Exploring Urban Green Spaces’ Effect against Traffic Exposure on Childhood Leukaemia Incidence
Abstract
1. Introduction
2. Materials and Methods Record
2.1. Study Design
2.2. Traffic Exposure Measure
2.3. UGS Selection and Measurement
2.4. Sociodemographic Covariates
2.5. Statistical Analysis
3. Results
3.1. Descriptive Analysis
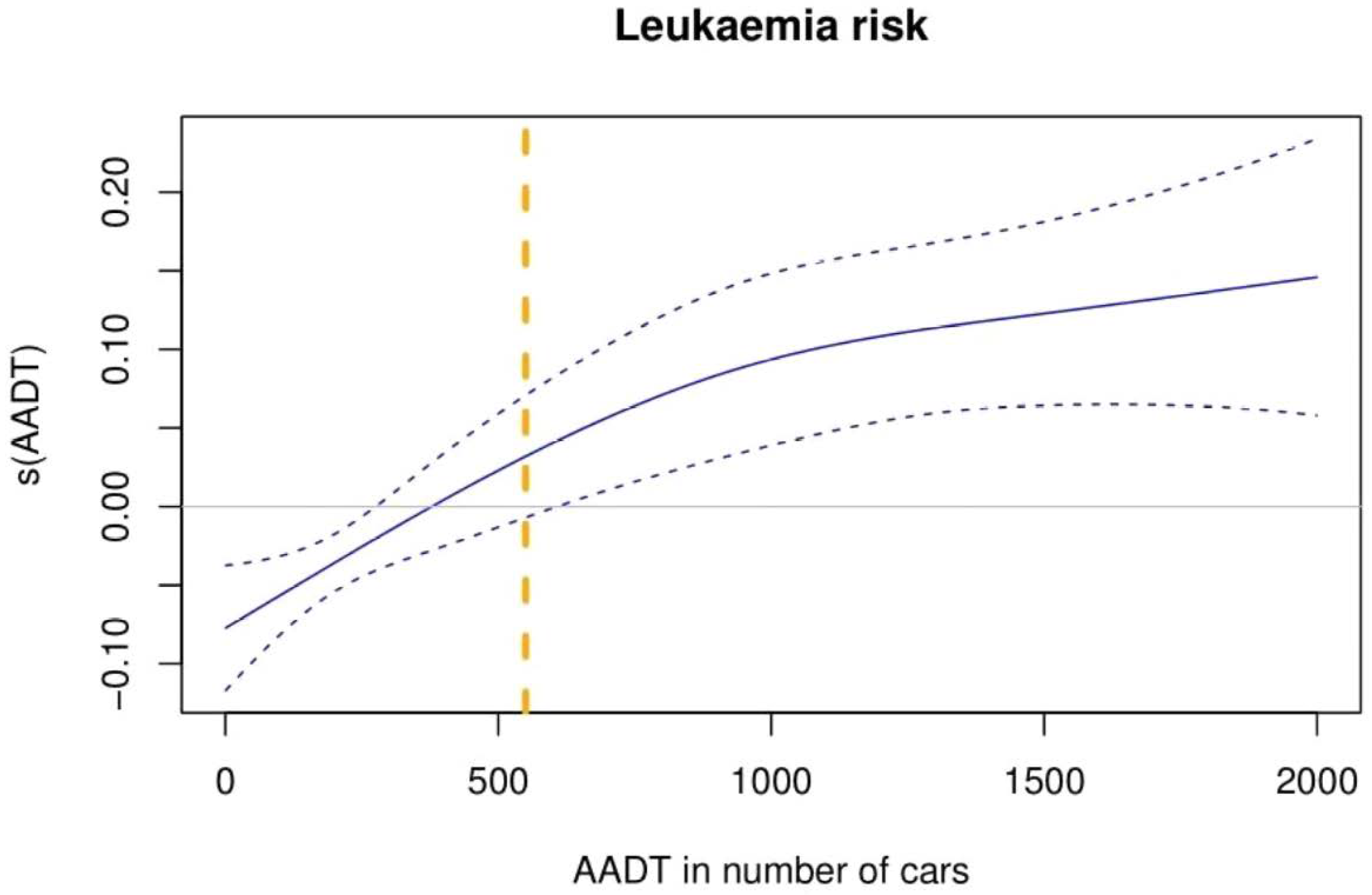
3.2. First Objective: Traffic Exposition and Childhood Leukaemia Incidence
3.3. Second Objective: UGS Exposure and Childhood Leukaemia Incidence
3.4. Third Objective: Traffic and UGS Exposure Effects on Childhood Leukaemia Incidence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peris-Bonet, R.; Salmerón, D.; Martínez-Beneito, M.A.; Galceran, J.; Marcos-Gragera, R.; Felipe, S.; González, V.; Codina, J.S.D.T. Childhood cancer incidence and survival in Spain. Ann. Oncol. 2010, 21, iii103–iii110. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Gragera, R.; Galceran, J.; Martos, C.; de Munain, A.L.; Vicente-Raneda, M.; Navarro, C.; Quirós-Garcia, J.R.; Sánchez, M.-J.; Ardanaz, E.; Ramos, M.; et al. Incidence and survival time trends for Spanish children and adolescents with leukaemia from 1983 to 2007. Clin. Transl. Oncol. 2017, 19, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, T.P.; Metayer, C.; Wiemels, J.L.; Singer, A.W.; Miller, M.D. Childhood Leukemia and Primary Prevention. Curr. Probl. Pediatr. Adolesc. Health Care 2016, 46, 317–352. [Google Scholar] [CrossRef]
- Urayama, K.Y. Epidemiology of childhood leukemia: A targeted overview. Rinsho Ketsueki 2021, 62, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Barroso, D.; García-Pérez, J.; López-Abente, G.; Tamayo-Uria, I.; Morales-Piga, A.; Romaguera, E.P.; Ramis, R. Agricultural crop exposure and risk of childhood cancer: New findings from a case–control study in Spain. Int. J. Health Geogr. 2016, 15, 18. [Google Scholar] [CrossRef]
- Tong, J.; Qin, L.; Cao, Y.; Li, J.; Zhang, J.; Nie, J.; An, Y. Environmental Radon Exposure and Childhood Leukemia. J. Toxicol. Environ. Health Part B 2012, 15, 332–347. [Google Scholar] [CrossRef]
- García-Pérez, J.; López-Abente, G.; Gómez-Barroso, D.; Morales-Piga, A.; Romaguera, E.P.; Tamayo, I.; Fernández-Navarro, P.; Ramis, R. Childhood leukemia and residential proximity to industrial and urban sites. Environ. Res. 2015, 140, 542–553. [Google Scholar] [CrossRef]
- Ambient (Outdoor) Air Pollution n.d. Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 25 January 2023).
- Vineis, P.; Husgafvel-Pursiainen, K. Air pollution and cancer: Biomarker studies in human populations. Carcinogenesis 2005, 26, 1846–1855. [Google Scholar] [CrossRef]
- Heck, J.E.; He, D.; Contreras, Z.A.; Ritz, B.; Olsen, J.; Hansen, J. Parental occupational exposure to benzene and the risk of childhood and adolescent acute lymphoblastic leukaemia: A population-based study. Occup. Env. Med. 2019, 76, 527–529. [Google Scholar] [CrossRef]
- Vlaanderen, J.; Lan, Q.; Kromhout, H.; Rothman, N.; Vermeulen, R. Occupational benzene exposure and the risk of chronic myeloid leukemia: A meta-analysis of cohort studies incorporating study quality dimensions. Am. J. Ind. Med. 2012, 55, 779–785. [Google Scholar] [CrossRef]
- Eguiluz-Gracia, I.; Mathioudakis, A.G.; Bartel, S.; Vijverberg, S.J.H.; Fuertes, E.; Comberiati, P.; Cai, Y.S.; Tomazic, P.V.; Diamant, Z.; Vestbo, J.; et al. The need for clean air: The way air pollution and climate change affect allergic rhinitis and asthma. Allergy 2020, 75, 2170–2184. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Jiao, R.; Nakyeyune, R.; Zang, Z.; Shao, Y.; Shen, Y.; Niu, C.; Zhu, L.; Ruan, X.; Liu, F. Exposure to outdoor air pollution at different periods and the risk of leukemia: A meta-analysis. Environ. Sci. Pollut. Res. 2021, 28, 35376–35391. [Google Scholar] [CrossRef] [PubMed]
- Kreis, C.; Héritier, H.; Scheinemann, K.; Hengartner, H.; de Hoogh, K.; Röösli, M.; Spycher, B.D. Childhood cancer and traffic-related air pollution in Switzerland: A nationwide census-based cohort study. Environ. Int. 2022, 166, 107380. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Hatch, E.E.; Rothman, K.J.; Heck, J.E.; Park, A.S.; Crippa, A.; Orsini, N.; Vinceti, M. Association between Outdoor Air Pollution and Childhood Leukemia: A Systematic Review and Dose-Response Meta-Analysis. Environ. Health Perspect. 2019, 127, 46002. [Google Scholar] [CrossRef] [PubMed]
- WHO. Urban Green Spaces and Health; Regional Office for Europe: Copenhagen, Denmark, 2016.
- Ampatzidis, P.; Kershaw, T. A review of the impact of blue space on the urban microclimate. Sci Total Environ. 2020, 730, 139068. [Google Scholar] [CrossRef]
- Klingberg, J.; Broberg, M.; Strandberg, B.; Thorsson, P.; Pleijel, H. Influence of urban vegetation on air pollution and noise exposure—A case study in Gothenburg, Sweden. Sci. Total Environ. 2017, 599–600, 1728–1739. [Google Scholar] [CrossRef]
- Nowak, D.J.; Crane, D.E. Carbon storage and sequestration by urban trees in the USA. Environ. Pollut. 2002, 116, 381–389. [Google Scholar] [CrossRef]
- Chen, S.; Wang, W.; Xu, W.; Wang, Y.; Wan, H.; Chen, D.; Tang, Z.; Tang, X.; Zhou, G.; Xie, Z.; et al. Plant diversity enhances productivity and soil carbon storage. Proc. Natl. Acad. Sci. USA 2018, 115, 4027–4032. [Google Scholar] [CrossRef]
- Diener, A.; Mudu, P. How can vegetation protect us from air pollution? A critical review on green spaces’ mitigation abilities for air-borne particles from a public health perspective—With implications for urban planning. Sci. Total Environ. 2021, 796, 148605. [Google Scholar] [CrossRef]
- Fyfe-Johnson, A.L.; Hazlehurst, M.F.; Perrins, S.P.; Bratman, G.N.; Thomas, R.; Garrett, K.A.; Hafferty, K.R.; Cullaz, T.M.; Marcuse, E.K.; Tandon, P.S. Nature and Children’s Health: A Systematic Review. Pediatrics 2021, 148, e2020049155. [Google Scholar] [CrossRef]
- Ye, T.; Yu, P.; Wen, B.; Yang, Z.; Huang, W.; Guo, Y.; Abramson, M.J.; Li, S. Greenspace and health outcomes in children and adolescents: A systematic review. Environ. Pollut. 2022, 314, 120193. [Google Scholar] [CrossRef]
- Ojeda Sánchez, C.; Segú-Tell, J.; Gomez-Barroso, D.; Pardo Romaguera, E.; Ortega-García, J.A.; Ramis, R. Urban green spaces and childhood leukemia incidence: A population-based case-control study in Madrid. Environ. Res. 2021, 202, 111723. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadistica. (Spanish Statistical Office) n.d. Available online: https://www.ine.es/welcome.shtml (accessed on 19 April 2019).
- Pardo Romaguera, E.; Muñoz López, A.; Valero Poveda, S.; Porta Cebolla, S.; Fernández-Delgado, R.; Barreda Reines, M.S.; Peris Bonet, R. Cáncer infantil en España. Estadísticas 1980–2021. Registro Español de Tumores Infantiles (RETI-SEHOP) 2022. Available online: https://www.uv.es/rnti/pdfs/Informe_RETI-SEHOP_1980-2021.pdf (accessed on 11 November 2022).
- Carreteras|Ministerio de Transportes, Movilidad y Agenda Urbana n.d. Available online: https://www.mitma.gob.es/carreteras (accessed on 23 November 2022).
- Centro de Descargas del CNIG (IGN). Centro de Descargas del CNIG n.d. Available online: http://centrodedescargas.cnig.es (accessed on 11 June 2019).
- Tamayo-Uria, I.; Boldo, E.; García-Pérez, J.; Gómez-Barroso, D.; Romaguera, E.P.; Cirach, M.; Ramis, R. Childhood leukaemia risk and residential proximity to busy roads. Environ. Int. 2018, 121, 332–339. [Google Scholar] [CrossRef]
- Loomis, D.; Guyton, K.Z.; Grosse, Y.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Vilahur, N.; Mattock, H.; Straif, K. Carcinogenicity of benzene. Lancet Oncol. 2017, 18, 1574–1575. [Google Scholar] [CrossRef]
- Schultz, E.S.; Litonjua, A.A.; Melén, E. Effects of Long-Term Exposure to Traffic-Related Air Pollution on Lung Function in Children. Curr. Allergy Asthma Rep. 2017, 17, 41. [Google Scholar] [CrossRef]
- Khreis, H.; Kelly, C.; Tate, J.; Parslow, R.; Lucas, K.; Nieuwenhuijsen, M. Exposure to traffic-related air pollution and risk of development of childhood asthma: A systematic review and meta-analysis. Environ. Int. 2017, 100, 1–31. [Google Scholar] [CrossRef]
- Lavigne, É.; Bélair, M.-A.; Do, M.T.; Stieb, D.M.; Hystad, P.; van Donkelaar, A.; Martin, R.V.; Crouse, D.L.; Crighton, E.; Chen, H.; et al. Maternal exposure to ambient air pollution and risk of early childhood cancers: A population-based study in Ontario, Canada. Environ. Int. 2017, 100, 139–147. [Google Scholar] [CrossRef]
- Raaschou-Nielsen, O.; Hvidtfeldt, U.A.; Roswall, N.; Hertel, O.; Poulsen, A.H.; Sørensen, M. Ambient benzene at the residence and risk for subtypes of childhood leukemia, lymphoma and CNS tumor. Int. J. Cancer 2018, 143, 1367–1373. [Google Scholar] [CrossRef]
- Huang, C.; Yang, J.; Lu, H.; Huang, H.; Yu, L. Green Spaces as an Indicator of Urban Health: Evaluating Its Changes in 28 Mega-Cities. Remote Sens. 2017, 9, 1266. [Google Scholar] [CrossRef]
- Manandhar, S.; Suksaroj, T.T.; Rattanapan, C. The Association between Green Space and the Prevalence of Overweight/Obesity among Primary School Children. Int. J. Occup. Environ. Med. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Dzhambov, A.M.; Dimitrova, D.D.; Dimitrakova, E.D. Association between residential greenness and birth weight: Systematic review and meta-analysis. Urban For. Urban Green. 2014, 13, 621–629. [Google Scholar] [CrossRef]
- Grazuleviciene, R.; Danileviciute, A.; Dedele, A.; Vencloviene, J.; Andrusaityte, S.; Uždanaviciute, I.; Nieuwenhuijsen, M.J. Surrounding greenness, proximity to city parks and pregnancy outcomes in Kaunas cohort study. Int. J. Hyg. Environ. Health 2015, 218, 358–365. [Google Scholar] [CrossRef]
- Beyer, K.M.M.; Kaltenbach, A.; Szabo, A.; Bogar, S.; Nieto, F.J.; Malecki, K.M. Exposure to Neighborhood Green Space and Mental Health: Evidence from the Survey of the Health of Wisconsin. Int. J. Environ. Res. Public Health 2014, 11, 3453–3472. [Google Scholar] [CrossRef] [PubMed]
- Dzhambov, A.M.; Browning, M.H.E.M.; Markevych, I.; Hartig, T.; Lercher, P. Analytical approaches to testing pathways linking greenspace to health: A scoping review of the empirical literature. Environ. Res. 2020, 186, 109613. [Google Scholar] [CrossRef]
- Porcherie, M.; Linn, N.; Le Gall, A.R.; Thomas, M.-F.; Faure, E.; Rican, S.; Simos, J.; Cantoreggi, N.; Vaillant, Z.; Cambon, L.; et al. Relationship between Urban Green Spaces and Cancer: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 1751. [Google Scholar] [CrossRef]
- Li, Q.; Morimoto, K.; Kobayashi, M.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Suzuki, H.; Li, Y.; Wakayama, Y.; et al. Visiting a Forest, but Not a City, Increases Human Natural Killer Activity and Expression of Anti-Cancer Proteins. Int. J. Immunopathol. Pharm. 2008, 21, 117–127. [Google Scholar] [CrossRef]
- Paz-Alberto, A.M.; Sigua, G.C. Phytoremediation: A Green Technology to Remove Environmental Pollutants. Am. J. Clim. Chang. 2013, 2013, 29110. [Google Scholar] [CrossRef]
- Oksanen, E.; Kontunen-Soppela, S. Plants have different strategies to defend against air pollutants. Curr. Opin. Environ. Sci. Health 2021, 19, 100222. [Google Scholar] [CrossRef]
- Sánchez, C.O.; García-Pérez, J.; Gómez-Barroso, D.; Domínguez-Castillo, A.; Romaguera, E.P.; Cañete, A.; Ortega-García, J.A.; Ramis, R. Exploring Blue Spaces’ Effects on Childhood Leukaemia Incidence: A Population-Based Case-Control Study in Spain. Int. J. Environ. Res. Public Health 2022, 19, 5232. [Google Scholar] [CrossRef]
- Grellier, J.; White, M.P.; Albin, M.; Bell, S.; Elliott, L.R.; Gascón, M.; Gualdi, S.; Mancini, L.; Nieuwenhuijsen, M.J.; A Sarigiannis, D.; et al. BlueHealth: A study programme protocol for mapping and quantifying the potential benefits to public health and well-being from Europe’s blue spaces. BMJ Open 2017, 7, e016188. [Google Scholar] [CrossRef]
- Bailey, H.D.; Fritschi, L.; Infante-Rivard, C.; Glass, D.C.; Miligi, L.; Dockerty, J.D.; Lightfoot, T.; Clavel, J.; Roman, E.; Spector, L.G.; et al. Parental occupational pesticide exposure and the risk of childhood leukemia in the offspring: Findings from the Childhood Leukemia International Consortium. Int. J. Cancer 2014, 135, 2157–2172. [Google Scholar] [CrossRef] [PubMed]
- Dadvand, P.; de Nazelle, A.; Triguero-Mas, M.; Schembari, A.; Cirach, M.; Amoly, E.; Figueras, F.; Basagaña, X.; Ostro, B.; Nieuwenhuijsen, M. Surrounding Greenness and Exposure to Air Pollution During Pregnancy: An Analysis of Personal Monitoring Data. Environ. Health Perspect. 2012, 120, 1286–1290. [Google Scholar] [CrossRef] [PubMed]
- Kehm, R.D.; Spector, L.G.; Poynter, J.N.; Vock, D.M.; Osypuk, T.L. Socioeconomic Status and Childhood Cancer Incidence: A Population-Based Multilevel Analysis. Am. J. Epidemiol. 2018, 187, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Interprovincial Migration Balance by Semester, Province, Sex, Age Group and Nationality. INE n.d. Available online: https://www.ine.es/jaxiT3/Tabla.htm?t=24450&L=1 (accessed on 20 May 2022).

| Characteristics | Controls (n = 15,156) | Cases (n = 2526) | ALL (n = 2015) | p Value a | AML(n = 401) | p Value a | Same Address (n = 1737) | p Value a |
|---|---|---|---|---|---|---|---|---|
| Sex, n (%) | ||||||||
| Boy | 9346 (55.1%) | 1391 (55.1%) | 1118 (55.5%) | 217 (54.1%) | 937 (56.0%) | |||
| Girl | 6810 (44.9%) | 1135 (44.9%) | 897 (44.5%) | 0.802 # | 184 (45.9%) | 0.763 # | 764 (44.0%) | 0.561 # |
| Age at diagnosis, median (IQR) | X | 4 (5) | 4 (4) | 0.126 ¥ | 3 (6) | <0.001 ¥ | 4 (5) | 0.574 ¥ |
| Activity rate, mean (SD) | 76.61 (5.29) | 76.33 (5.50) | 76.2 (5.6) | 0.417 * | 76.7 (5.0) | 0.218 * | 76.6 (5.34) | 0.073 * |
| SES, mean (SD) | 1.03 (0.14) | 1.04 (0.13) | 1.04 (0.14) | 0.706 * | 1.04 (0.13) | 0.785 * | 1.04 (0.13) | 0.748 * |
| AADT, mean (SD) | 698 (650) | 640 (650) | 632 (645) | 0.685 * | 661 (658) | 0.555 * | 714 (679) | <0.001 * |
| Histologic subtype, n (%) | ||||||||
| ALL | x | 2015 (79.8%) | 1375 (79.2%) | |||||
| AML | x | 401 (15.9%) | 287 (16.5%) | |||||
| CML | x | 28 (1.1%) | 19 (1.1%) | |||||
| Other specific leukaemia | x | 48 (1.9%) | 31 (1.8%) | |||||
| Non-specific leukaemia | x | 34 (1.3%) | 25 (1.4%) | 0.978 # |
| Childhood Leukaemias | ALL Subtype | AML Subtype | Same Address | |||||
|---|---|---|---|---|---|---|---|---|
| Cases/Controls (n) | Adjusted OR (95% CI) | Cases/Controls (n) | Adjusted OR (95% CI) | Cases/Controls (n) | Adjusted OR (95% CI) | Cases/Controls (n) | Adjusted OR (95% CI) | |
| UGS.Q1 | 658/3031 | 1 | 523/3031 | 1 | 105/3031 | 1 | 394/3031 | 1 |
| UGS.Q2 | 497/3031 | 0.74 (0.65–0.84) | 407/3031 | 0.77 (0.67–0.88) | 76/3031 | 0.71 (0.52–0.96) | 364/3031 | 0.91 (0.78–1.06) |
| UGS.Q3 | 464/3032 | 0.69 (0.61–0.78) | 385/3032 | 0.73 (0.63–0.84) | 60/3032 | 0.55 (0.40–0.77) | 337/3032 | 0.84 (0.72–0.98) |
| UGS.Q4 | 482/3030 | 0.72 (0.63–0.82) | 364/3030 | 0.69 (0.59–0.80) | 93/3030 | 0.85 (0.64–1.14) | 350/3030 | 0.87 (0.74–1.01) |
| UGS.Q5 | 425/3032 | 0.63 (0.54–0.72) | 336/3032 | 0.63 (0.54–0.74) | 67/3032 | 0.61 (0.44–0.84) | 292/3032 | 0.72 (0.61–0.85) |
| Childhood Leukaemias | ALL Subtype | AML Subtype | Same Address | |||||
|---|---|---|---|---|---|---|---|---|
| Quintile | Cases/Controls (n) | Adjusted OR (95% CI) | Cases/Controls (n) | Adjusted OR (95% CI) | Cases/Controls (n) | Adjusted OR (95% CI) | Cases/Controls (n) | Adjusted OR (95% CI) |
| TE.Q1 | 159/1359 | 1 | 120/1359 | 1 | 28/1359 | 1 | 110/1359 | 1 |
| TE.Q2 | 212/1359 | 1.34 (1.08–1.67) | 173/1359 | 1.45 (1.14–1.85) | 33/1359 | 1.17 (0.71–1.97) | 152/1359 | 1.38 (1.07–1.79) |
| TE.Q3 | 197/1360 | 1.25 (1.00–1.57) | 164/1360 | 1.38 (1.08–1.78) | 26/1360 | 0.93 (0.54–1.59) | 160/1360 | 1.46 (1.13–1.89) |
| TE.Q4 | 195/1358 | 1.25 (1.00–1.57) | 147/1358 | 1.26 (0.98–1.62) | 36/1358 | 1.27 (0.77–2.12) | 155/1358 | 1.41 (1.10–1.84) |
| TE.Q5 | 216/1360 | 1.40 (1.12–1.76) | 169/1360 | 1.47 (1.14–1.89) | 36/1360 | 1.23 (0.76–2.12) | 177/1360 | 1.63 (1.26–2.10) |
| UGS.Q1 | 195/1359 | 1 | 156/1359 | 1 | 33/1359 | 1 | 149/1359 | 1 |
| UGS.Q2 | 190/1359 | 0.95 (0.77–1.18) | 151/1359 | 0.95 (0.75–1.20) | 28/1359 | 0.84 (0.50–1.41) | 151/1359 | 0.99 (0.78–1.26) |
| UGS.Q3 | 215/1359 | 1.07 (0.87–1.32) | 170/1360 | 1.06 (0.84–1.34) | 33/1359 | 0.99 (0.60–1.63) | 163/1359 | 1.07 (0.84–1.36) |
| UGS.Q4 | 199/1359 | 0.99 (0.80–1.23) | 155/1358 | 0.97 (0.77–1.24) | 36/1359 | 1.07 (0.65–1.75) | 161/1359 | 1.05 (0.83–1.34) |
| UGS.Q5 | 180/1360 | 0.90 (0.72–1.12) | 141/1360 | 0.89 (0.70–1.14) | 29/1360 | 0.87 (0.51–1.46) | 130/1360 | 0.86 (0.66–1.10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojeda Sánchez, C.; García-Pérez, J.; Gómez-Barroso, D.; Domínguez-Castillo, A.; Pardo Romaguera, E.; Cañete, A.; Ortega-García, J.A.; Ramis, R. Exploring Urban Green Spaces’ Effect against Traffic Exposure on Childhood Leukaemia Incidence. Int. J. Environ. Res. Public Health 2023, 20, 2506. https://doi.org/10.3390/ijerph20032506
Ojeda Sánchez C, García-Pérez J, Gómez-Barroso D, Domínguez-Castillo A, Pardo Romaguera E, Cañete A, Ortega-García JA, Ramis R. Exploring Urban Green Spaces’ Effect against Traffic Exposure on Childhood Leukaemia Incidence. International Journal of Environmental Research and Public Health. 2023; 20(3):2506. https://doi.org/10.3390/ijerph20032506
Chicago/Turabian StyleOjeda Sánchez, Carlos, Javier García-Pérez, Diana Gómez-Barroso, Alejandro Domínguez-Castillo, Elena Pardo Romaguera, Adela Cañete, Juan Antonio Ortega-García, and Rebeca Ramis. 2023. "Exploring Urban Green Spaces’ Effect against Traffic Exposure on Childhood Leukaemia Incidence" International Journal of Environmental Research and Public Health 20, no. 3: 2506. https://doi.org/10.3390/ijerph20032506
APA StyleOjeda Sánchez, C., García-Pérez, J., Gómez-Barroso, D., Domínguez-Castillo, A., Pardo Romaguera, E., Cañete, A., Ortega-García, J. A., & Ramis, R. (2023). Exploring Urban Green Spaces’ Effect against Traffic Exposure on Childhood Leukaemia Incidence. International Journal of Environmental Research and Public Health, 20(3), 2506. https://doi.org/10.3390/ijerph20032506








