Developing Forest Therapy Programmes Based on the Health Benefits of Terpenes in Dominant Tree Species in Tara National Park (Serbia)
Abstract
:1. Introduction
2. Materials and Methods
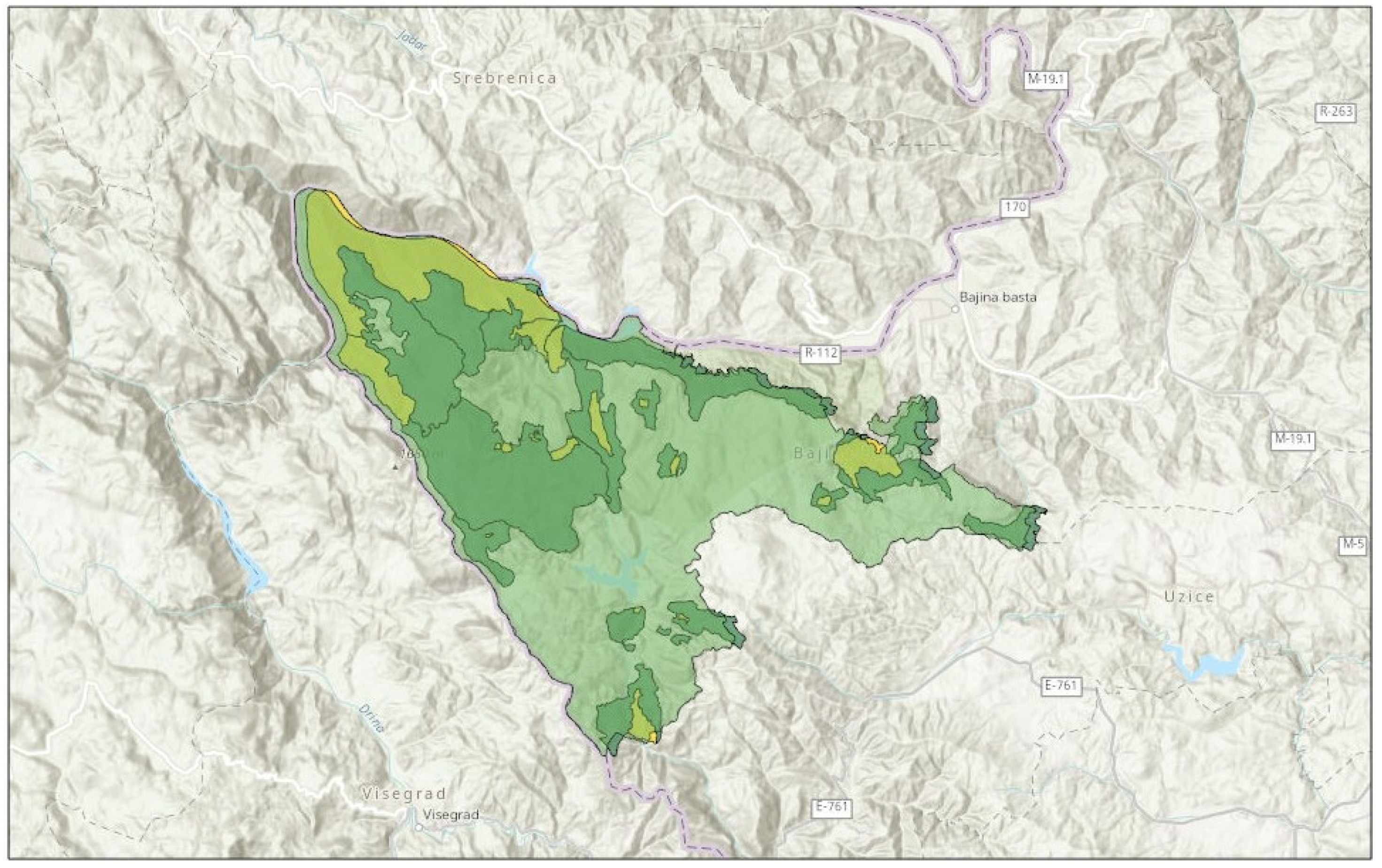
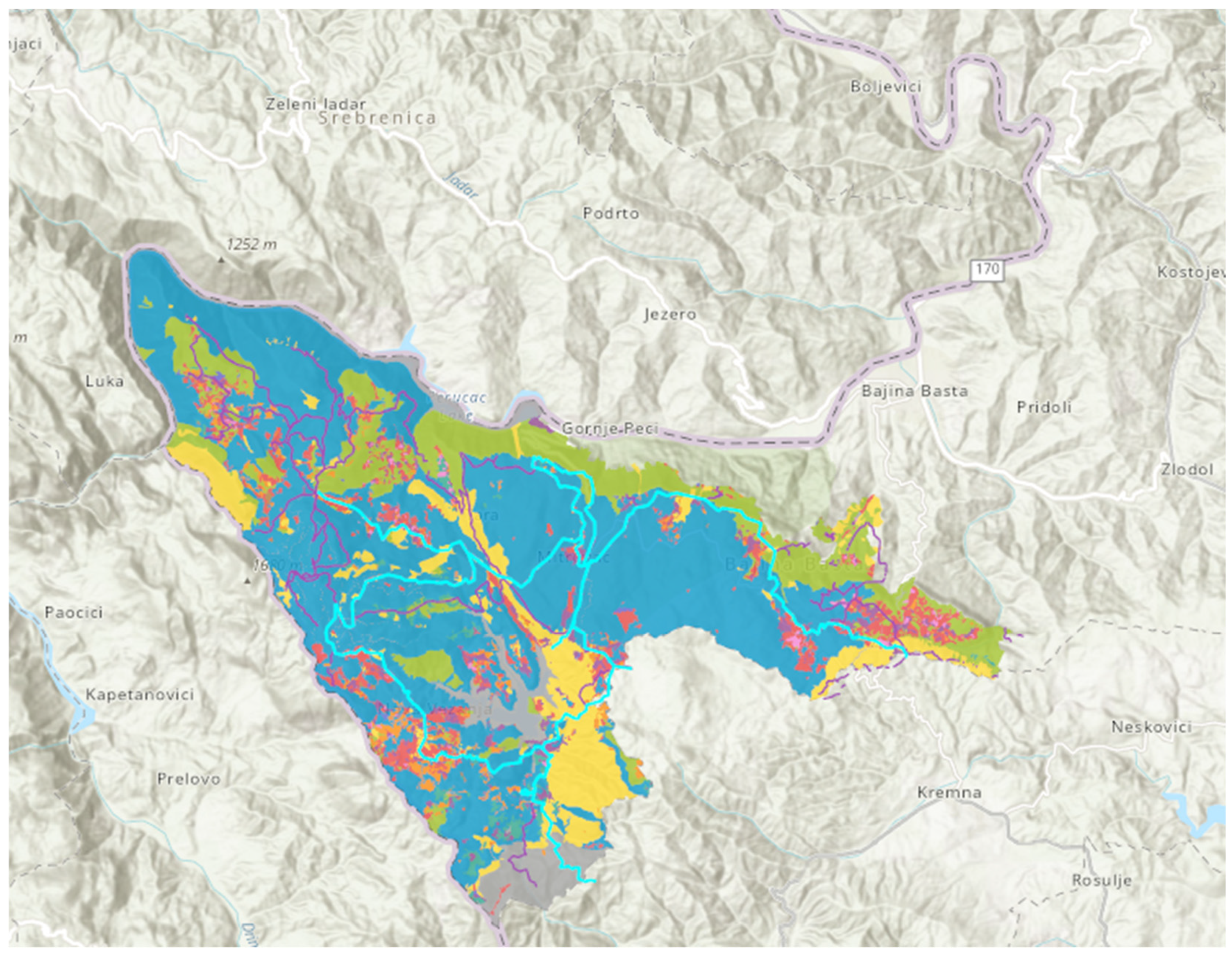
2.1. Study Area
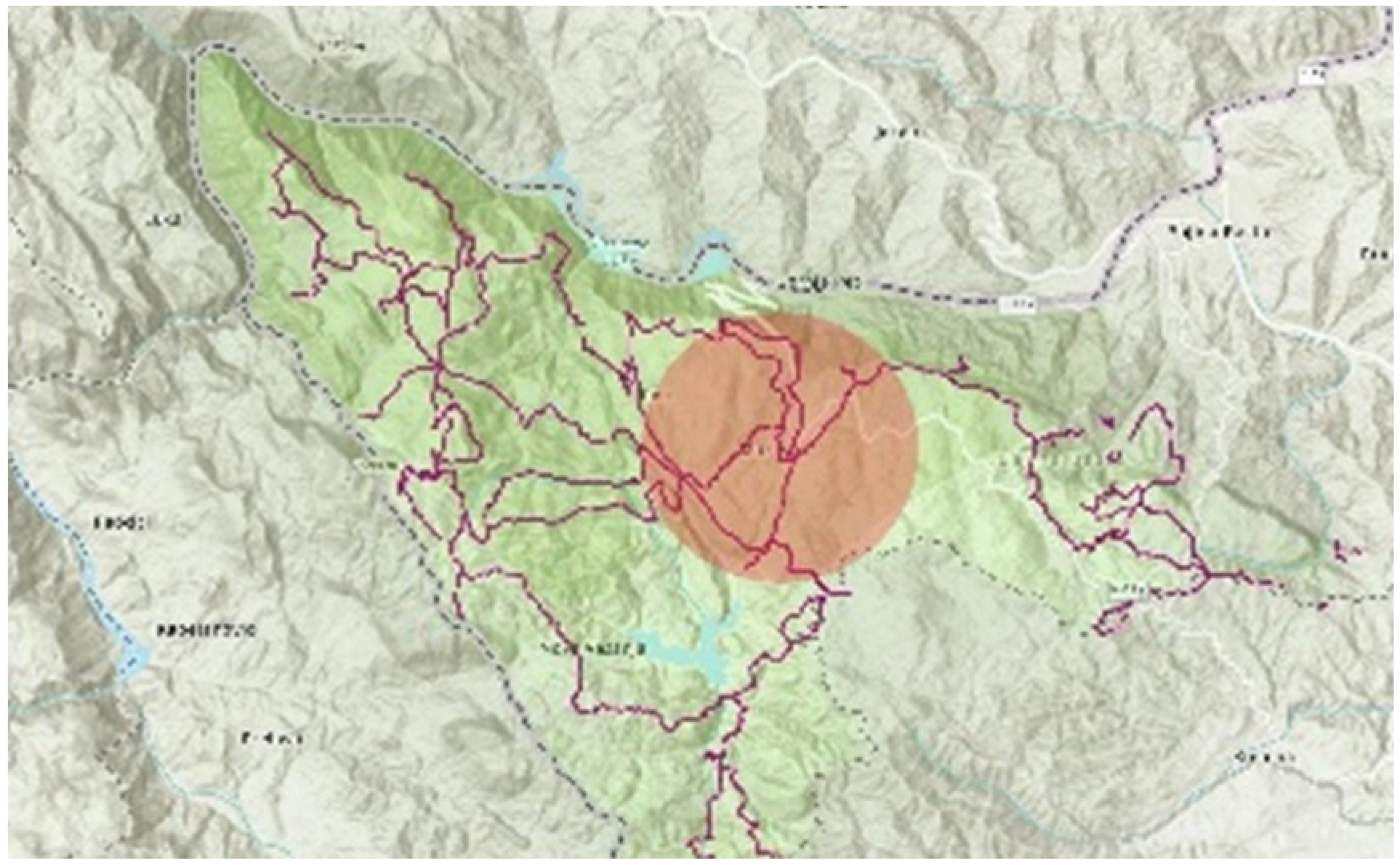
2.2. Hiking Trails in NP Tara
- Hiking route length (km)
- Hiking route elevation (m)
- Hiking route duration (hours)
- Conditional and technical difficulty (ranged from 1 to 10, 1 as no difficulty to 10 as highly difficult)
- Accessibility (if the hiking trail is partially on or near the local road for the security of the users).
2.3. Plant Material and Terpenes Analysis
3. Results
Terpenes Composition in Dominant Species of the Area
4. Discussion
4.1. Health Benefits of Terpenes
4.2. The Development of Forest Therapy Programmes Based on Health Properties of Terpenes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rantala, O.; Puhakka, R. Engaging with Nature: Nature Affords Well-Being for Families and Young People in Finland. Child. Geogr. 2020, 18, 490–503. [Google Scholar] [CrossRef]
- Keniger, L.E.; Gaston, K.J.; Irvine, K.N.; Fuller, R.A. What Are the Benefits of Interacting with Nature? Int. J. Environ. Res. Public Health 2013, 10, 913–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larenas-Linnemann, D.; Rodríguez-Pérez, N.; Arias-Cruz, A.; Blandón-Vijil, M.V.; del Río-Navarro, B.E.; Estrada-Cardona, A.; Gereda, J.E.; Luna-Pech, J.A.; Navarrete-Rodríguez, E.M.; Onuma-Takane, E.; et al. Enhancing Innate Immunity against Virus in Times of COVID-19: Trying to Untangle Facts from Fictions. World Allergy Organ. J. 2020, 13, 100476. [Google Scholar] [CrossRef]
- Tyrväinen, L.; Ojala, A.; Korpela, K.; Lanki, T.; Tsunetsugu, Y.; Kagawa, T. The Influence of Urban Green Environments on Stress Relief Measures: A Field Experiment. J. Environ. Psychol. 2014, 38, 1–9. [Google Scholar] [CrossRef]
- Maund, P.R.; Irvine, K.N.; Dallimer, M.; Fish, R.; Austen, G.E.; Davies, Z.G. Do Ecosystem Service Frameworks Represent People’s Values? Ecosyst. Serv. 2020, 46, 101221. [Google Scholar] [CrossRef] [PubMed]
- Lenaerts, A.; Heyman, S.; de Decker, A.; Lauwers, L.; Sterckx, A.; Remmen, R.; Bastiaens, H.; Keune, H. Vitamin Nature: How Coronavirus Disease 2019 Has Highlighted Factors Contributing to the Frequency of Nature Visits in Flanders, Belgium. Front. Public Health 2021, 9, 389. [Google Scholar] [CrossRef]
- Antonelli, M.; Donelli, D.; Barbieri, G.; Valussi, M.; Maggini, V.; Firenzuoli, F. Forest Volatile Organic Compounds and Their Effects on Human Health: A State-of-the-Art Review. Int. J. Environ. Res. Public Health 2020, 17, 6506. [Google Scholar] [CrossRef]
- Zorić, M.; Kostić, S.; Kladar, N.; Božin, B.; Vasić, V.; Kebert, M.; Orlović, S. Phytochemical Screening of Volatile Organic Compounds in Three Common Coniferous Tree Species in Terms of Forest Ecosystem Services. Forests 2021, 12, 928. [Google Scholar] [CrossRef]
- Farkic, J.; Isailovic, G.; Taylor, S. Forest Bathing as a Mindful Tourism Practice. Ann. Tour. Res. Empir. Insights 2021, 2, 100028. [Google Scholar] [CrossRef]
- Li, Q. Effect of Forest Bathing Trips on Human Immune Function. Environ. Health Prev. Med. 2010, 15, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Kagawa, T.; Miyazaki, Y. The Physiological Effects of Shinrin-Yoku (Taking in the Forest Atmosphere or Forest Bathing): Evidence from Field Experiments in 24 Forests across Japan. Environ. Health Prev. Med. 2010, 15, 18–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, K.S.; Lim, Y.R.; Lee, K.; Lee, J.; Lee, J.H.; Lee, I.S. Terpenes from Forests and Human Health. Toxicol. Res. 2017, 33, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Upadhyay, S.; Orhan, I.E.; Jugran, A.K.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α-and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, A.L.; Figueiredo, C.R.; Arruda, D.C.; Pereira, F.V.; Borin Scutti, J.A.; Massaoka, M.H.; Travassos, L.R.; Sartorelli, P.; Lago, J.H.G. α-Pinene Isolated from Schinus Terebinthifolius raddi (Anacardiaceae) Induces Apoptosis and Confers Antimetastatic Protection in a Melanoma Model. Biochem. Biophys. Res. Commun. 2011, 411, 449–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoshnazar, M.; Bigdeli, M.R.; Parvardeh, S.; Pouriran, R. Attenuating Effect of α-Pinene on Neurobehavioural Deficit, Oxidative Damage and Inflammatory Response Following Focal Ischaemic Stroke in Rat. J. Pharm. Pharmacol. 2019, 71, 1725–1733. [Google Scholar] [CrossRef]
- Rahbar, I.; Abbasnejad, M.; Haghani, J.; Raoof, M.; Kooshki, R.; Esmaeili-Mahani, S. The Effect of Central Administration of Alpha-Pinene on Capsaicin-Induced Dental Pulp Nociception. Int. Endod. J. 2019, 52, 307–317. [Google Scholar] [CrossRef]
- Yoshida, N.; Takada, T.; Yamamura, Y.; Adachi, I.; Suzuki, H.; Kawakami, J. Inhibitory Effects of Terpenoids on Multidrug Resistance-Associated Protein 2-and Breast Cancer Resistance Protein-Mediated Transport. Drug Metab. Dispos. 2008, 36, 1206–1211. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Liu, Y.; Li, M.; Mao, J.; Zhang, L.; Huang, R.; Jin, X.; Ye, L. Anti-Tumor Effect of α-Pinene on Human Hepatoma Cell Lines through Inducing G2/M Cell Cycle Arrest. J. Pharmacol. Sci. 2015, 127, 332–338. [Google Scholar] [CrossRef] [Green Version]
- Pereira da Silva, H.N.; dos Santos Machado, S.D.; de Andrade Siqueira, A.M.; Cardoso Costa da Silva, E.; de Oliveira Canto, M.Â.; Jensen, L.; Vargas Flores da Silva, L.; Sena Fugimura, M.M.; de Sousa Barroso, A.; Veras Mourão, R.H.; et al. Sedative and Anesthetic Potential of the Essential Oil and Hydrolate from the Fruit of Protium Heptaphyllum and Their Isolated Compounds in Colossoma Macropomum Juveniles. Aquaculture 2020, 529, 735629. [Google Scholar] [CrossRef]
- Woo, J.L.C. Sleep-Enhancing Effects of Phytoncide Via Behavioral, Electrophysiological, and Molecular Modeling Approaches. Exp. Neurobiol. 2020, 29, 120. [Google Scholar] [CrossRef]
- Hafidh, R.R.; Hussein, S.Z.; MalAllah, M.Q.; Abdulamir, A.S.; Bakar, F.A. A High-Throughput Quantitative Expression Analysis of Cancer-Related Genes in Human HepG2 Cells in Response to Limonene, a Potential Anticancer Agent. Curr. Cancer Drug Targets 2018, 18, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lin, H.; Wang, Y.; Lv, W.; Zhang, S.; Qian, Y.; Deng, X.; Feng, N.; Yu, H.; Qian, B. D-Limonene Exhibits Antitumor Activity by Inducing Autophagy and Apoptosis in Lung Cancer. OncoTargets Ther. 2018, 11, 1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Christo Scherer, M.M.; Marques, F.M.; Figueira, M.M.; Peisino, M.C.O.; Schmitt, E.F.P.; Kondratyuk, T.P.; Endringer, D.C.; Scherer, R.; Fronza, M. Wound Healing Activity of Terpinolene and α-Phellandrene by Attenuating Inflammation and Oxidative Stress in Vitro. J. Tissue Viability 2019, 28, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.C.; Kuo, Y.H.; Jan, J.T.; Liang, P.H.; Wang, S.Y.; Liu, H.G.; Lee, C.K.; Chang, S.T.; Kuo, C.J.; Lee, S.S.; et al. Specific Plant Terpenoids and Lignoids Possess Potent Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus. J. Med. Chem. 2007, 50, 4087–4095. [Google Scholar] [CrossRef] [Green Version]
- Kesselmeier, J.; Staudt, M. Biogenic Volatile Organic Compounds (VOC): An Overview on Emission, Physiology and Ecology. J. Atmos. Chem. 1999, 33, 23–88. [Google Scholar] [CrossRef]
- Kim, T.; Song, B.; Cho, K.S.; Lee, I.S. Therapeutic Potential of Volatile Terpenes and Terpenoids from Forests for Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 2187. [Google Scholar] [CrossRef] [Green Version]
- Calfapietra, C.; Fares, S.; Manes, F.; Morani, A.; Sgrigna, G.; Loreto, F. Role of Biogenic Volatile Organic Compounds (BVOC) Emitted by Urban Trees on Ozone Concentration in Cities: A Review. Environ. Pollut. 2013, 183, 71–80. [Google Scholar] [CrossRef]
- Fitzky, A.C.; Sandén, H.; Karl, T.; Fares, S.; Calfapietra, C.; Grote, R.; Saunier, A.; Rewald, B. The Interplay Between Ozone and Urban Vegetation—BVOC Emissions, Ozone Deposition, and Tree Ecophysiology. Front. For. Glob. Chang. 2019, 2, 50. [Google Scholar] [CrossRef]
- Ben Derbassi, N.; Pedrosa, M.C.; Heleno, S.; Carocho, M.; Ferreira, I.C.F.R.; Barros, L. Plant Volatiles: Using Scented Molecules as Food Additives. Trends Food Sci. Technol. 2022, 122, 97–103. [Google Scholar] [CrossRef]
- Wang, M. Characteristics of BVOC Emissions from a Swedish Boreal Forest. Ph.D. Thesis, Lund University, Lund, Sweden, 2018. [Google Scholar]
- Kopaczyk, J.M.; Warguła, J.; Jelonek, T. The Variability of Terpenes in Conifers under Developmental and Environmental Stimuli. Environ. Exp. Bot. 2020, 180, 104197. [Google Scholar] [CrossRef]
- Ormeño, E.; Goldstein, A.; Niinemets, Ü. Extracting and Trapping Biogenic Volatile Organic Compounds Stored in Plant Species. TrAC-Trends Anal. Chem. 2011, 30, 978–989. [Google Scholar] [CrossRef]
- Đurić, A.; Jevtić, S.; Josipović, M.; Kafedžić, I.; Karaklić, D.; Malinić, M.; Mitrović, N.; Milanović, R.; Stanković, J.; Tomić, M.; et al. Plan Upravljanja Nacionalnog Parka Tara za Period 2018–2027 Godine; JP Nacionalni Park Tara: Bajina Bašta, Serbia, 2018. [Google Scholar]
- Available online: https://www.nptara.rs/en/for-visitors/2019-02-14-13-01-58/hiking-trails.html (accessed on 27 January 2022).
- Zorić, M.; Kostić, S.; Kebert, M.; Kladar, N.; Božin, B.; Orlović, S. Volatile Organic Compounds of Tilia Cordata Mill. from Serbia, in Terms of Ecosystem Services. Topola 2020, 4, 21–28. [Google Scholar] [CrossRef]
- Grote, R.; Sharma, M.; Ghirardo, A.; Schnitzler, J.-P. A New Modeling Approach for Estimating Abiotic and Biotic Stress-Induced de Novo Emissions of Biogenic Volatile Organic Compounds From Plants. Front. For. Glob. Chang. 2019, 2, 26. [Google Scholar] [CrossRef]
- Peñuelas, J.; Llusià, J. The Complexity of Factors Driving Volatile Organic Compound Emissions by Plants. Biol. Plant. 2001, 44, 481–487. [Google Scholar] [CrossRef]
- Bach, A.; Yáñez-Serrano, A.M.; Llusià, J.; Filella, I.; Maneja, R.; Penuelas, J. Human Breathable Air in a Mediterranean Forest: Characterization of Monoterpene Concentrations under the Canopy. Int. J. Environ. Res. Public Health 2020, 17, 4391. [Google Scholar] [CrossRef]
- Greenberg, J.P.; Peñuelas, J.; Guenther, A.; Seco, R.; Turnipseed, A.; Jiang, X.; Filella, I.; Estiarte, M.; Sardans, J.; Ogaya, R.; et al. A Tethered-Balloon PTRMS Sampling Approach for Surveying of Landscape-Scale Biogenic VOC Fluxes. Atmos. Meas. Tech. 2014, 7, 2263–2271. [Google Scholar] [CrossRef] [Green Version]
- Guenther, A.B.; Zimmerman, P.R.; Harley, P.C.; Monson, R.K. Isoprene and Monoterpene Emission Rate Variability’ Model Evaluations and Sensitivity Analyses. J. Geophys. Res. Atmos. 1993, 98, 12609–12617. [Google Scholar] [CrossRef] [Green Version]
- Esposito, R.; Lusini, I.; VečeřOvá, K.; Holi, P.; Pallozzi, E.; Guidolotti, G.; Urban, O.; Calfapietra, C. Shoot-Level Terpenoids Emission in Norway Spruce (Picea abies) under Natural Field and Manipulated Laboratory Conditions. Plant Physiol. Biochem. 2016, 108, 530–538. [Google Scholar] [CrossRef]
- Pokorska, O.; Dewulf, J.; Amelynck, C.; Schoon, N.; Šimpraga, M.; Steppe, K.; van Langenhove, H. Isoprene and Terpenoid Emissions from Abies alba: Identification and Emission Rates under Ambient Conditions. Atmos. Environ. 2012, 59, 501–508. [Google Scholar] [CrossRef]
- Paduch, R.; Kandefer-Szerszeń, M.; Trytek, M.; Fiedurek, J. Terpenes: Substances Useful in Human Healthcare. Arch. Immunol. Ther. Exp. 2007, 55, 315–327. [Google Scholar] [CrossRef]
- Stojanović, D.; Orlović, S.; Zlatković, M.; Kostić, S.; Vasić, V.; Miletić, B.; Kesić, L.; Matović, B.; Božanić, D.; Pavlović, L.; et al. Climate Change within Serbian Forests: Current State and Future Perspectives. Topola 2021, 208, 39–56. [Google Scholar] [CrossRef]
- Sunjaya, A.F.; Sunjaya, A.P. Protective Effects of Phytoncides Against Cancer. Adv. Sci. Lett. 2018, 24, 6837–6840. [Google Scholar] [CrossRef]
- Li, Q.; Nakadai, A.; Matsushima, H.; Miyazaki, Y.; Krensky, A.; Kawada, T.; Morimoto, K. Phytoncides (Wood Essential Oils) Induce Human Natural Killer Cell Activity. Immunopharmacol. Immunotoxicol. 2006, 28, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kawada, T. Healthy Forest Parks Make Healthy People: Forest Environments Enhance Human Immune Function; Department of Hygiene and Public Health, Nippon Medical School: Tokyo, Japan, 2010. [Google Scholar]
- Li, Q.; Kobayashi, M.; Wakayama, Y.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Shimizu, T.; Kawada, T.; Ohira, J.P.T. Effect of phytoncide from trees on human natural killer cell function. Int. J. Immunopathol. Pharmacol. 2009, 22, 951–959. [Google Scholar] [CrossRef]
- Lee, J.; Tsunetsugu, Y.; Takayama, N.; Park, B.J.; Li, Q.; Song, C.; Komatsu, M.; Ikei, H.; Tyrväinen, L.; Kagawa, T.; et al. Influence of Forest Therapy on Cardiovascular Relaxation in Young Adults. Evid.-Based Complement. Altern. Med. 2014, 2014, 834360. [Google Scholar] [CrossRef]
- Ochiai, H.; Ikei, H.; Song, C.; Kobayashi, M.; Takamatsu, A.; Miura, T.; Kagawa, T.; Li, Q.; Kumeda, S.; Imai, M.; et al. Physiological and Psychological Effects of Forest Therapy on Middle-Aged Males with High-Normal Blood Pressure. Int. J. Environ. Res. Public Health 2015, 12, 2532–2542. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Ikei, H.; Miyazaki, Y. Sustained Effects of a Forest Therapy Program on the Blood Pressure of Office Workers. Urban For. Urban Green. 2017, 27, 246–252. [Google Scholar] [CrossRef]
- Ohtsuka, Y.; Yabunaka, N.; Takayama, S. Shinrin-Yoku (Forest-Air Bathing and Walking) Effectively Decreases Blood Glucose Levels in Diabetic Patients. Int. J. Biometeorol. 1998, 41, 125–127. [Google Scholar] [CrossRef]
- Han, J.W.; Choi, H.; Jeon, Y.H.; Yoon, C.H.; Woo, J.M.; Kim, W. The Effects of Forest Therapy on Coping with Chronic Widespread Pain: Physiological and Psychological Differences between Participants in a Forest Therapy Program and a Control Group. Int. J. Environ. Res. Public Health 2016, 13, 255. [Google Scholar] [CrossRef] [Green Version]
- Jung, W.H.; Woo, J.M.; Ryu, J.S. Effect of a Forest Therapy Program and the Forest Environment on Female Workers’ Stress. Urban For. Urban Green. 2015, 14, 274–281. [Google Scholar] [CrossRef]
- Bielinis, E.; Takayama, N.; Boiko, S.; Omelan, A.; Bielinis, L. The Effect of Winter Forest Bathing on Psychological Relaxation of Young Polish Adults. Urban For. Urban Green. 2018, 29, 276–283. [Google Scholar] [CrossRef]
- Chun, M.H.; Chang, M.C.; Lee, S.J. The Effects of Forest Therapy on Depression and Anxiety in Patients with Chronic Stroke. Int. J. Neurosci. 2017, 127, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Kamran, S.; Sinniah, A.; Abdulghani, M.A.M.; Alshawsh, M.A. Therapeutic Potential of Certain Terpenoids as Anticancer Agents: A Scoping Review. Cancers 2022, 14, 1100. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.M.; Abbas, M.A.; Kandil, Y.I. Cytotoxic Activity of Varthemia Iphionoides Essential Oil against Various Human Cancer Cell Lines. Acta Pol. Pharm.-Drug Res. 2019, 76, 701–706. [Google Scholar] [CrossRef]
- Ziaei, A.; Ramezani, M.; Wright, L.; Paetz, C.; Schneider, B.; Amirghofran, Z. Identification of Spathulenol in Salvia Mirzayanii and the Immunomodulatory Effects. Phytother. Res. 2011, 25, 557–562. [Google Scholar] [CrossRef]
- Patil, A.; Jadhav, V. GC-MS Analysis of Bioactive Components from Methanol Leaf Extract of Toddalia asiatica (L.). Int. J. Pharm. Sci. Rev. Res. 2014, 29, 18–20. [Google Scholar]
- Hosseini, K.; Jasori, S.; Delazar, A.; Asgharian, P.; Tarhriz, V. Chemical Characterization and Evaluation of Anticancer Effect of Falcaria Vulgaris via Induction of Apoptosis. 2021. preprint. [CrossRef]
- Zardi-Bergaoui, A.; Znati, M.; Harzallah-Skhiri, F.; Jannet, H.B. Caryophyllene Sesquiterpenes from Pulicaria vulgaris Gaertn.: Isolation, Structure Determination, Bioactivity and Structure−Activity Relationship. Chem. Biodivers. 2019, 16, e1800483. [Google Scholar] [CrossRef]
- Zhuang, S.-R.; Chen, S.-L.; Tsai, J.-H.; Huang, C.-C.; Wu, T.-C.; Liu, W.-S.; Tseng, H.-C.; Lee, H.-S.; Huang, M.-C.; Shane, G.-T.; et al. Citronellol and the Chinese Medical Herb Complex on Cellular Immunity 785 Effect of Citronellol and the Chinese Medical Herb Complex on Cellular Immunity of Cancer Patients Receiving Chemotherapy/Radiotherapy. Phytother. Res. 2009, 23, 785–790. [Google Scholar] [CrossRef]
- Guzmán-Gutiérrez, S.L.; Gómez-Cansino, R.; García-Zebadúa, J.C.; Jiménez-Pérez, N.C.; Reyes-Chilpa, R. Antidepressant Activity of Litsea Glaucescens Essential Oil: Identification of β-Pinene and Linalool as Active Principles. J. Ethnopharmacol. 2012, 143, 673–679. [Google Scholar] [CrossRef]
- Linck, V.M.; da Silva, A.L.; Figueiró, M.; Caramão, E.B.; Moreno, P.R.H.; Elisabetsky, E. Effects of Inhaled Linalool in Anxiety, Social Interaction and Aggressive Behavior in Mice. Phytomedicine 2010, 17, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Donelli, D.; Carlone, L.; Maggini, V.; Firenzuoli, F.; Bedeschi, E. Effects of Forest Bathing (Shinrin-Yoku) on Individual Well-Being: An Umbrella Review. Int. J. Environ. Health Res. 2021, 2021, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Doimo, I.; Masiero, M.; Gatto, P. Forest and Wellbeing: Bridging Medical and Forest Research for Effective Forest-Based Initiatives. Forests 2020, 11, 791. [Google Scholar] [CrossRef]
- Pagès, A.B.; Peñuelas, J.; Clarà, J.; Llusià, J.; López, F.C.I.; Maneja, R. How Should Forests Be Characterized in Regard to Human Health? Evidence from Existing Literature. Int. J. Environ. Res. Public Health 2020, 17, 1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenman, T.S.; Churkina, G.; Jariwala, S.P.; Kumar, P.; Lovasi, G.S.; Pataki, D.E.; Weinberger, K.R.; Whitlow, T.H. Urban Trees, Air Quality, and Asthma: An Interdisciplinary Review. Landsc. Urban Plan. 2019, 187, 47–59. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Albanese, L.; Bartolini, G.; Zabini, F. Temporal and Spatial Variability of Volatile Organic Compounds in the Forest Atmosphere. Int. J. Environ. Res. Public Health 2019, 16, 4915. [Google Scholar] [CrossRef] [Green Version]
- Clifford, M.A. Your Guide to Forest Bathing (Expanded Edition): Experience the Healing Power of Nature; Red Wheel/Weiser: Newburyport, MA, USA, 2021. [Google Scholar]
- Konu, H. Developing a Forest-Based Wellbeing Tourism Product Together with Customers—An Ethnographic Approach. Tour. Manag. 2015, 49, 1–16. [Google Scholar] [CrossRef]
- Komppula, R.; Konu, H. Designing Forest-Based Wellbeing Tourism Services for Japanese Customers; Taylor & Francis: Abingdon, UK, 2017. [Google Scholar]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and Medicinal Uses of Terpenes. In Medicinal Plants; Springer International Publishing: Cham, Switzerland, 2019; pp. 333–359. [Google Scholar]
- Kennedy, D.; Okello, E.; Chazot, P.; Howes, M.J.; Ohiomokhare, S.; Jackson, P.; Haskell-Ramsay, C.; Khan, J.; Forster, J.; Wightman, E. Volatile Terpenes and Brain Function: Investigation of the Cognitive and Mood Effects of Mentha × Piperita L. Essential Oil with In Vitro Properties Relevant to Central Nervous System Function. Nutrients 2018, 10, 1029. [Google Scholar] [CrossRef] [Green Version]
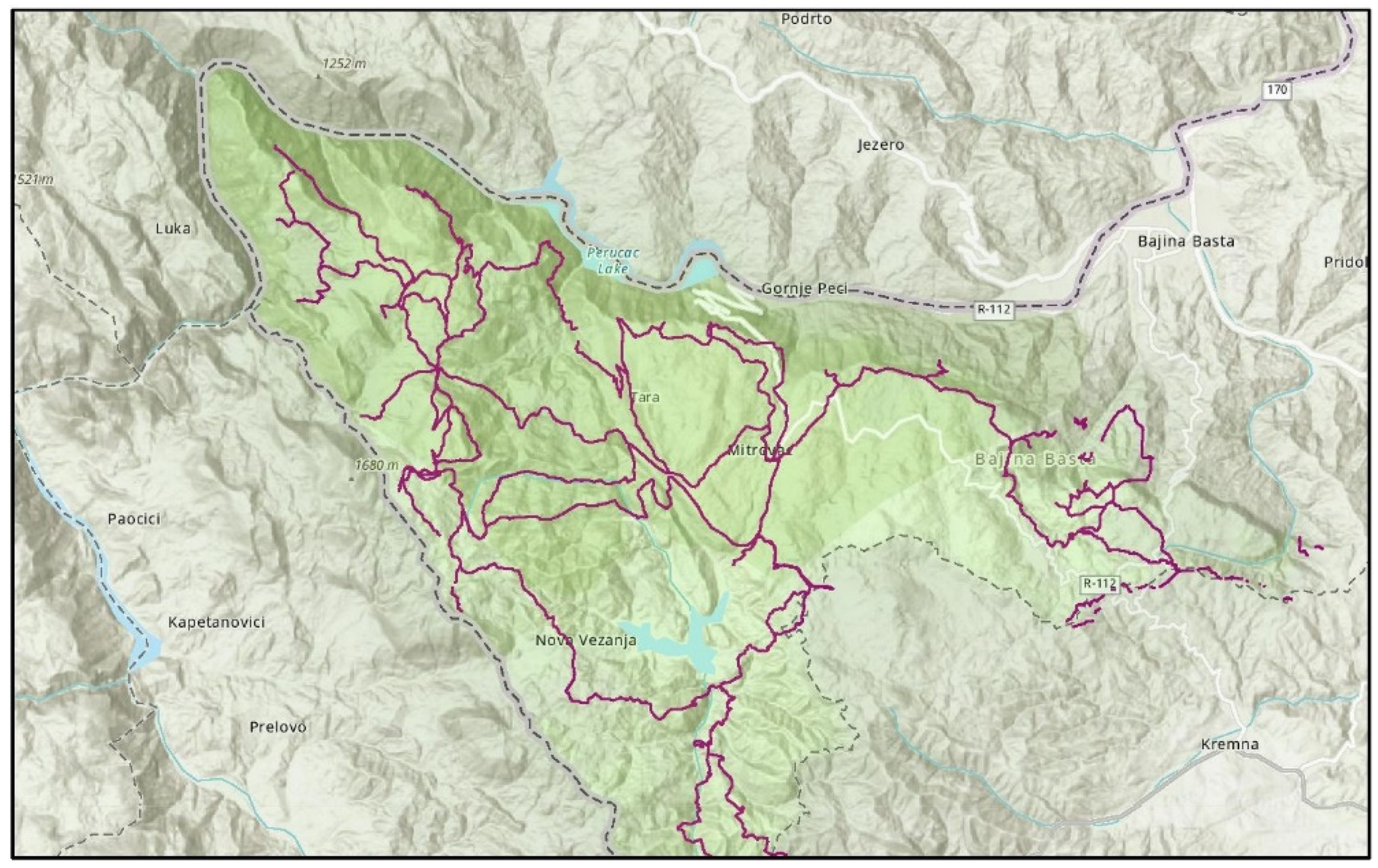
| Evaluated Hiking Trails | Hiking Route Length (km) | Hiking Route Elevation (m) | Hiking Route Duration (Hours) | Conditional Difficulty (out of 10) | Technical Difficulty (out of 10) |
|---|---|---|---|---|---|
| 06 Mitrovac—Zborište | 11.5 | 600 | 3 | 4 | 2 |
| 07 Mitrovac—Donji Jelisavčići | 3.6 | 240 | 1 | 2 | 2 |
| 08 Mitrovac—Predov Krst | 14.6 | 680 | 5 | 5 | 2 |
| 09 Mitrovac—Banjska stena | 6 | 105 | 2 | 1 | 1 |
| 09a Mitrovac—Velika livada—Banjska stena | 6.2 | 200 | 2 | 2 | 3 |
| 10 Mitrovac—Luke | 12.8 | 780 | 3 | 3 | 5 |
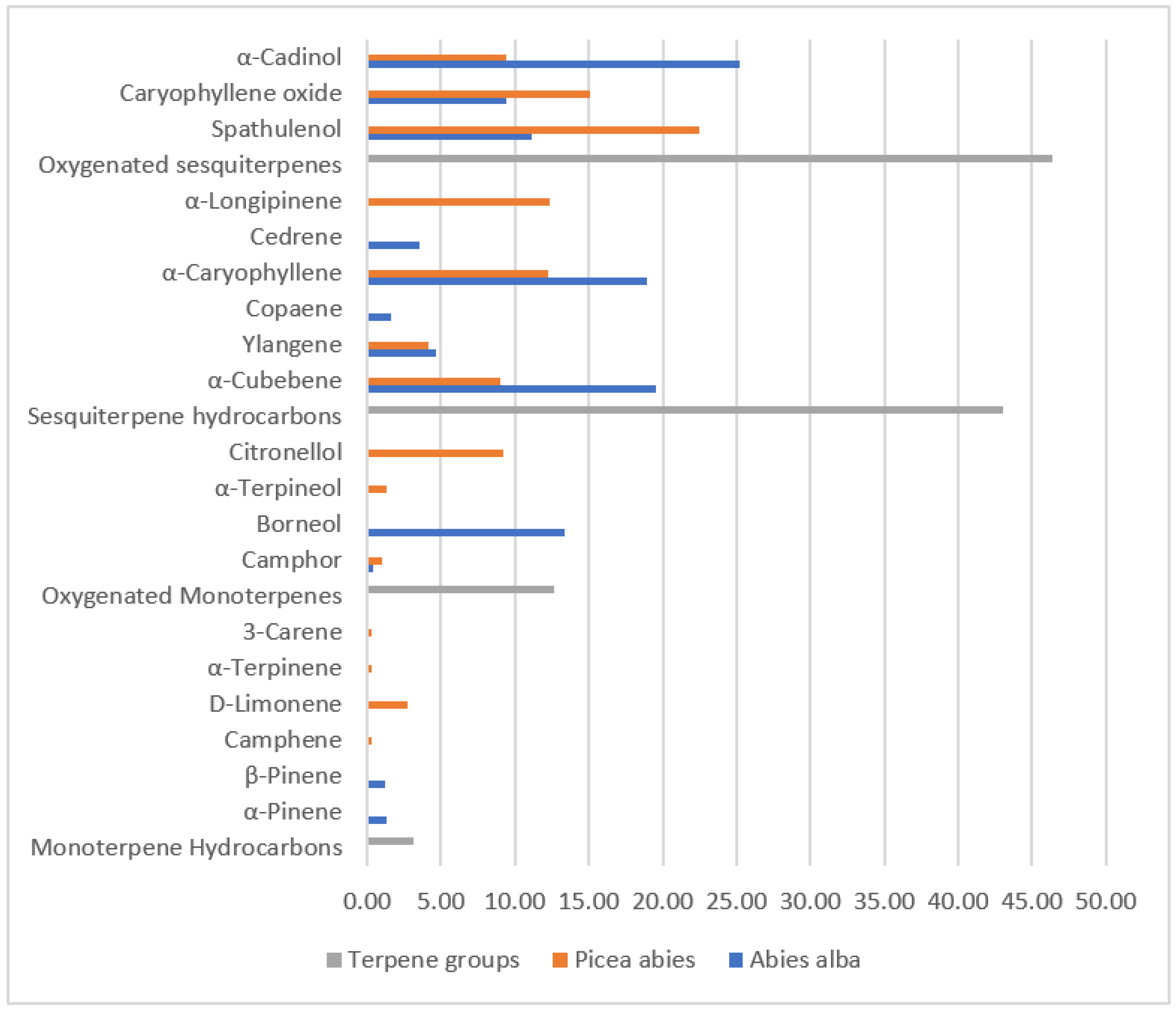
| Compound | Abies alba | Picea abies |
|---|---|---|
| Monoterpene Hydrocarbons | ||
| α-Pinene | 1.27 | 0.00 |
| β-Pinene | 1.22 | 0.00 |
| Camphene | 0.00 | 0.32 |
| D-Limonene | 0.00 | 2.76 |
| α-Terpinene | 0.00 | 0.28 |
| 3-Carene | 0.00 | 0.35 |
| Oxygenated Monoterpenes | ||
| Camphor | 0.40 | 1.03 |
| Borneol | 13.40 | 0.00 |
| α-Terpineol | 0.00 | 1.33 |
| Citronellol | 0.00 | 9.22 |
| Sesquiterpene hydrocarbons | ||
| α-Cubebene | 19.55 | 9.00 |
| Ylangene | 4.62 | 4.12 |
| Copaene | 1.59 | 0.00 |
| α-Caryophyllene | 18.92 | 12.23 |
| Cedrene | 3.54 | 0.00 |
| α-Longipinene | 0.00 | 12.39 |
| Oxygenated sesquiterpenes | ||
| Spathulenol | 11.18 | 22.44 |
| Caryophyllene oxide | 9.39 | 15.12 |
| α-Cadinol | 25.18 | 9.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorić, M.; Farkić, J.; Kebert, M.; Mladenović, E.; Karaklić, D.; Isailović, G.; Orlović, S. Developing Forest Therapy Programmes Based on the Health Benefits of Terpenes in Dominant Tree Species in Tara National Park (Serbia). Int. J. Environ. Res. Public Health 2022, 19, 5504. https://doi.org/10.3390/ijerph19095504
Zorić M, Farkić J, Kebert M, Mladenović E, Karaklić D, Isailović G, Orlović S. Developing Forest Therapy Programmes Based on the Health Benefits of Terpenes in Dominant Tree Species in Tara National Park (Serbia). International Journal of Environmental Research and Public Health. 2022; 19(9):5504. https://doi.org/10.3390/ijerph19095504
Chicago/Turabian StyleZorić, Martina, Jelena Farkić, Marko Kebert, Emina Mladenović, Dragić Karaklić, Gorana Isailović, and Saša Orlović. 2022. "Developing Forest Therapy Programmes Based on the Health Benefits of Terpenes in Dominant Tree Species in Tara National Park (Serbia)" International Journal of Environmental Research and Public Health 19, no. 9: 5504. https://doi.org/10.3390/ijerph19095504
APA StyleZorić, M., Farkić, J., Kebert, M., Mladenović, E., Karaklić, D., Isailović, G., & Orlović, S. (2022). Developing Forest Therapy Programmes Based on the Health Benefits of Terpenes in Dominant Tree Species in Tara National Park (Serbia). International Journal of Environmental Research and Public Health, 19(9), 5504. https://doi.org/10.3390/ijerph19095504









