yeon, P.-S.; Jeon, J.-Y.; Jung, M.-S.; Min, G.-M.; Kim, G.-Y.; Han, K.-M.; Shin, M.-J.; Jo, S.-H.; Kim, J.-G.; Shin, W.-S. Effect of Forest Therapy on Depression and Anxiety: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 12685. [Google Scholar] [CrossRef]
- Andersen, L.; Corazon, S.S.; Stigsdotter, U.K. Nature Exposure and Its Effects on Immune System Functioning: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 1416. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Donelli, D.; Carlone, L.; Maggini, V.; Firenzuoli, F.; Bedeschi, E. Effects of forest bathing (shinrin-yoku) on individual well-being: An umbrella review. Int. J. Environ. Health Res. 2021, 32, 1842–1867. [Google Scholar] [CrossRef]
- Stier-Jarmer, M.; Throner, V.; Kirschneck, M.; Immich, G.; Frisch, D.; Schuh, A. The Psychological and Physical Effects of Forests on Human Health: A Systematic Review of Systematic Reviews and Meta-Analyses. Int. J. Environ. Res. Public Health 2021, 18, 1770. [Google Scholar] [CrossRef]
- Chae, Y.; Lee, S.; Jo, Y.; Kang, S.; Park, S.; Kang, H. The Effects of Forest Therapy on Immune Function. Int. J. Environ. Res. Public Health 2021, 18, 8440. [Google Scholar] [CrossRef]
- Tomasi, S.; Di Nuovo, S.; Hidalgo, M.C. Environment and mental health: Empirical study on the relationship between contact with nature and symptoms of anxiety and depression (Ambiente y salud mental: Estudio empírico sobre la relación entre contacto con la naturaleza, síntomas de ansiedad y de depresión). Psyecology 2020, 11, 319–341. [Google Scholar] [CrossRef]
- Song, C.; Ikei, H.; Kagawa, T.; Miyazaki, Y. Effects of Walking in a Forest on Young Women. Int. J. Environ. Res. Public Health 2019, 16, 229. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Jiang, T.; Guo, L.; Mingyan, J.; Liu, A.; Jiang, Z.; Liu, Z.; Chen, Q. Effects of Walking in Bamboo Forest and City Environments on Brainwave Activity in Young Adults. Evid.-Based Complement. Altern. Med. 2018, 2018, 9653857. [Google Scholar] [CrossRef]
- Song, C.; Ikei, H.; Park, B.-J.; Lee, J.; Kagawa, T.; Miyazaki, Y. Psychological Benefits of Walking through Forest Areas. Int. J. Environ. Res. Public Health 2018, 15, 2804. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Albanese, L.; Antonelli, M.; Baraldi, R.; Becheri, F.R.; Centritto, F.; Donelli, D.; Finelli, F.; Firenzuoli, F.; Margheritini, G.; et al. Short-Term Effects of Forest Therapy on Mood States: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 9509. [Google Scholar] [CrossRef] [PubMed]
- Farrow, M.R.; Washburn, K. A Review of Field Experiments on the Effect of Forest Bathing on Anxiety and Heart Rate Variability. Glob. Adv. Health Med. 2019, 8, 2164956119848654. [Google Scholar] [CrossRef] [PubMed]
- Kotera, Y.; Richardson, M.; Sheffield, D. Effects of Shinrin-Yoku (Forest Bathing) and Nature Therapy on Mental Health: A Systematic Review and Meta-analysis. Int. J. Ment. Health Addict. 2020, 20, 337–361. [Google Scholar] [CrossRef]
- Antonelli, M.; Donelli, D.; Barbieri, G.; Valussi, M.; Maggini, V.; Firenzuoli, F. Forest Volatile Organic Compounds and Their Effects on Human Health: A State-of-the-Art Review. Int. J. Environ. Res. Public Health 2020, 17, 6506. [Google Scholar] [CrossRef]
- Li, Q.; Kobayashi, M.; Wakayama, Y.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Shimizu, T.; Kawada, T.; Park, B.-J.; et al. Effect of Phytoncide from Trees on Human Natural Killer Cell Function. Int. J. Immunopathol. Pharmacol. 2009, 22, 951–959. [Google Scholar] [CrossRef]
- Lee, K.J.; Hur, J.; Yang, K.-S.; Lee, M.-K.; Lee, S.-J. Acute Biophysical Responses and Psychological Effects of Different Types of Forests in Patients With Metabolic Syndrome. Environ. Behav. 2018, 50, 298–323. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, J.; Wu, Q.; Chen, Z.; Wang, G. Seasonal dynamics of VOCs released from Cinnamomun camphora forests and the associated adjuvant therapy for geriatric hypertension. Ind. Crop. Prod. 2021, 174, 114131. [Google Scholar] [CrossRef]
- Li, Q.; Nakadai, A.; Matsushima, H.; Miyazaki, Y.; Krensky, A.M.; Kawada, T.; Morimoto, K. Phytoncides (Wood Essential Oils) Induce Human Natural Killer Cell Activity. Immunopharmacol. Immunotoxicol. 2006, 28, 319–333. [Google Scholar] [CrossRef]
- Li, Q.; Morimoto, K.; Nakadai, A.; Inagaki, H.; Katsumata, M.; Shimizu, T.; Hirata, Y.; Hirata, K.; Suzuki, H.; Miyazaki, Y.; et al. Forest Bathing Enhances Human Natural Killer Activity and Expression of Anti-Cancer Proteins. Int. J. Immunopathol. Pharmacol. 2007, 20, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Morimoto, K.; Kobayashi, M.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Suzuki, H.; Li, Y.; Wakayama, Y.; et al. Visiting a Forest, but Not a City, Increases Human Natural Killer Activity and Expression of Anti-Cancer Proteins. Int. J. Immunopathol. Pharmacol. 2008, 21, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kobayashi, M.; Inagaki, H.; Hirata, Y.; Li, Y.J.; Hirata, K.; Shimizu, T.; Suzuki, H.; Katsumata, M.; Wakayama, Y.; et al. A Forest Bathing Trip Increases Human Natural Killer Activity and Expression of Anti-Cancer Proteins in Females Subjects. J. Biol. Regul. Homeost. Agents 2010, 24, 157–165. [Google Scholar]
- Tsao, T.-M.; Tsai, M.-J.; Hwang, J.-S.; Cheng, W.-F.; Wu, C.-F.; Chou, C.-C.; Su, T.-C. Health effects of a forest environment on natural killer cells in humans: An observational pilot study. Oncotarget 2018, 9, 16501–16511. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Dicke, M.; Schnitzler, J.-P.; Turlings, T.C.J. Plant volatiles and the environment. Plant Cell Environ. 2014, 37, 1905–1908. [Google Scholar] [CrossRef]
- Laothawornkitkul, J.; Taylor, J.E.; Paul, N.; Hewitt, C.N. Biogenic volatile organic compounds in the Earth system. New Phytol. 2009, 183, 27–51. [Google Scholar] [CrossRef] [PubMed]
- Šimpraga, M.; Ghimire, R.P.; Van Der Straeten, D.; Blande, J.D.; Kasurinen, A.; Sorvari, J.; Holopainen, T.; Adriaenssens, S.; Holopainen, J.K.; Kivimäenpää, M. Unravelling the functions of biogenic volatiles in boreal and temperate forest ecosystems. Eur. J. For. Res. 2019, 138, 763–787. [Google Scholar] [CrossRef]
- Loreto, F.; Bagnoli, F.; Fineschi, S. One species, many terpenes: Matching chemical and biological diversity. Trends Plant Sci. 2009, 14, 416–420. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Loreto, F.; Reichstein, M. Physiological and physicochemical controls on foliar volatile organic compound emissions. Trends Plant Sci. 2004, 9, 180–186. [Google Scholar] [CrossRef]
- Choi, Y.; Park, S.; Kim, S.; Kim, E.; Kim, G. A Model Combining Forest Environment Images and Online Microclimate Data Instead of On-Site Measurements to Predict Phytoncide Emissions. Forests 2022, 13, 1895. [Google Scholar] [CrossRef]
- Choi, K.; Ko, D.W.; Kim, K.W.; Shin, M.Y. A Modeling Approach for Quantifying Human-Beneficial Terpene Emission in the Forest: A Pilot Study Applying to a Recreational Forest in South Korea. Int. J. Environ. Res. Public Health 2022, 19, 8278. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Albanese, L.; Bartolini, G.; Zabini, F. Temporal and Spatial Variability of Volatile Organic Compounds in the Forest Atmosphere. Int. J. Environ. Res. Public Health 2019, 16, 4915. [Google Scholar] [CrossRef] [PubMed]
- Zorić, M.; Farkić, J.; Kebert, M.; Mladenović, E.; Karaklić, D.; Isailović, G.; Orlović, S. Developing Forest Therapy Programmes Based on the Health Benefits of Terpenes in Dominant Tree Species in Tara National Park (Serbia). Int. J. Environ. Res. Public Health 2022, 19, 5504. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.; Yáñez-Serrano, A.M.; Llusià, J.; Filella, I.; Maneja, R.; Penuelas, J. Human Breathable Air in a Mediterranean Forest: Characterization of Monoterpene Concentrations under the Canopy. Int. J. Environ. Res. Public Health 2020, 17, 4391. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Song, B.; Cho, K.S.; Lee, I.-S. Therapeutic Potential of Volatile Terpenes and Terpenoids from Forests for Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 2187. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, G.; Park, S.; Lee, S.; Kim, S.; Kim, E. Statistical Evidence for Managing Forest Density in Consideration of Natural Volatile Organic Compounds. Atmosphere 2021, 12, 1113. [Google Scholar] [CrossRef]
- Wu, J.; Long, J.; Liu, H.; Sun, G.; Li, J.; Xu, L.; Xu, C. Biogenic volatile organic compounds from 14 landscape woody species: Tree species selection in the construction of urban greenspace with forest healthcare effects. J. Environ. Manag. 2021, 300, 113761. [Google Scholar] [CrossRef]
- Zhu, S.-X.; Hu, F.-F.; He, S.-Y.; Qiu, Q.; Su, Y.; He, Q.; Li, J.-Y. Comprehensive Evaluation of Healthcare Benefits of Different Forest Types: A Case Study in Shimen National Forest Park, China. Forests 2021, 12, 207. [Google Scholar] [CrossRef]
- Simkin, J.; Ojala, A.; Tyrväinen, L. Restorative effects of mature and young commercial forests, pristine old-growth forest and urban recreation forest—A field experiment. Urban For. Urban Green. 2020, 48, 126567. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; Liu, J.; An, C.; Liu, Y.; Fan, X.; Hu, Y. Physiological and Psychological Effects of Nature Experiences in Different Forests on Young People. Forests 2021, 12, 1391. [Google Scholar] [CrossRef]
- Pagès, A.B.; Peñuelas, J.; Clarà, J.; Llusià, J.; Campillo i López, F.; Maneja, R. How Should Forests Be Characterized in Regard to Human Health? Evidence from Existing Literature. Int. J. Environ. Res. Public Health 2020, 17, 1027. [Google Scholar] [CrossRef] [PubMed]
- Imamura, C.; Sakakibara, K.; Arai, K.; Ohira, H.; Yamaguchi, Y.; Yamada, H. Effect of Indoor Forest Bathing on Reducing Feelings of Fatigue Using Cerebral Activity as an Indicator. Int. J. Environ. Res. Public Health 2022, 19, 6672. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.; Maneja, R.; Zaldo-Aubanell, Q.; Romanillos, T.; Llusià, J.; Eustaquio, A.; Palacios, O.; Penuelas, J. Human absorption of monoterpenes after a 2-h forest exposure: A field experiment in a Mediterranean holm oak forest. J. Pharm. Biomed. Anal. 2021, 200, 114080. [Google Scholar] [CrossRef]
- Kim, J.; Sin, C.; Park, J.-O.; Lee, H.; Kim, D.; Kim, S. Physiological and psychological effects of forest healing focused on plant fragrance therapy for maladjusted soldiers. J. People Plants Environ. 2021, 24, 429–439. [Google Scholar] [CrossRef]
- Liang, R.; Zhang, R.; Wen, H.; Mo, J.; Huang, M.; Chen, H. Effects of Volatile Organic Compounds (VOCs) of Cinnamomum burmannii in Its Natural State on Physical and Mental Health. Pol. J. Environ. Stud. 2022, 32, 133–144. [Google Scholar] [CrossRef]
- Schuh, A.; Immich, G. Forest Therapy—The Potential of the Forest for Your Health; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Sumitomo, K.; Akutsu, H.; Fukuyama, S.; Minoshima, A.; Kukita, S.; Yamamura, Y.; Sato, Y.; Hayasaka, T.; Osanai, S.; Funakoshi, H.; et al. Conifer-Derived Monoterpenes and Forest Walking. Mass Spectrom. 2015, 4, A0042. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Koyama, S.; Heinbockel, T. The Effects of Essential Oils and Terpenes in Relation to Their Routes of Intake and Application. Int. J. Mol. Sci. 2020, 21, 1558. [Google Scholar] [CrossRef]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Bharathi, M.; Kunaviktikul, W.; Klunklin, A.; Chanthapoon, C.; Chaiyasut, C. Essential Oils, Phytoncides, Aromachology, and Aromatherapy—A Review. Appl. Sci. 2022, 12, 4495. [Google Scholar] [CrossRef]
- Bandelow, B.; Michaelis, S. Epidemiology of anxiety disorders in the 21st century. Dialog.-Clin. Neurosci. 2015, 17, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2018.
- Lim, L.-F.; Solmi, M.; Cortese, S. Association between anxiety and hypertension in adults: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2021, 131, 96–119. [Google Scholar] [CrossRef] [PubMed]
- Celano, C.M.; Villegas, A.C.; Albanese, A.M.; Gaggin, H.K.; Huffman, J.C. Depression and Anxiety in Heart Failure: A Review. Harv. Rev. Psychiatry 2018, 26, 175–184. [Google Scholar] [CrossRef]
- Li, J.; Ji, F.; Song, J.; Gao, X.; Jiang, D.; Chen, G.; Chen, S.; Lin, X.; Zhuo, C. Anxiety and clinical outcomes of patients with acute coronary syndrome: A meta-analysis. BMJ Open 2020, 10, e034135. [Google Scholar] [CrossRef] [PubMed]
- Watkins, L.L.; Koch, G.G.; Sherwood, A.; Blumenthal, J.A.; Davidson, J.R.T.; O’Connor, C.; Sketch, J.M.H. Association of Anxiety and Depression With All—Cause Mortality in Individuals With Coronary Heart Disease. J. Am. Heart Assoc. 2013, 2, e000068. [Google Scholar] [CrossRef]
- Gaibazzi, N.; Ugo, F.; Ardissino, D. Summertime loneliness as a trigger for all variants of stress-cardiomyopathy in the elderly. Eur. Heart J. 2007, 29, 956. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Sydeman, S.J.; Owen, A.E.; Marsh, B.J. Measuring Anxiety and Anger with the State-Trait Anxiety Inventory (STAI) and the State-Trait Anger Expression Inventory (STAXI). In The Use of Psychological Testing for Treatment Planning and Outcomes Assessment; Maruish, M.E., Ed.; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 1999; pp. 993–1021. [Google Scholar]
- Grove, J.R.; Prapavessis, H. Preliminary Evidence for the Reliability and Validity of an Abbreviated Profile of Mood States. Int. J. Sport Psychol. 1992, 23, 93–109. [Google Scholar]
- Rossi, V.; Pourtois, G. Transient state-dependent fluctuations in anxiety measured using STAI, POMS, PANAS or VAS: A comparative review. Anxiety Stress. Coping 2012, 25, 603–645. [Google Scholar] [CrossRef] [PubMed]
- Rapparini, F.; Baraldi, R.; Miglietta, F.; Loreto, F. Isoprenoid emission in trees of Quercus pubescens and Quercus ilex with lifetime exposure to naturally high CO2 environment+. Plant Cell Environ. 2004, 27, 381–391. [Google Scholar] [CrossRef]
- Ahn, J.-W.; Pandey, S.K.; Kim, K.-H. Comparison of GC-MS Calibration Properties of Volatile Organic Compounds and Relative Quantification Without Calibration Standards. J. Chromatogr. Sci. 2011, 49, 19–28. [Google Scholar] [CrossRef]
- Carriero, G.; Neri, L.; Famulari, D.; Di Lonardo, S.; Piscitelli, D.; Manco, A.; Esposito, A.; Chirico, A.; Facini, O.; Finardi, S.; et al. Composition and emission of VOC from biogas produced by illegally managed waste landfills in Giugliano (Campania, Italy) and potential impact on the local population. Sci. Total. Environ. 2018, 640–641, 377–386. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; ISBN 3-900051-07-0. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; RStudio Team: Boston, MA, USA, 2020. [Google Scholar]
- Ho, D.; Imai, K.; King, G.; Stuart, E.A. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J. Stat. Softw. 2011, 42, 1–28. [Google Scholar] [CrossRef]
- Bang, K.-S.; Kim, S.; Song, M.K.; Kang, K.I.; Jeong, Y. The Effects of a Health Promotion Program Using Urban Forests and Nursing Student Mentors on the Perceived and Psychological Health of Elementary School Children in Vulnerable Populations. Int. J. Environ. Res. Public Health 2018, 15, 1977. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]
- Rosenbaum, P.R.; Rubin, D.B. The central role of the propensity score in observational studies for causal effects. Biometrika 1983, 70, 41–55. [Google Scholar] [CrossRef]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef]
- Rubin, D.B. Matching to Remove Bias in Observational Studies. Biometrics 1973, 29, 159. [Google Scholar] [CrossRef]
- VanderWeele, T.J. Principles of confounder selection. Eur. J. Epidemiol. 2019, 34, 211–219. [Google Scholar] [CrossRef]
- Fung, T.; Lau, B.; Ngai, S.; Tsang, H. Therapeutic Effect and Mechanisms of Essential Oils in Mood Disorders: Interaction between the Nervous and Respiratory Systems. Int. J. Mol. Sci. 2021, 22, 4844. [Google Scholar] [CrossRef]
- Donelli, D.; Antonelli, M.; Bellinazzi, C.; Gensini, G.F.; Firenzuoli, F. Effects of lavender on anxiety: A systematic review and meta-analysis. Phytomedicine 2019, 65, 153099. [Google Scholar] [CrossRef]
- Antonelli, M.; Barbieri, G.; Donelli, D. Defining a new perspective in Environmental Health: The healing environment. Int. J. Biometeorol. 2022, 66, 1039–1044. [Google Scholar] [CrossRef]
- Sancho-Knapik, D.; Gil-Pelegrín, E.; Ferrio, J.P.; Alonso-Forn, D.; Martín-Sánchez, R.; Silva, J.V.d.S.; Imanishi, J.; Peguero-Pina, J.J.; Sanz, M. Changes in the Abundance of Monoterpenes from Breathable Air of a Mediterranean Conifer Forest: When Is the Best Time for a Human Healthy Leisure Activity? Forests 2022, 13, 965. [Google Scholar] [CrossRef]
- Shim, S.R.; Chang, J.; Lee, J.; Byeon, W.; Lee, J.; Lee, K.J. Perspectives on the Psychological and Physiological Effects of Forest Therapy: A Systematic Review with a Meta-Analysis and Meta-Regression. Forests 2022, 13, 2029. [Google Scholar] [CrossRef]
- Yi, Y.; Seo, E.; An, J. Does Forest Therapy Have Physio-Psychological Benefits? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2022, 19, 10512. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhu, Y.; Lv, J.; Guo, Y.; Yang, L.; Chen, Y.; Tang, W.; Xiang, S.; Sun, X.; Chen, J.; et al. Association of anxiety with cardiovascular disease in a Chinese cohort of 0.5 million adults. J. Affect. Disord. 2022, 315, 291–296. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).