Chemical Composition and Antimicrobial Activity of New Honey Varietals
Abstract
1. Introduction
2. Results
2.1. Melissopalynological Analysis
2.2. Physicochemical Properties of Honey
2.3. Antimicrobial Activity of Honey
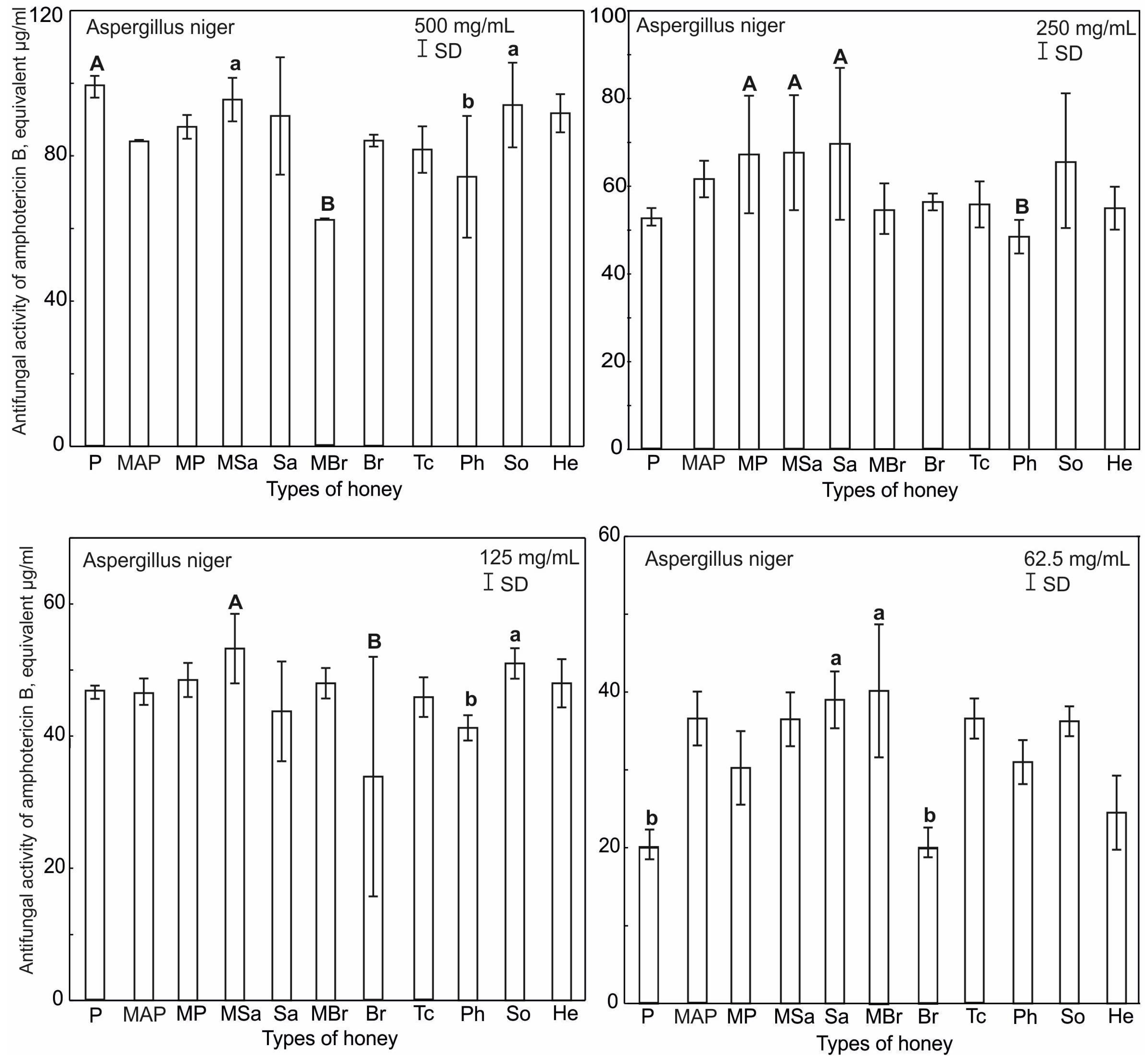
2.4. Antifungal Activity of Honey
2.5. Catalase
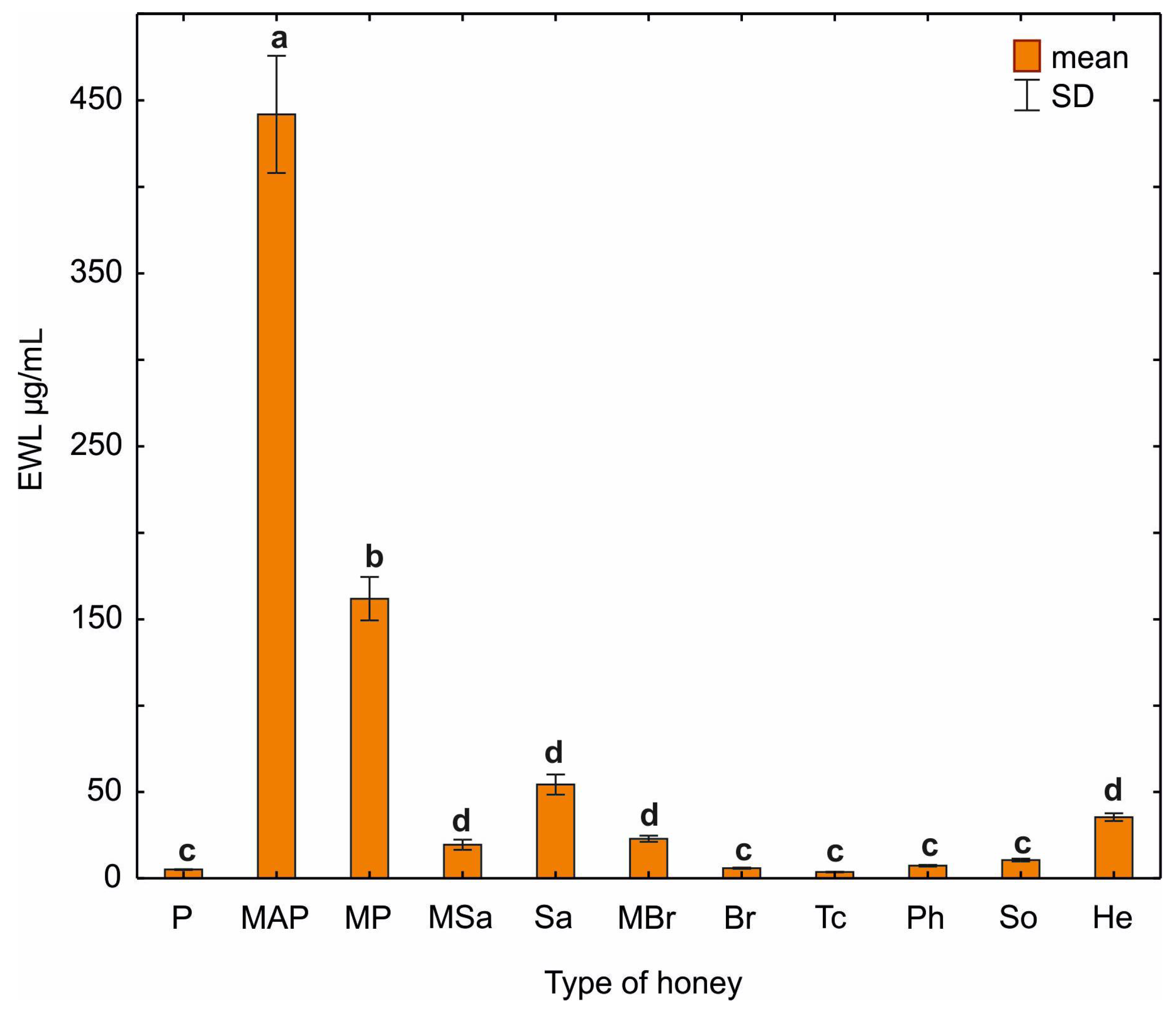
2.6. Lysozyme-like Activity of Honey
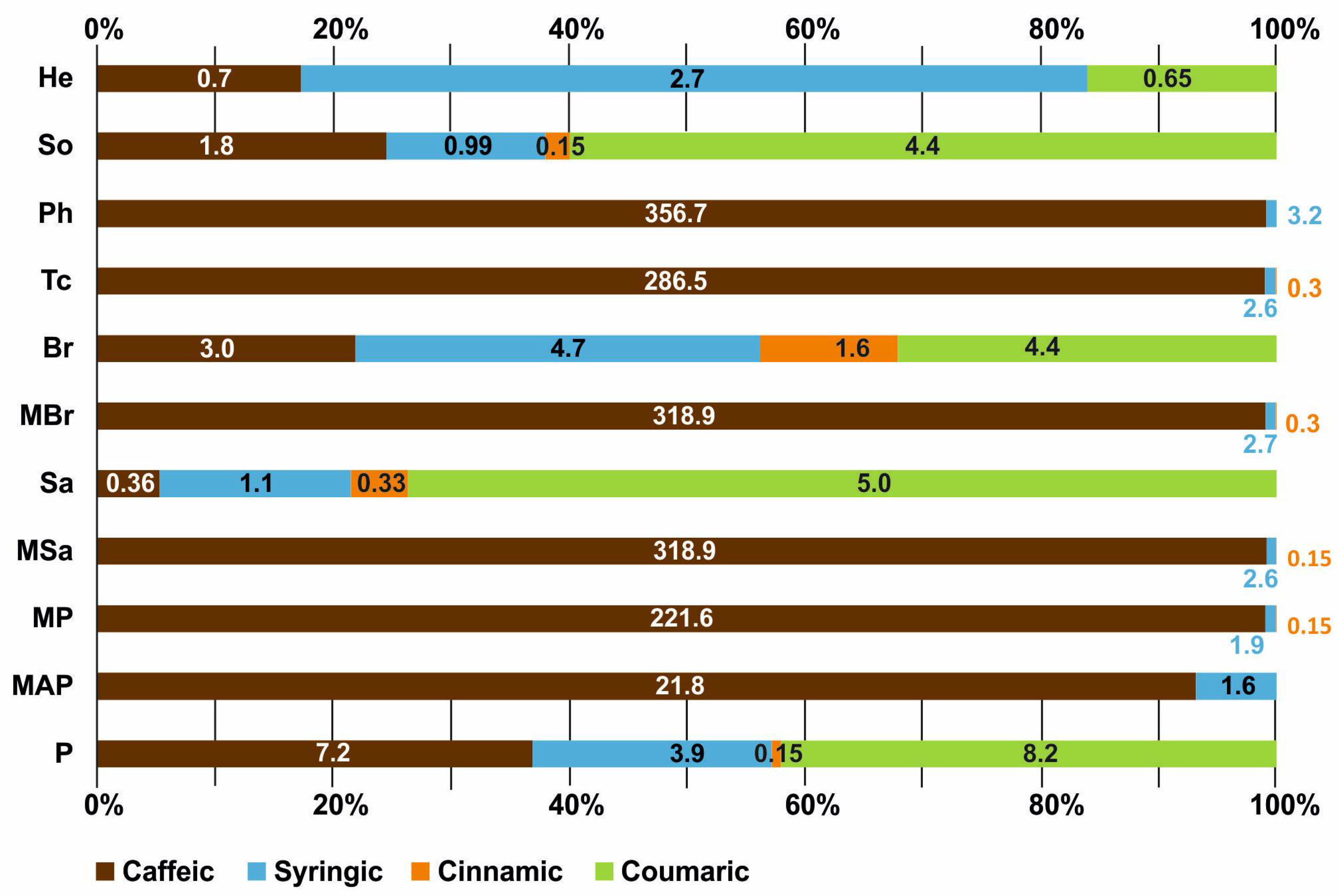
2.7. HPLC Analysis of Phenolic Compounds in Honey Samples
3. Discussion
4. Materials and Methods
4.1. Honey Sample Collection and Classification
4.2. Honey Sample Classification Using Pollen Analysis
4.3. Honey Sample Preparation
4.4. Physicochemical Properties of Honeys
4.4.1. Water Content
4.4.2. Electrical Conductivity
4.4.3. pH
4.4.4. Color Intensity
4.4.5. Total Phenolic Content
4.4.6. Sugar Analysis in Honey Samples
4.4.7. Protein Content
4.5. Microorganisms Used in the Antimicrobial Assays
- Escherichia coli D31 (CGSC 5165; Genetic Stock Centre, New Haven, CT, USA)
- Bacillus circulans strain ATCC 61;
- Staphylococcus aureus, clinical strain 1-KI, obtained from The Department of Immunobiology, Maria Curie-Sklodowska University in Lublin, Poland.
- Pseudomonas aeruginosa strain ATCC 27853;
- Pseudomonas aeruginosa clinical strain 02/18, obtained from The Department of Microbiology and Epidemiology, Military Institute of Hygiene and Epidemiology in Warsaw, Poland.
- Aspergillus niger 71, grown in PDA broth (5% potato extract; 0.5% dextrose; 1.7% agar);
- Saccharomyces cerevisiae, subcultured in sterile Sabouraud broth (1% pepton; 4% glucose; 1.5% agar);
- Candida albicans, grown in YPD broth (1% yeast extract; 2% pepton; 2% glucose; 1.6% agar).
4.6. Antibacterial Activity Assay
4.7. Antifungal Activity Assay
4.8. Lysozyme-like Activity of Honey Samples
4.9. Antimicrobial Activity Connected with Hydrogen Peroxide in Honey Samples
4.10. Solid Phase Extraction of Honey Samples
4.11. HPLC Analysis of Phenolic Compounds in Honey Samples
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and health: A review of recent clinical research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar] [CrossRef]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.A.; Al-Asiri, S.A.; Mahesar, A.L.; Ansari, M.J. Role of honey in modern medicine. Saudi J. Biol. Sci. 2017, 24, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Abubaker, K.; St-Martin, L.; Castle, A. Re-examining the role of hydrogen peroxide in bacteriostatic and bactericidal activities of honey. Front. Microbiol. 2011, 25, 213. [Google Scholar] [CrossRef]
- Samad, A.; Khalid, A.; Sattar, S.; Rafique, N.; Rashid, M.A.; Khan, M.A.; Sattar, S.; Rafique, N.; Javed, H. Fungicidal activity of honey originating from different phytogeographic regions against Aspergillus niger and Penicillium chrysogenum. J. Entomol. Zool. Stud. 2016, 4, 339–342. [Google Scholar]
- Halagarda, M.; Groth, S.; Popek, S.; Rohn, S.; Pedan, V. Antioxidant activity and phenolic profile of selected organic and conventional honeys from Poland. Antioxidants 2020, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Kwakman, P.H.S.; Zaat, S.A.J. Antibacterial components of honey. IUBMB Life 2012, 64, 48–55. [Google Scholar] [CrossRef]
- Sojka, M.; Valachova, I.; Bucekova, M.; Majtan, J. Antibiofilm efficacy of honey and bee-derived defensin-1 on multispecies wound biofilm. J. Med. Microbiol. 2016, 65, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Bucekova, M.; Jardekova, L.; Juricova, V.; Bugarova, V.; Di Marco, G.; Gismondi, A.; Leonardi, D.; Farkasovska, J.; Godocikova, J.; Laho, M.; et al. Antibacterial activity of different blossom honeys: New findings. Molecules 2019, 24, 1573. [Google Scholar] [CrossRef]
- Mohrig, W.; Messner, B. Lyzozym als antibakterielles agents im bienenhonig und bienengift. Acta Biol. Med. Germ. 1968, 21, 85–90. [Google Scholar]
- Kwakman, P.H.; te Velde, A.A.; de Boer, L.; Speijer, D.; Vandenbroucke-Grauls, C.M.; Zaat, S.A. How honey kills bacteria. FASEB J. 2010, 24, 2576–2582. [Google Scholar] [CrossRef]
- Piotr, S. Antimicrobial activity of honey. Agricultural and biological sciences. In Honey Analysis; IntechOpen: London, UK, 2017; pp. 215–232. [Google Scholar] [CrossRef]
- Wang, R.; Starkey, M.; Hazan, R.; Rahme, L.G. Honey’s ability to counter bacterial infections arises from both bactericidal compounds and QS inhibition. Front. Microbiol. 2012, 3, 144. [Google Scholar] [CrossRef]
- Anand, S.; Deighton, M.; Livanos, G.; Morrison, P.D.; Pang, E.C.K.; Mantri, N. Antimicrobial activity of agastache honey and characterization of its bioactive compounds in comparison with important commercial honeys. Front. Microbiol. 2019, 25, 263. [Google Scholar] [CrossRef]
- Abuharfeil, N.; Al-Oran, R.; Abo-Shehada, M. The Effect of bee honey on the proliferative activity of human B-and T-lymphocytes and the activity of phagocytes. Food Agric. Immunol. 1999, 11, 169–177. [Google Scholar] [CrossRef]
- Eyarefe, D.O.; Kuforiji, D.I.; Jarikre, T.A.; Emikpe, B.O. Enhanced electroscalpel incisional wound healing potential of honey in wistar rat. Int. J. Vet. Sci. Med. 2017, 13, 128–134. [Google Scholar] [CrossRef]
- Obia, O.; Chuemere, A.N.; Chike, C.P.R.; Nyeche, S. Effect of supplementation of natural honey on serum albumin and total protein of alloxan induced diabetic wistar rats. Am. J. Phytomed. Clin. Ther. 2017, 5, 21. [Google Scholar] [CrossRef]
- Draiaia, R.; Chefrour, A.; Dainese, N.; Borin, A.; Manziello, C.; Gallina, A.; Mutinelli, F. Physicochemical parameters and antibiotics residuals in Algerian honey. Afr. J. Biotechnol. 2015, 14, 1242–1251. [Google Scholar] [CrossRef]
- El-Sohaimy, S.; Masry, S.H.D.; Shehata, M.G. Physicochemical characteristics of honey from different origins. Ann. Agric. Sci. 2015, 60, 279–287. [Google Scholar] [CrossRef]
- Aljohar, H.I.; Maher, H.M.; Albaqami, J.; Al-Mehaizie, M.; Orfali, R.; Orfali, R.; Alrubia, S. Physical and chemical screening of honey samples available in the Saudi market: An important aspect in the authentication process and quality assessment. Saudi Pharm. J. 2018, 26, 932–942. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.; Fresno, J.M.; Estevinho, M.M.; Sousa-Pimenta, M.; Tornadijo, M.E.; Estevinho, L.M. Honey: Another alternative in the fight against antibiotic-resistant bacteria? Antibiotics 2020, 9, 774. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernandez, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Lamas, L.B.; Flórez, S.M.; Toyos, P.A.; et al. Phenolic compounds in honey and their associated health benefits: A review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef]
- Khan, S.U.; Anjum, S.I.; Rahman, K.; Ansari, M.J.; Khan, W.U.; Kamal, S.; Khattak, B.; Muhammad, A.; Khan, H.U. Honey: Single food stuff comprises many drugs. Saudi J. Biol. Sci. 2018, 25, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Ranzato, E. Honey, wound repair and regenerative medicine. J. Funct. Biomater. 2018, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Albaridi, N.A. Antibacterial potency of honey. Int. J. Microbiol. 2019, 2019, 2464507. [Google Scholar] [CrossRef] [PubMed]
- Almasaudi, S.B.; Al-Nahari, A.A.M.; Abd El-Ghany, E.S.M.; Barbour, E.; Al Muhayawi, S.M.; Al-Jaouni, S.; Azhar, E.; Qari, M.; Qari, Y.A.; Harakeh, S. Antimicrobial effect of different types of honey on Staphylococcus aureus. Saudi J. Biol. Sci. 2017, 24, 1255–1261. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.H.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Wahdan, H.A. Causes of the antimicrobial activity of honey. Infection 1998, 26, 26–31. [Google Scholar] [CrossRef]
- Chong, K.P.; Rossall, S.; Atong, M. In vitro antimicrobial activity and fungitoxicity of syringic acid, caffeic acid and 4-hydroxybenzoic acid against Ganoderma boninense. J. Agric. Sci. 2009, 1, 15. [Google Scholar] [CrossRef]
- Stojković, D.; Petrović, J.; Soković, M.; Glamočlija, J.; Kukić-Marković, J.; Petrović, S. In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J. Sci. Food Agric. 2019, 93, 3205–3208. [Google Scholar] [CrossRef]
- Chalabaev, S.; Turlin, E.; Bay, S.; Ganneau, C.; Brito-Fravallo, E.; Charles, J.-F.; Danchin, A.; Biville, F. Cinnamic acid, an autoinducer of its own biosynthesis, is processed via Hca enzymes in Photorhabdus luminescens. Appl. Environ. Microbiol. 2008, 74, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, H.; Muhammad, S. The use of Lawsonia inermis Linn. (Henna) in the management of burn wound infections. Afr. J. Biotechnol. 2011, 4, 934–937. [Google Scholar]
- Anyanwu, C. Investigation of in vitro antifungal activity of honey. J. Med. Plant Res. 2012, 6, 3512–3516. [Google Scholar] [CrossRef]
- Koc, A.N.; Silici, S.; Ercal, B.D.; Kasap, F.; Hörmet-Oz, H.T.; Mavus-Buldu, H. Antifungal activity of Turkish honey against Candida spp. and Trichosporon spp: An in vitro evaluation. Med. Mycol. 2009, 47, 707–712. [Google Scholar] [CrossRef]
- Bucekova, M.; Buriova, M.; Pekarik, L.; Majtan, V.; Majtan, J. Phytochemicals-mediated production of hydrogen peroxide is crucial for high antibacterial activity of honeydew honey. Sci. Rep. 2018, 8, 9061. [Google Scholar] [CrossRef] [PubMed]
- Osés, S.; Pascual Maté, A.; Fuente, D.; Pablo, A.; Muiño, M.A.; Sancho, M. Comparison of methods to determine antibacterial activity of honeys against Staphylococcus aureus. NJAS-Wagening. J. Life Sci. 2016, 78, 29–33. [Google Scholar] [CrossRef]
- Sagdic, O.; Silici, S.; Ekici, L. Evaluation of the phenolic content, antiradical, antioxidant and antimicrobial activity of different floral sources of honey. Int. J. Food Prop. 2012, 16, 658–666. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Giamperi, F.; Brenciani, A.; Mazzoni, L.; Gasparrini, M.; González-Paramás, A.M.; Santos-Buelga, C.; Morroni, G.; Simoni, S.; Forbes-Hernández, T.Y.; et al. Apis mellifera vs Melipona beecheii Cuban polifloral honeys: A comparison based on their physicochemical parameters, chemical composition and biological properties. LWT Food Sci. Technol. 2018, 87, 272–279. [Google Scholar] [CrossRef]
- Grecka, K.; Kuś, P.M.; Worobo, R.W.; Szweda, P. Study of the anti-ataphylococcal potential of honeys produced in Northern Poland. Molecules 2018, 23, 260. [Google Scholar] [CrossRef]
- Moussa, A.; Noureddine, D.; Saad, A.; Abdelmelek, M.; Abdelkader, B. Antifungal activity of four honeys of different types from Algeria against pathogenic yeast: Candida albicans and Rhodotorula sp. Asian Pac. J. Trop. Biomed. 2012, 2, 554–557. [Google Scholar] [CrossRef]
- Irish, J.; Carter, D.A.; Shokohi, T.; Blair, S. Honey has an antifungal effect against Candida species. Med. Mycol. 2006, 44, 289–291. [Google Scholar] [CrossRef]
- Mizzi, L.; Maniscalco, D.; Gaspari, S.; Chatzitzika, C.; Gatt, R.; Valdramidis, V.P. Assessing the individual microbial inhibitory capacity of different sugars against pathogens commonly found in food systems. Lett. Appl. Microbiol. 2020, 71, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Proaño, A.; Coello, D.; Villacrés-Granda, I.; Ballesteros, I.; Debut, A.; Vizuete, K.; Brenciani, A.; Álvarez-Suarez, J.M. The osmotic action of sugar combined with hydrogen peroxide and bee-derived antibacterial peptide Defensin-1 is crucial for the antibiofilm activity of eucalyptus honey. LWT. 2021, 136, 110379. [Google Scholar] [CrossRef]
- International Honey Commission (IHC). Harmonized Methods of the International Honey Commission. 2009. Available online: https://www.ihc-platform.net/ihcmethods2009.pdf (accessed on 1 July 2022).
- Doner, L.W. The sugars of honey—A review. J. Sci. Food Agric. 1978, 28, 443–456. [Google Scholar] [CrossRef]
- Pascual-Mate, A.; Osés, S.M.; Marcazzan, G.L.; Gardini, S.; Fernández Muiño, M.A.; Sancho, T. Sugar composition and sugar-related parameters of honeys from the northern Iberian Plateau. J. Food Compos. Anal. 2018, 74, 34–43. [Google Scholar] [CrossRef]
- Szczesna, T.; Rybak-Chmielewska, H.; Waś, E.; Kachaniuk, K.; Teper, D. Characteristics of Polish unifloral honeys. I. Rape honey (Brassica napus L. var. Oleifera metzger). J. Apic. Sci. 2011, 55, 111–119. [Google Scholar]
- Münstedt, K.; Böhme, M.; Hauenschild, A.; Hrgovic, I. Consumption of rapeseed honey leads to higher serum fructose levels compared with analogue glucose/fructose solutions. Eur. J. Clin. Nutr. 2011, 65, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M. Bee Products—Chemical and Biological Properties; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Wild, P.; Gabrieli, A.; Schraner, E.M.; Pellegrini, A.; Thomas, U.; Frederik, P.; Stuart, M.; Von Fellenberg, R. Reevaluation of the effect of lysoyzme on Escherichia coli employing ultrarapid freezing followed by cryoelectronmicroscopy or freeze substitution. Microsc. Res. Tech. 1997, 1, 297–304. [Google Scholar] [CrossRef]
- Pellegrini, A.; Thomas, U.; Wild, P.; Schraner, E.; von Fellenberg, R. Effect of lysozyme or modified lysozyme fragments on DNA and RNA synthesis and membrane permeability of Escherichia coli. Microbiol. Res. 2000, 155, 69–77. [Google Scholar] [CrossRef]
- Song, W.; Zhang, M.; Huang, X.; Chen, B.; Ding, Y.; Zhang, Y.; Yu, D.G.; Kim, I. Smart l-borneol-loaded hierarchical hollow polymer nanospheres with antipollution and antibacterial capabilities. Mater. Today Chem. 2022, 26, 101252. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Xu, Y.; Shi, X.; Zhang, M.; Huang, Y.; Liang, Y.; Chen, Y.; Ji, W.; Kim, J.R.; et al. Engineering of hollow polymeric nano-sphere-supported imidazolium-based ionic liquids with enhanced antimicrobial activities. Nano Res. 2022, 15, 5556–5568. [Google Scholar] [CrossRef]
- Council Directive 2001/110/EC of 20 December 2001 Relating to Honey. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32001L0110 (accessed on 1 July 2022).
- Kühn, I.; Durka, W.; Klotz, S. BiolFlor—A new plant-trait database as a tool for plant invasion ecology. Divers. Distrib. 2004, 10, 363–365. [Google Scholar] [CrossRef]
- Frasco, D. Analysis of Honey Color and HMF Content Using a Genesys UV-Visible Spectrophotometer; Thermo Fisher Scientific: Madison, WI, USA, 2018; Available online: https://assets.thermofisher.com/TFS-Assets/MSD/Application-Notes/honey-color-hmf-content-analysis-using-genesys-uv-visible-spectrophotometer-AN53025.pdf (accessed on 1 July 2022).
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 1999, 299, 265–275. [Google Scholar] [CrossRef]
- Kek, S.P.; Chin, N.L.; Yusof, Y.A.; Tan, S.W.; Chua, L.S. Total phenolic contents and colour intensity of Malaysian honeys from the Apis spp. and Trigona spp. Bees. Agric. Agric. Sci. Proc. 2014, 2, 150–155. [Google Scholar] [CrossRef]
- Bradford, M.M. Rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Michałkiewicz, A.; Biesaga, M.; Pyrzynska, K. Solid-phase extraction procedure for determination of phenolic acids and some flavonols in honey. J. Chromatogr. A 2008, 11, 18–24. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Smilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination; Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using CANOCO 5, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar] [CrossRef]
- Baroni, M.V.; Chiabrando, G.A.; Costa, C.; Wunderlin, D.A. Assessment of the floral origin of honey by SDS-page immunoblot techniques. J. Agric. Food Chem. 2002, 50, 1362–1367. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
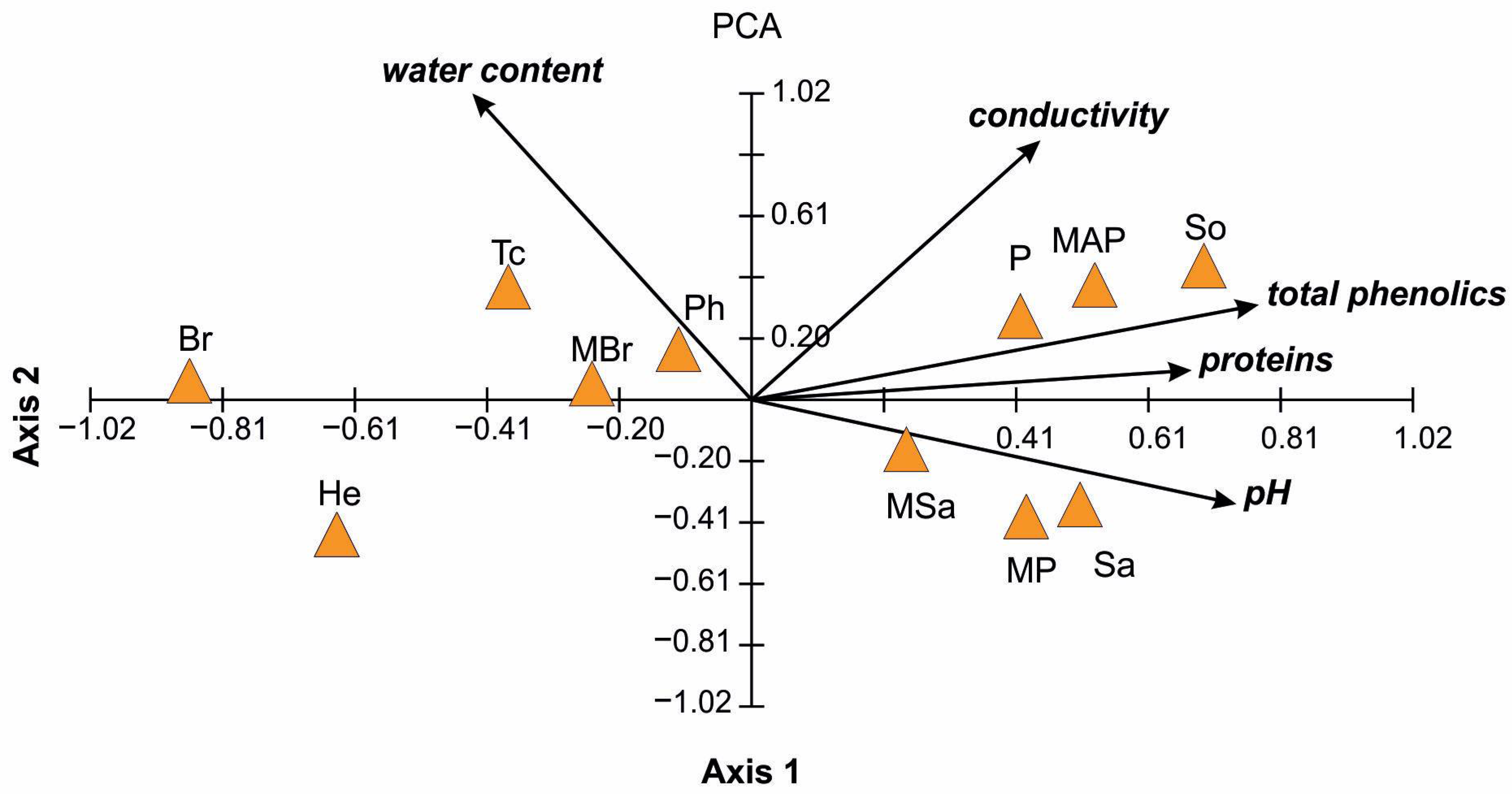

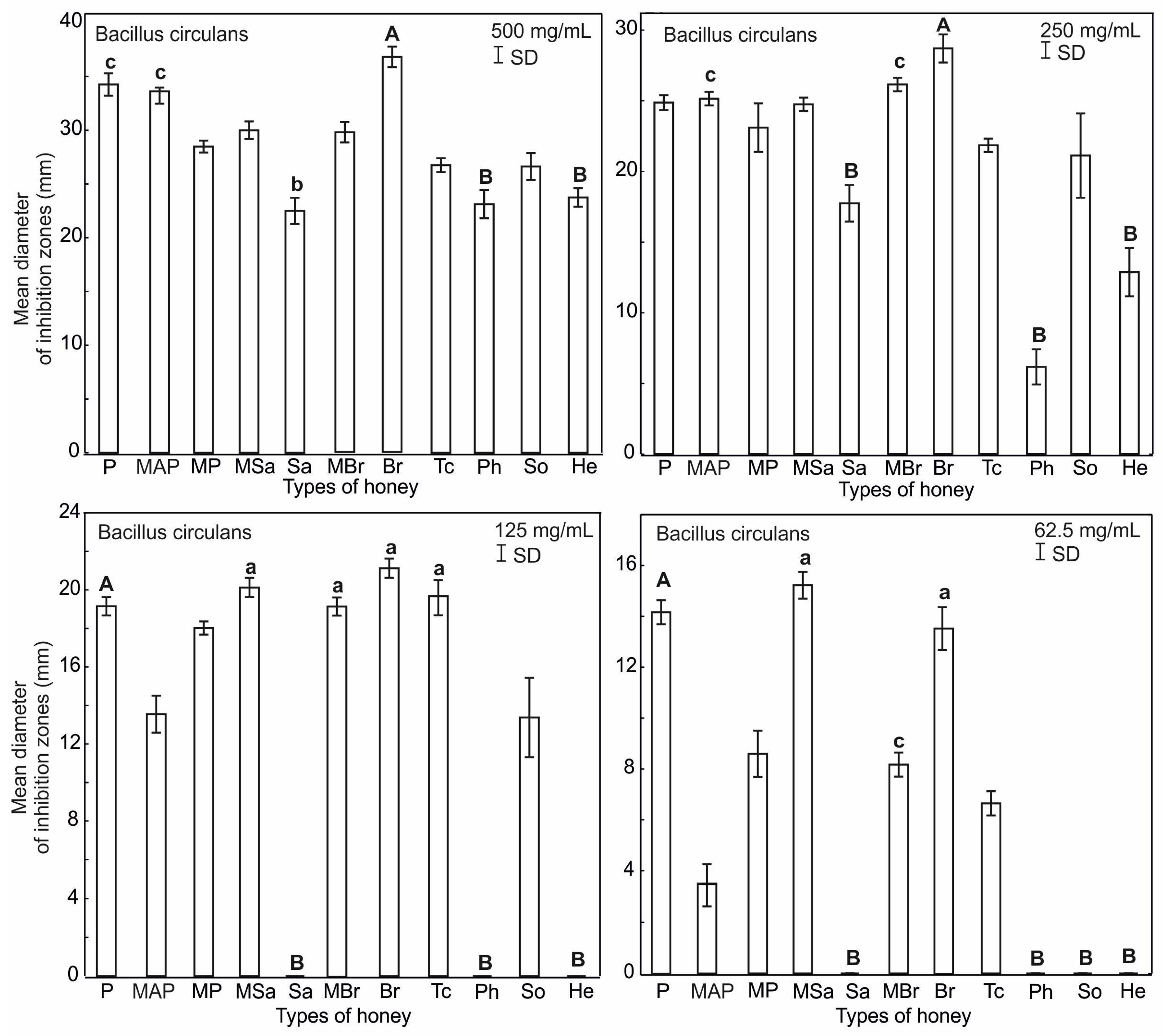
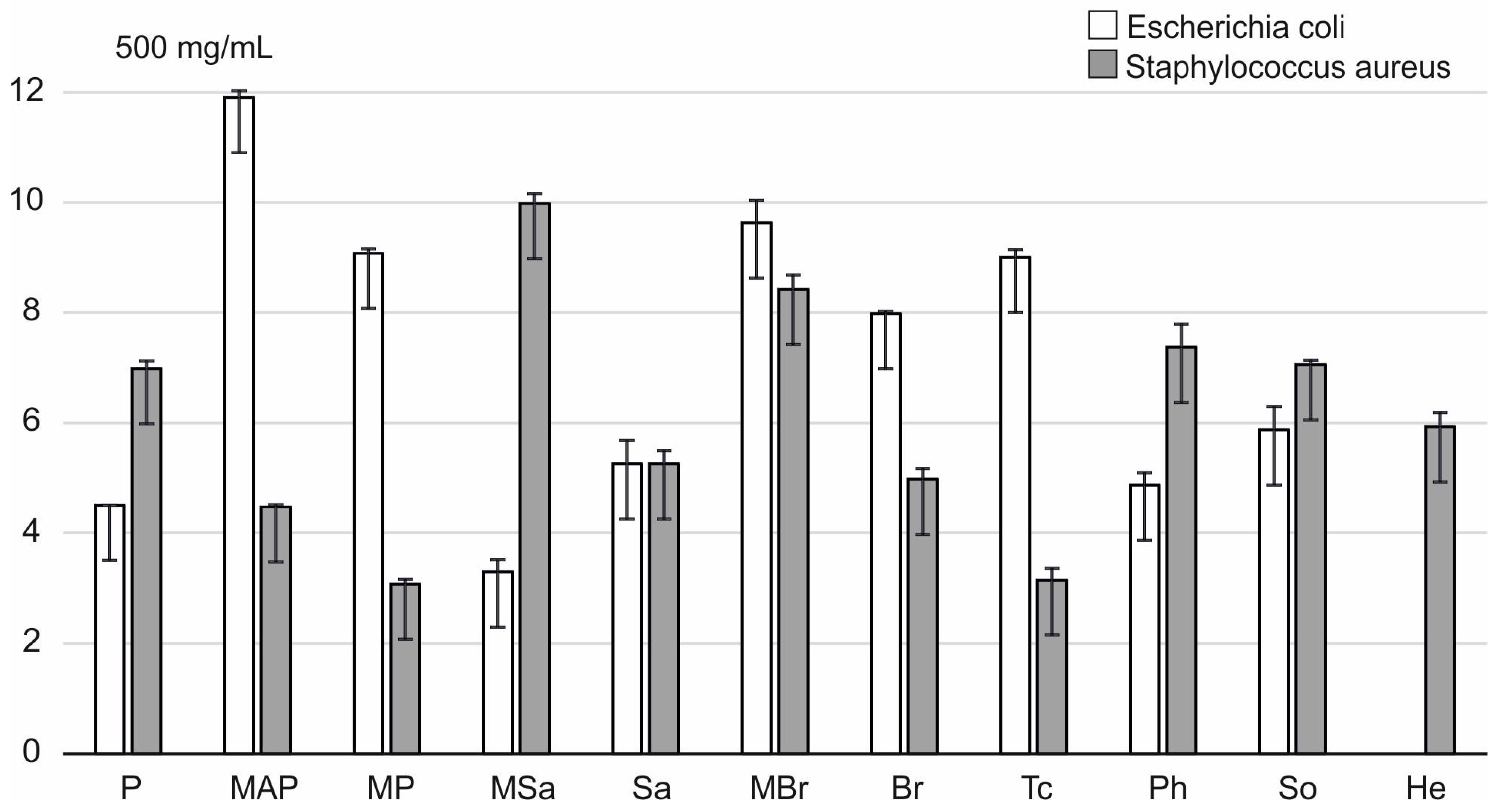
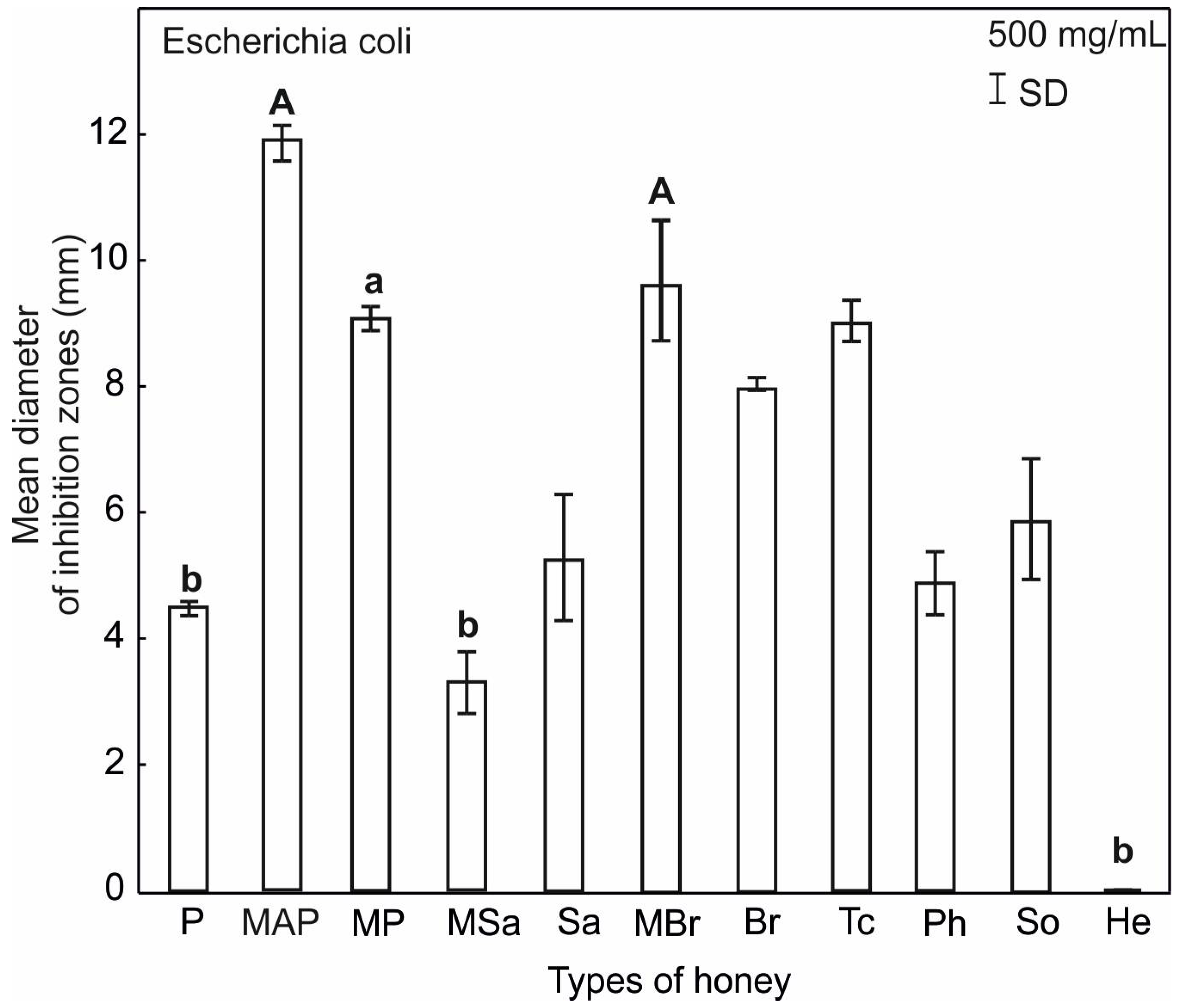

| Honey Type | Water Content (%) | pH | Electrical Conductivity (mS/cm) | Total Phenol (μg GAEs/g) | Proteins (mg/mL) |
|---|---|---|---|---|---|
| P | 16.33 ± 0.47 | 4.43 ± 0.005 | 0.29 ± 0.008 | 670.20 ± 18.96 | 112.40 ± 2.47 |
| MAP | 18.00 ± 0.00 | 4.62 ± 0.012 | 0.35 ± 0.009 | 776.54 ± 9.12 | 116.80 ± 0.57 |
| MP | 14.67 ± 0.47 | 4.79 ± 0.009 | 0.29 ± 0.005 | 700.47 ± 18.18 | 103.60 ± 0.57 |
| MSa | 15.67 ± 0.47 | 4.68 ± 0.017 | 0.46 ± 0.014 | 606.54 ± 27.66 | 96.80 ± 2.27 |
| Sa | 15.00 ± 0.00 | 4.96 ± 0.029 | 0.29 ± 0.005 | 667.14 ± 4.79 | 107.60 ±0.57 |
| MBr | 18.00 ± 0.00 | 4.67 ± 0.014 | 0.36 ± 0.012 | 404.74 ± 9.12 | 95.20 ± 2.27 |
| Br | 17.67 ± 0.47 | 4.22 ± 0.012 | 0.27 ± 0.005 | 378.27 ± 7.30 | 49.20 ± 3.40 |
| Tc | 18.00 ± 0.00 | 4.08 ± 0.017 | 0.42 ± 0.012 | 624.40 ± 15.43 | 85.60 ± 3.00 |
| Ph | 17.67 ± 0.47 | 4.62 ± 0.005 | 0.4 ± 0.005 | 524.40 ± 18.58 | 90.80 ± 0.57 |
| So | 16.33 ± 0.47 | 4.85 ± 0.009 | 0.91 ± 0.008 | 808.05 ± 7.20 | 85.20 ± 0.00 |
| He | 15.67 ± 0.47 | 4.35 ± 0.012 | 0.25 ± 0.005 | 431.27 ± 5.45 | 41.20 ± 2.47 |
| Honey Type | Sugars Content (g/100 g), Average ± SD, N = 3 | Fructose/ Glucose (Ratio) | |||||
|---|---|---|---|---|---|---|---|
| Glucose | Fructose | Sucrose | Rhamnose | Erlose | Fucose | ||
| P | 38.80 ± 0.42 | 39.28 ± 0.12 | 3.26 ± 0.07 | 1.18 ± 0.04 | 3.02 ± 0.10 | 0.00 ± 0.00 | 1.01 |
| MAP | 44.77 ± 0.10 | 43.57 ± 0.28 | 2.42 ± 0.08 | 1.79 ± 0.07 | 0.66 ± 0.02 | 0.23 ± 0.01 | 0.97 |
| MP | 38.80 ± 0.17 | 38.71 ± 0.23 | 2.79 ± 0.08 | 1.24 ± 0.07 | 2.00 ± 0.07 | 0.00 ± 0.00 | 1.00 |
| MSa | 40.75 ± 0.21 | 42.74 ± 0.39 | 3.08 ± 0.09 | 1.58 ± 0.04 | 1.74 ± 0.10 | 0.00 ± 0.00 | 1.05 |
| Sa | 37.51 ± 0.18 | 39.24 ± 0.15 | 3.46 ± 0.07 | 1.71 ± 0.03 | 2.92 ± 0.10 | 0.00 ± 0.00 | 1.05 |
| MBr | 39.56 ± 0.17 | 42.1 ± 0.58 | 2.45 ± 0.07 | 1.01 ± 0.08 | 1.57 ± 0.06 | 0.00 ± 0.00 | 1.06 |
| Br | 39.05 ± 0.35 | 38.53 ± 0.47 | 4.96 ± 0.08 | 1.46 ± 0.05 | 8.26 ± 0.31 | 0.00 ± 0.00 | 0.99 |
| Tc | 45.10 ± 0.27 | 38.23 ± 0.28 | 1.31 ± 0.07 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.85 |
| Ph | 52.53 ± 0.46 | 37.17 ± 0.34 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.71 |
| So | 39.51 ± 0.35 | 40.36 ± 0.47 | 2.89 ± 0.02 | 1.20 ± 0.05 | 1.77 ± 0.13 | 0.00 ± 0.00 | 1.02 |
| He | 37.27 ± 0.29 | 42.61 ± 0.20 | 2.91 ± 0.08 | 1.55 ± 0.02 | 0.69 ± 0.02 | 0.35 ± 0.02 | 1.14 |
| Honey Sample | Lysozyme-like Activity Expressed as µg/mL EWL |
|---|---|
| (1) after centrifugation | 2.3 ± 0.47 |
| (2) after dialysis | 0.71 ± 0.11 |
| (3) after lyophilization | 1.5 ± 0.26 |
| Honey Type | Phenolic Acids (μg/g) | |||
|---|---|---|---|---|
| Coumaric | Caffeic | Cinnamic | Syringic | |
| P | 8.2 | 7.2 | 0.15 | 3.96 |
| MAP | – | 210.78 | – | 1.6 |
| MP | – | 221.6 | 0.15 | 1.99 |
| MSa | – | 318.9 | 0.15 | 2.6 |
| Sa | 5.0 | 0.36 | 0.33 | 1.1 |
| MBr | – | 318.9 | 0.3 | 2.7 |
| Br | 4.4 | 3.0 | 1.6 | 4.7 |
| Tc | – | 286.45 | 0.3 | 2.6 |
| Ph | – | 356.72 | – | 3.2 |
| So | 4.4 | 10.8 | 0.15 | 0.99 |
| He | 0.65 | 0.7 | – | 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunat-Budzyńska, M.; Rysiak, A.; Wiater, A.; Grąz, M.; Andrejko, M.; Budzyński, M.; Bryś, M.S.; Sudziński, M.; Tomczyk, M.; Gancarz, M.; et al. Chemical Composition and Antimicrobial Activity of New Honey Varietals. Int. J. Environ. Res. Public Health 2023, 20, 2458. https://doi.org/10.3390/ijerph20032458
Kunat-Budzyńska M, Rysiak A, Wiater A, Grąz M, Andrejko M, Budzyński M, Bryś MS, Sudziński M, Tomczyk M, Gancarz M, et al. Chemical Composition and Antimicrobial Activity of New Honey Varietals. International Journal of Environmental Research and Public Health. 2023; 20(3):2458. https://doi.org/10.3390/ijerph20032458
Chicago/Turabian StyleKunat-Budzyńska, Magdalena, Anna Rysiak, Adrian Wiater, Marcin Grąz, Mariola Andrejko, Michał Budzyński, Maciej S. Bryś, Marcin Sudziński, Michał Tomczyk, Marek Gancarz, and et al. 2023. "Chemical Composition and Antimicrobial Activity of New Honey Varietals" International Journal of Environmental Research and Public Health 20, no. 3: 2458. https://doi.org/10.3390/ijerph20032458
APA StyleKunat-Budzyńska, M., Rysiak, A., Wiater, A., Grąz, M., Andrejko, M., Budzyński, M., Bryś, M. S., Sudziński, M., Tomczyk, M., Gancarz, M., Rusinek, R., & Ptaszyńska, A. A. (2023). Chemical Composition and Antimicrobial Activity of New Honey Varietals. International Journal of Environmental Research and Public Health, 20(3), 2458. https://doi.org/10.3390/ijerph20032458











