Birth Outcomes in DES Children and Grandchildren: A Multigenerational National Cohort Study on Informative Families
Abstract
1. Introduction
2. Results
3. Discussion
4. Subjects and Methods
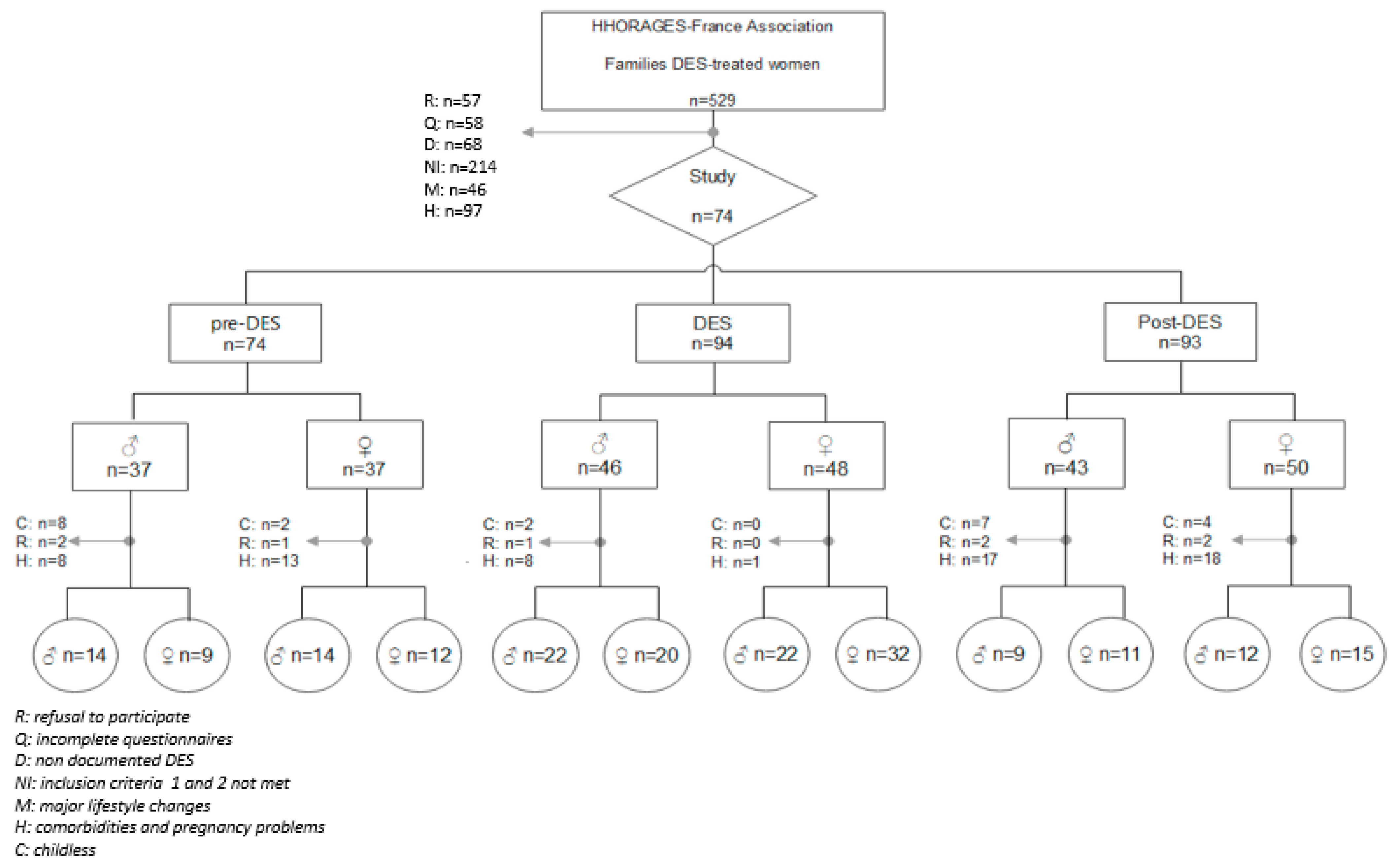
4.1. Study Population
4.2. Covariates
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, Z.R.; Xu, X.L.; Deng, S.L.; Lian, Z.X.; Yu, K. Oestrogenic Endocrine Disruptors in the Placenta and the Fetus. Int. J. Mol. Sci. 2020, 21, 1519. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.E.; Sadler-Riggleman, I.; Skinner, M.K. Environmentally Induced Epigenetic Transgenerational Inheritance of Disease. Environ. Epigenet. 2018, 4, dvy016. [Google Scholar] [CrossRef]
- Miller, R.K.; Heckmann, M.E.; McKenzie, R.C. Diethylstilbestrol: Placental Transfer, Metabolism, Covalent Binding and Fetal Distribution in the Wistar Rat. J. Pharmacol. Exp. Ther. 1982, 220, 358–365. [Google Scholar] [PubMed]
- Tournaire, M.; Epelboin, S.; Devouche, E. Diethylstilbestrol Story. Therapie 2014, 69, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Herbst, A.L.; Ulfelder, H.; Poskanzer, D.C. Adenocarcinoma of the Vagina. Association of Maternal Stilbestrol Therapy with Tumor Appearance in Young Women. N. Engl. J. Med. 1971, 284, 878–881. [Google Scholar] [CrossRef]
- Giusti, R.M.; Iwamoto, K.; Hatch, E.E. Diethylstilbestrol Revisited: A Review of the Long-Term Health Effects. Ann. Intern. Med. 1995, 122, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Hatch, E.E.; Troisi, R.; Wise, L.; Titus-Ernstoff, L.; Hyer, M.; Palmer, J.; Strohsnitter, W.C.; Robboy, S.J.; Anderson, D.; Kaufman, R. Preterm Birth, Fetal Growth, and Age at Menarche among Women Exposed Prenatally to Diethylstilbestrol (Des). Reprod. Toxicol. 2011, 31, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Troisi, R.; Hatch, E.; Titus-Ernstoff, L.; Hyer, M.; Palmer, J.; Robboy, S.J.; Strohsnitter, W.C.; Kaufman, R.; Herbst, A.L.; Hoover, R.N. Cancer Risk in Women Prenatally Exposed to Diethylstilbestrol. Int. J. Cancer 2007, 121, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Missmer, S.A.; Hankinson, S.E.; Spiegelman, D.; Barbieri, R.L.; Michels, K.B.; Hunter, D.J. In Utero Exposures and the Incidence of Endometriosis. Fertil. Steril. 2004, 82, 1501–1508. [Google Scholar] [CrossRef]
- Hoover, R.N.; Hyer, M.; Pfeiffer, R.M.; Adam, E.; Bond, B.; Cheville, A.L.; Colton, T.; Hartge, P.; Hatch, E.E.; Herbst, A.L.; et al. Adverse Health Outcomes in Women Exposed in Utero to Diethylstilbestrol. N. Engl. J. Med. 2011, 365, 1304–1314. [Google Scholar] [CrossRef]
- Upson, K.; Sathyanarayana, S.; Scholes, D.; Holt, V.L. Early-Life Factors and Endometriosis Risk. Fertil. Steril. 2015, 104, 964–971.e5. [Google Scholar] [CrossRef] [PubMed]
- Wolff, E.F.; Sun, L.; Hediger, M.L.; Sundaram, R.; Peterson, C.M.; Chen, Z.; Louis, G.M.B. In Utero Exposures and Endometriosis: The Endometriosis, Natural History, Disease, Outcome (Endo) Study. Fertil. Steril. 2013, 99, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Tournaire, M.; Epelboin, S.; Devouche, E.; Viot, G.; Le Bidois, J.; Cabau, A.; Dunbavand, A.; Levadou, A. Adverse Health Effects in Children of Women Exposed in Utero to Diethylstilbestrol (Des). Therapie 2016, 71, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, L.; Paris, F.; Cassel-Knipping, N.; Villeret, J.; Verschuur, A.; Soyer-Gobillard, M.-O.; Carcopino-Tusoli, X.; Hamamah, S.; Kalfa, N.; Sultan, C. Diethylstilbestrol Exposure During Pregnancy with Primary Clear Cell Carcinoma of the Cervix in an 8-Year-Old Granddaughter: A Multigenerational Effect of Endocrine Disruptors? Hum. Reprod. 2021, 36, 82–86. [Google Scholar] [CrossRef]
- Gaspari, L.; Soyer-Gobillard, M.O.; Paris, F.; Kalfa, N.; Hamamah, S.; Sultan, C. Multigenerational Endometriosis: Consequence of Fetal Exposure to Diethylstilbestrol? Environ. Health 2021, 20, 96. [Google Scholar] [CrossRef]
- Kalfa, N.; Paris, F.; Soyer-Gobillard, M.O.; Daures, J.P.; Sultan, C. Prevalence of Hypospadias in Grandsons of Women Exposed to Diethylstilbestrol During Pregnancy: A Multigenerational National Cohort Study. Fertil. Steril. 2011, 95, 2574–2577. [Google Scholar] [CrossRef]
- Gaspari, L.; Paris, F.; Soyer-Gobillard, M.O.; Hamamah, S.; Kalfa, N.; Sultan, C. “Idiopathic” Partial Androgen Insensitivity Syndrome in 11 Grandsons of Women Treated by Diethylstilbestrol During Gestation: A Multi-Generational Impact of Endocrine Disruptor Contamination? J. Endocrinol. Investig. 2021, 44, 379–381. [Google Scholar] [CrossRef]
- O’Reilly, E.J.; Mirzaei, F.; Forman, M.R.; Ascherio, A. Diethylstilbestrol Exposure in Utero and Depression in Women. Am. J. Epidemiol. 2010, 171, 876–882. [Google Scholar] [CrossRef]
- Kioumourtzoglou, M.A.; Coull, B.A.; O’Reilly, E.J.; Ascherio, A.; Weisskopf, M.G. Association of Exposure to Diethylstilbestrol During Pregnancy with Multigenerational Neurodevelopmental Deficits. JAMA Pediatr. 2018, 172, 670–677. [Google Scholar] [CrossRef]
- Soyer-Gobillard, M.O.; Paris, F.; Gaspari, L.; Courtet, P.; Sultan, C. Association between Fetal Des-Exposure and Psychiatric Disorders in Adolescence/Adulthood: Evidence from a French Cohort of 1002 Prenatally Exposed Children. Gynecol. Endocrinol. 2016, 32, 25–29. [Google Scholar] [CrossRef]
- Soyer-Gobillard, M.-O.; Gaspari, L.; Paris, F.; Kalfa, N.; Hamamah, S.; Courtet, P.; Sultan, C. Prenatal Exposure to Diethylstilbestrol and Multigenerational Psychiatric Disorders: An Informative Family. Int. J. Environ. Res. Public Health 2021, 18, 9965. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, W.J.; Davis, M.E.; Rynkiewicz, L.M.; Pottinger, R.E. Does the Administration of Diethylstilbestrol During Pregnancy Have Therapeutic Value? Am. J. Obstet. Gynecol. 1953, 66, 1062–1081. [Google Scholar] [CrossRef] [PubMed]
- Bamigboye, A.A.; Morris, J. Oestrogen Supplementation, Mainly Diethylstilbestrol, for Preventing Miscarriages and Other Adverse Pregnancy Outcomes. Cochrane Database Syst. Rev. 2003, 3, CD004353. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.H. Effect of Stilbestrol on Pregnancy Compared to the Effect of a Placebo. Am. J. Obstet. Gynecol. 1953, 65, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. Developmental Origins of Adult Health and Disease. J. Epidemiol. Community Health 2004, 58, 114–115. [Google Scholar] [CrossRef] [PubMed]
- Street, M.E.; Bernasconi, S. Endocrine-Disrupting Chemicals in Human Fetal Growth. Int. J. Mol. Sci. 2020, 21, 1430. [Google Scholar] [CrossRef]
- Kolan, A.S.; Hall, J.M. Association of Preterm Birth and Exposure to Endocrine Disrupting Chemicals. Int. J. Mol. Sci. 2023, 24, 1952. [Google Scholar] [CrossRef]
- Shah, P.S. Knowledge Synthesis Group on Determinants of LBWPTb. Parity and Low Birth Weight and Preterm Birth: A Systematic Review and Meta-Analyses. Acta Obstet. Gynecol. Scand. 2010, 89, 862–875. [Google Scholar] [CrossRef]
- Saeed, M.; Rogan, E.; Cavalieri, E. Mechanism of Metabolic Activation and DNA Adduct Formation by the Human Carcinogen Diethylstilbestrol: The Defining Link to Natural Estrogens. Int. J. Cancer 2009, 124, 1276–1284. [Google Scholar] [CrossRef]
- Smith, O.W.; Smith, G.V.; Hurwitz, D. Increased Excretion of Pregnanediol in Pregnancy from Diethylstilbestrol with Special Reference to the Prevention of Late Pregnancy Accidents. Am. J. Obstet. Gynecol. 1946, 51, 411–415. [Google Scholar] [CrossRef]
- Smith, R.; Smith, J.I.; Shen, X.; Engel, P.J.; Bowman, M.; McGrath, S.A.; Bisits, A.M.; McElduff, P.; Giles, W.B.; Smith, D. Patterns of Plasma Corticotropin-Releasing Hormone, Progesterone, Estradiol, and Estriol Change and the Onset of Human Labor. J. Clin. Endocrinol. Metab. 2009, 94, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- McLean, M.; Bisits, A.; Davies, J.; Woods, R.; Lowry, P.; Smith, R. A Placental Clock Controlling the Length of Human Pregnancy. Nat. Med. 1995, 1, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Smith, R. Alterations in the Hypothalamic Pituitary Adrenal Axis During Pregnancy and the Placental Clock That Determines the Length of Parturition. J. Reprod. Immunol. 1998, 39, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Fowden, A.L.; Szemere, J.; Hughes, P.; Gilmour, R.S.; Forhead, A.J. The Effects of Cortisol on the Growth Rate of the Sheep Fetus During Late Gestation. J. Endocrinol. 1996, 151, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Santolaya-Forgas, J. Current Concepts of Fetal Growth Restriction: Part I. Causes, Classification, and Pathophysiology. Obstet. Gynecol. 1998, 92, 1044–1055. [Google Scholar] [CrossRef]
- Titus, L.; Hatch, E.E.; Drake, K.M.; Parker, S.E.; Hyer, M.; Palmer, J.R.; Strohsnitter, W.C.; Adam, E.; Herbst, A.L.; Huo, D.; et al. Reproductive and Hormone-Related Outcomes in Women Whose Mothers Were Exposed in Utero to Diethylstilbestrol (Des): A Report from the Us National Cancer Institute Des Third Generation Study. Reprod. Toxicol. 2019, 84, 32–38. [Google Scholar] [CrossRef]
- Palanza, P.; Parmigiani, S.; vom Saal, F.S. Effects of Prenatal Exposure to Low Doses of Diethylstilbestrol, O,P’ddt, and Methoxychlor on Postnatal Growth and Neurobehavioral Development in Male and Female Mice. Horm. Behav. 2001, 40, 252–265. [Google Scholar] [CrossRef]
- Reed, C.E.; Fenton, S.E. Exposure to Diethylstilbestrol During Sensitive Life Stages: A Legacy of Heritable Health Effects. Birth Defects Res. C Embryo Today 2013, 99, 134–146. [Google Scholar] [CrossRef]
- Al Jishi, T.; Sergi, C. Current Perspective of Diethylstilbestrol (Des) Exposure in Mothers and Offspring. Reprod. Toxicol. 2017, 71, 71–77. [Google Scholar] [CrossRef]
- Tournaire, M.; Devouche, E.; Epelboin, S.; Cabau, A. Diethylstilbestrol Exposure: Evaluation of the Doses Received in France. Eur. J. Epidemiol. 2012, 27, 315–316, author reply 8. [Google Scholar] [CrossRef]
- Yim, G.; Roberts, A.; Wypij, D.; Kioumourtzoglou, M.A.; Weisskopf, M.G. Grandmothers’ Endocrine Disruption During Pregnancy, Low Birth Weight, and Preterm Birth in Third Generation. Int. J. Epidemiol. 2022, 50, 1886–1896. [Google Scholar] [CrossRef] [PubMed]
- Newbold, R.R. Lessons Learned from Perinatal Exposure to Diethylstilbestrol. Toxicol. Appl. Pharmacol. 2004, 199, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Newbold, R.R.; McLachlan, J.A. Transplacental Hormonal Carcinogenesis: Diethylstilbestrol as an Example. Prog. Clin. Biol. Res. 1996, 394, 131–147. [Google Scholar] [PubMed]
- Klip, H.; Verloop, J.; van Gool, J.D.; Koster, M.E.; Burger, C.W.; E van Leeuwen, F. Hypospadias in Sons of Women Exposed to Diethylstilbestrol in Utero: A Cohort Study. Lancet 2002, 359, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Pons, J.C.; Papiernik, E.; Billon, A.; Hessabi, M.; Duyme, M. Hypospadias in Sons of Women Exposed to Diethylstilbestrol in Utero. Prenat. Diagn. 2005, 25, 418–419. [Google Scholar] [CrossRef]
- Brouwers, M.M.; Feitz, W.F.; Roelofs, L.A.; Kiemeney, L.A.; de Gier, R.P.; Roeleveld, N. Hypospadias: A Transgenerational Effect of Diethylstilbestrol? Hum. Reprod. 2006, 21, 666–669. [Google Scholar] [CrossRef]
- Titus-Ernstoff, L.; Troisi, R.; E Hatch, E.; A Wise, L.; Palmer, J.; Hyer, M.; Kaufman, R.; Adam, E.; Strohsnitter, W.; Noller, K.; et al. Menstrual and Reproductive Characteristics of Women Whose Mothers Were Exposed in Utero to Diethylstilbestrol (Des). Int. J. Epidemiol. 2006, 35, 862–868. [Google Scholar] [CrossRef]
- Titus-Ernstoff, L.; Troisi, R.; Hatch, E.; Palmer, J.; Hyer, M.; Kaufman, R.; Adam, E.; Noller, K.; Hoover, R.N. Birth Defects in the Sons and Daughters of Women Who Were Exposed in Utero to Diethylstilbestrol (Des). Int. J. Androl. 2010, 33, 377–384. [Google Scholar] [CrossRef]
- Jefferson, T.B.; Wang, T.; Jefferson, W.N.; Li, Y.; Hamilton, K.J.; Wade, P.A.; Williams, C.J.; Korach, K.S. Multiple Tissue-Specific Epigenetic Alterations Regulate Persistent Gene Expression Changes Following Developmental Des Exposure in Mouse Reproductive Tissues. Epigenetics 2022, 1–21. [Google Scholar] [CrossRef]
- Van Tongeren, M.; Nieuwenhuijsen, M.J.; Gardiner, K.; Armstrong, B.; Vrijheid, M.; Dolk, H.; Botting, B. A Job-Exposure Matrix for Potential Endocrine-Disrupting Chemicals Developed for a Study into the Association between Maternal Occupational Exposure and Hypospadias. Ann. Occup. Hyg. 2002, 46, 465–477. [Google Scholar] [CrossRef]
- Brouwers, M.M.; van Tongeren, M.; Hirst, A.A.; Bretveld, R.W.; Roeleveld, N. Occupational Exposure to Potential Endocrine Disruptors: Further Development of a Job Exposure Matrix. Occup. Environ. Med. 2009, 66, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Fenichel, P.; Brucker-Davis, F.; Chevalier, N. The History of Distilbene(R) (Diethylstilbestrol) Told to Grandchildren--the Transgenerational Effect. Ann. Endocrinol. 2015, 76, 253–259. [Google Scholar] [CrossRef] [PubMed]
| Exclusion Criteria | n |
|---|---|
| Declined to participate | 57 |
| Incomplete questionnaire | 54 |
| Only one delivery | 49 |
| Only two deliveries | 60 |
| Three or more deliveries, but not pre-DES, DES, and post-DES conditions | 47 |
| Three or more deliveries with pre-DES, DES, and post-DES conditions, but at least one condition represented only by twins, stillbirth, or neonatal death | 14 |
| Three or more deliveries with pre-DES, DES, and post-DES conditions, but without at least one grandchild born from one of her pre-DES or post-DES children and one from her DES child | 33 |
| Changed partners | 11 |
| Not documented DES exposure | 68 |
| Comorbidities | |
| Diabetes mellitus | 5 |
| Thyroid disease | 7 |
| BMI <18.5 or >25 | 9 |
| Severe anemia | 1 |
| Chronic hypertension | 1 |
| Chronic renal disease | 1 |
| Chronic cardiorespiratory disease | 1 |
| Autoimmune disease | 7 |
| Epilepsy with anti-epileptic drugs | 2 |
| Cancer | 3 |
| History of caesarean section | 15 |
| Severe depression | 5 |
| Changed habitat between pregnancies (city, countryside) | 12 |
| Changed lifestyle habits between pregnancies (smoke, alcohol, illicit drugs) | 14 |
| Changed job with EDC exposure between pregnancies (beautician, cleaner, hairdresser, laboratory technician) | 17 |
| Changed anti-epileptic drugs between pregnancies | 3 |
| Presented pregnancy-related problems | |
| Gestational diabetes mellitus | 5 |
| Intrahepatic cholestasis | 2 |
| Gestational hypertension | 8 |
| Preeclampsia | 12 |
| Eclampsia | 1 |
| Infections | 0 |
| Traumatisms | 0 |
| Major congenital anomalies in fetus | 3 |
| Major life event during pregnancy (divorce, a death in the family, injury, or job loss) | 9 |
| Pre-DES | DES | Post-DES | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | n | Mean ± SD | Median | Min | Max | n | Mean ± SD | Median | Min | Max | n | Mean ± SD | Median | Min | Max |
| Mean DES dose | - | - | - | - | - | 94 | 3982.4 ± 412.4 | 4200 | 2100 | 4250 | - | - | - | - | - |
| IPI | - | - | - | - | - | 94 | 1.4 ± 0.4 | 1.4 | 0.4 | 2.3 | 93 | 1.4 ± 0.5 | 1.4 | 0.6 | 2.4 |
| Maternal age | 74 | 23.6 ± 2.7 | 23.55 | 19.4 | 31.1 | 94 | 26.3 ± 3.1 | 25.9 | 21.2 | 34.2 | 93 | 29.1 ± 3.3 | 28.7 | 23.2 | 38.2 |
| GW | 74 | 39.8 ± 1.1 | 40 | 33 | 40 | 94 | 38.5 ± 3.1 | 40 | 28 | 40 | 93 | 39.3 ± 2.1 | 40 | 30 | 40 |
| BW | 74 | 3203.4 ± 423.5 | 3100 | 1850 | 4200 | 94 | 3141.1 ± 854.1 | 3335 | 980 | 4900 | 93 | 3347.4 ± 634 | 3345 | 1260 | 4200 |
| BW ≥37 GW | 72 | 3240.3 ± 365.1 | 3100 | 2650 | 4200 | 80 | 3421.0 ± 543.9 | 3500 | 2200 | 4900 | 83 | 3485.3 ± 493.0 | 3401 | 1700 | 4200 |
| Pre-DES | DES | Post-DES | p Value Cochran-Armitage Test for Trend | ||||
|---|---|---|---|---|---|---|---|
| BOYS (n = 37) | GIRLS (n = 37) | BOYS (n = 46) | GIRLS (n = 48) | BOYS (n = 43) | GIRLS (n = 50) | ||
| PTB (< 37 GW), n (%) | 2 (2.7) | 14 (14.9) | 10 (10.8) | 0.0095 | |||
| LBW < 2499 g, n (%) | 0 | 2 (2.9) | 2 (2.5) | 0.0006 | |||
| HBW > 4000 g, n (%) | 3 (4.17) | 8 (11.6) | 18 (22.5) | 0.0033 | |||
| PTB | LBW < 2499 g | HBW > 4000 g | |
|---|---|---|---|
| Variables | p-Value | p-Value | p-Value |
| Current smoker | 0.7362 | 0.3932 | 0.3845 |
| Sex | 0.8542 | 0.3608 | 0.7394 |
| GW | <0.0001 | 0.0616 | |
| Mother age | 0.5596 | 0.7272 | 0.5772 |
| IPI | 0.7800 | 0.6678 | 0.0948 |
| Exposure group | 0.4480 | 0.0269 | 0.0005 |
| Prescribed DES dose | 0.1304 | 0.0661 | 0.3456 |
| Number of pregnancies | 0.1395 | 0.0640 | 0.0087 |
| Pre-DES | DES | Post-DES | |||||
|---|---|---|---|---|---|---|---|
| BOYS | GIRLS | BOYS | GIRLS | BOYS | GIRLS | ||
| Comorbidities | BMI < 18.5 or > 25 | 2 | 1 | 3 | 2 | 3 | |
| Severe anemia | 1 | ||||||
| Chronic hypertension | 1 | 1 | |||||
| Chronic cardiorespiratory disease | 1 | ||||||
| Chronic renal disease | 1 | ||||||
| Autoimmune disease | 1 | 2 | 2 | ||||
| Diabetes mellitus | 1 | ||||||
| Thyroid problem | 2 | 1 | 2 | ||||
| Epilepsy with anti-epileptic drugs | 1 | 1 | |||||
| Cancer | 1 | ||||||
| Severe depression | 2 | 4 | 3 | ||||
| Pregnancy-related problems | Gestational diabetes mellitus | 2 | 3 | 3 | 1 | 1 | 2 |
| Gestational hypertension | 2 | 2 | 2 | 3 | |||
| Preeclampsia | 1 | 2 | 2 | 1 | |||
| Intrahepatic cholestasis | 1 | ||||||
| Major congenital anomalies in fetus | |||||||
| Pre-DES | DES | Post-DES | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Paternal Germline | Maternal Germline | Paternal Germline | Maternal Germline | Paternal Germline | Maternal Germline | |||||||
| Mothers (n) | 19 | 21 | 35 | 47 | 17 | 26 | ||||||
| Maternal age | 24.5 ± 2.3 | 25.6 ± 3.0 | 24.6 ± 2.4 | 25.8 ± 2.9 | 26.7 ± 2.8 | 25.8 ± 3.0 | ||||||
| G2 children (n) | 23 | 26 | 42 | 54 | 20 | 27 | ||||||
| Sex G2 children (n) | 14  | 9  | 14  | 12  | 22  | 20  | 22  | 32  | 9  | 11  | 12  | 15  |
| PTB (GW < 37 weeks) n (%) | 2 | (8.7) | 2 | (7.7) | 0 | (0) | 8 | (14.8) | 2 | (10.0) | 4 | (14.8) |
| LBW n (%) | 2 | (8.7) | 2 | (7.7) | 0 | (0) | 8 | (14.8) | 2 | (10.0) | 3 | (11.1) |
| LBW in ≥ 37 GW n (%) | 0 | (0) | 2 | (8.3) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| HBW n (%) | 4 | (17.4) | 2 | (7.7) | 2 | (4.8) | 6 | (11.1) | 0 | (0) | 0 | (0) |
| HBW in ≥ 37 GW n (%) | 4 | (19.1) | 2 | (8.3) | 2 | (4.8) | 6 | (13.0) | 0 | (0) | 0 | (0) |
| BW (n), mean ± SD (g) | 23 | 3313.0 ± 611.9 | 26 | 3218.5 ± 415.6 | 42 | 3329.2 ± 418.6 | 54 | 3214.3 ± 693.5 | 20 | 3228.5 ± 473.3 | 27 | 3185.4 ± 516.5 |
| BW ≥ 37GW (n), mean ± SD (g) | 21 | 3443.8 ± 450.4 | 24 | 3245.8 ± 421.4 | 42 | 3329.2 ± 418.6 | 46 | 3422.9 ± 500.0 | 18 | 3353.3 ± 289.0 | 23 | 3344.6 ± 332.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaspari, L.; Soyer-Gobillard, M.-O.; Rincheval, N.; Paris, F.; Kalfa, N.; Hamamah, S.; Sultan, C. Birth Outcomes in DES Children and Grandchildren: A Multigenerational National Cohort Study on Informative Families. Int. J. Environ. Res. Public Health 2023, 20, 2542. https://doi.org/10.3390/ijerph20032542
Gaspari L, Soyer-Gobillard M-O, Rincheval N, Paris F, Kalfa N, Hamamah S, Sultan C. Birth Outcomes in DES Children and Grandchildren: A Multigenerational National Cohort Study on Informative Families. International Journal of Environmental Research and Public Health. 2023; 20(3):2542. https://doi.org/10.3390/ijerph20032542
Chicago/Turabian StyleGaspari, Laura, Marie-Odile Soyer-Gobillard, Nathalie Rincheval, Françoise Paris, Nicolas Kalfa, Samir Hamamah, and Charles Sultan. 2023. "Birth Outcomes in DES Children and Grandchildren: A Multigenerational National Cohort Study on Informative Families" International Journal of Environmental Research and Public Health 20, no. 3: 2542. https://doi.org/10.3390/ijerph20032542
APA StyleGaspari, L., Soyer-Gobillard, M.-O., Rincheval, N., Paris, F., Kalfa, N., Hamamah, S., & Sultan, C. (2023). Birth Outcomes in DES Children and Grandchildren: A Multigenerational National Cohort Study on Informative Families. International Journal of Environmental Research and Public Health, 20(3), 2542. https://doi.org/10.3390/ijerph20032542






