Early Origins of Chronic Obstructive Pulmonary Disease: Prenatal and Early Life Risk Factors
Abstract
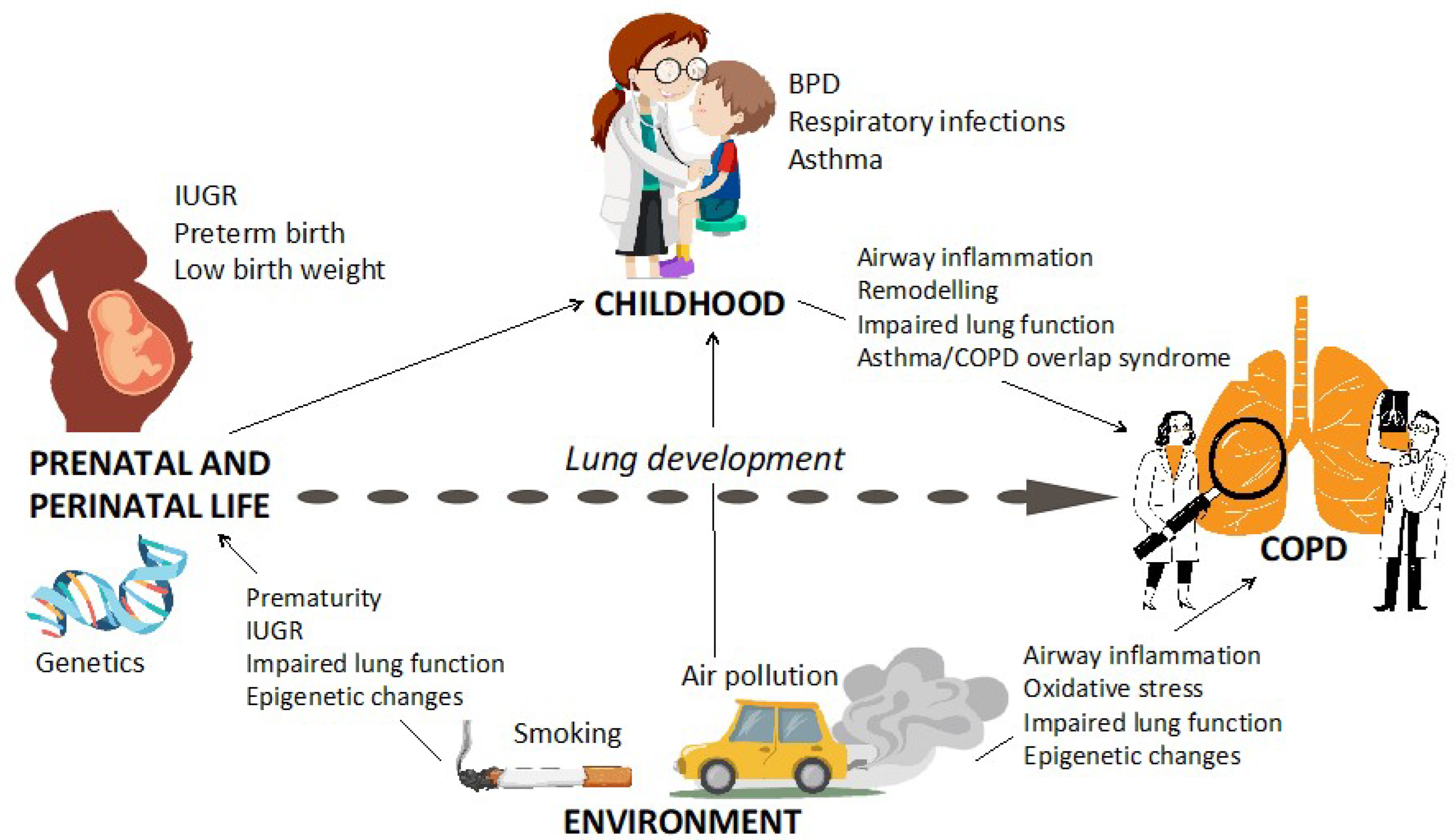
1. Introduction
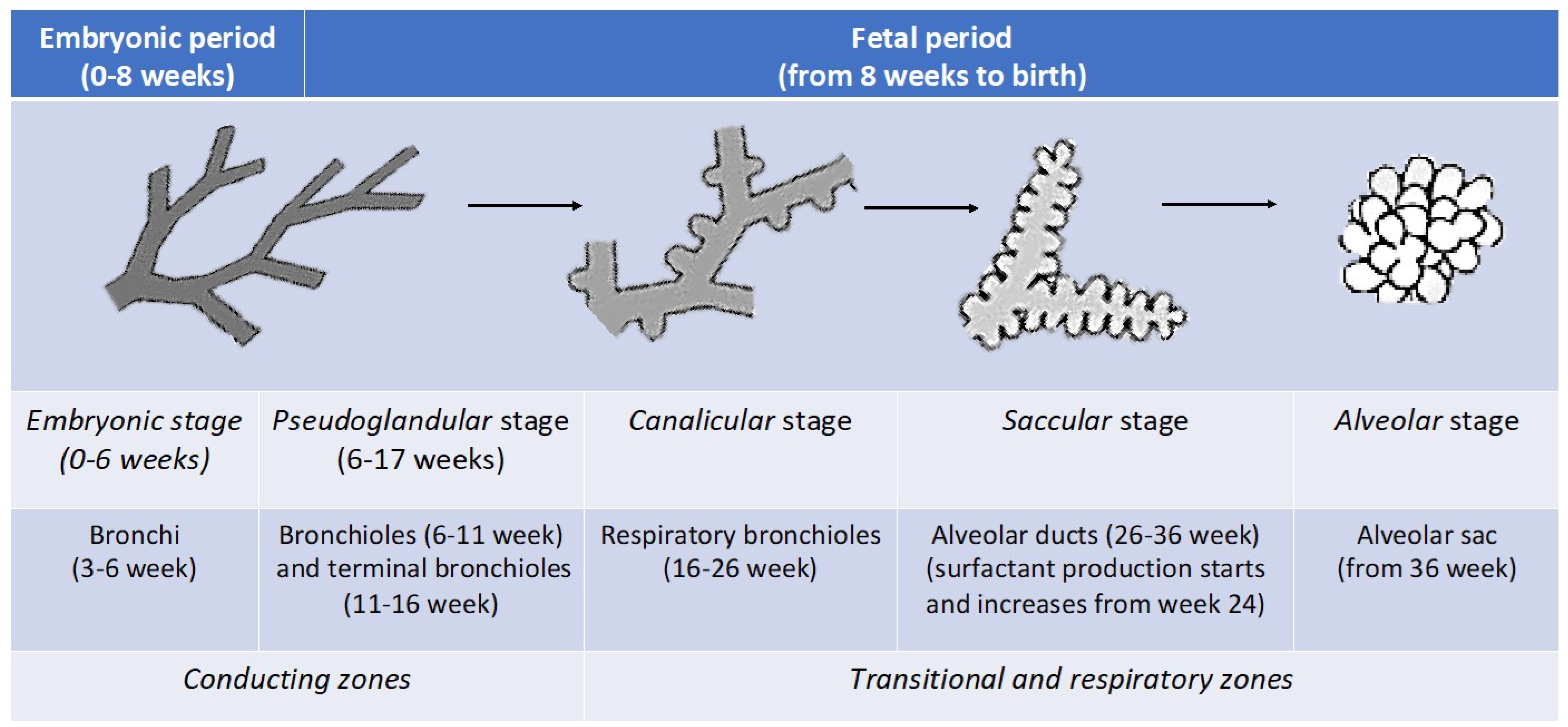
1.1. Behind the Scenes: The Development of the Respiratory System
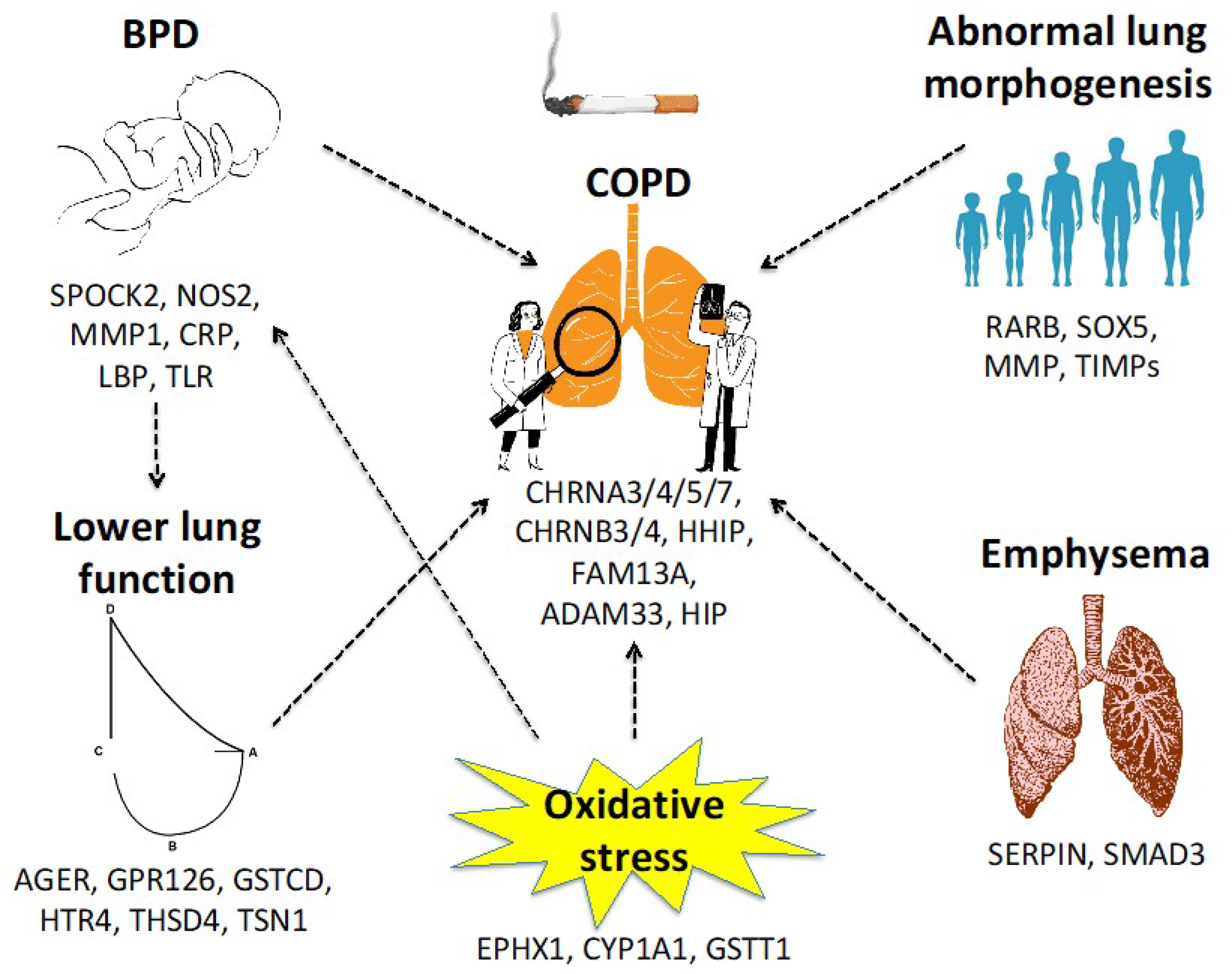
1.2. How Genes Influence the Respiratory System and Lung Function through Life
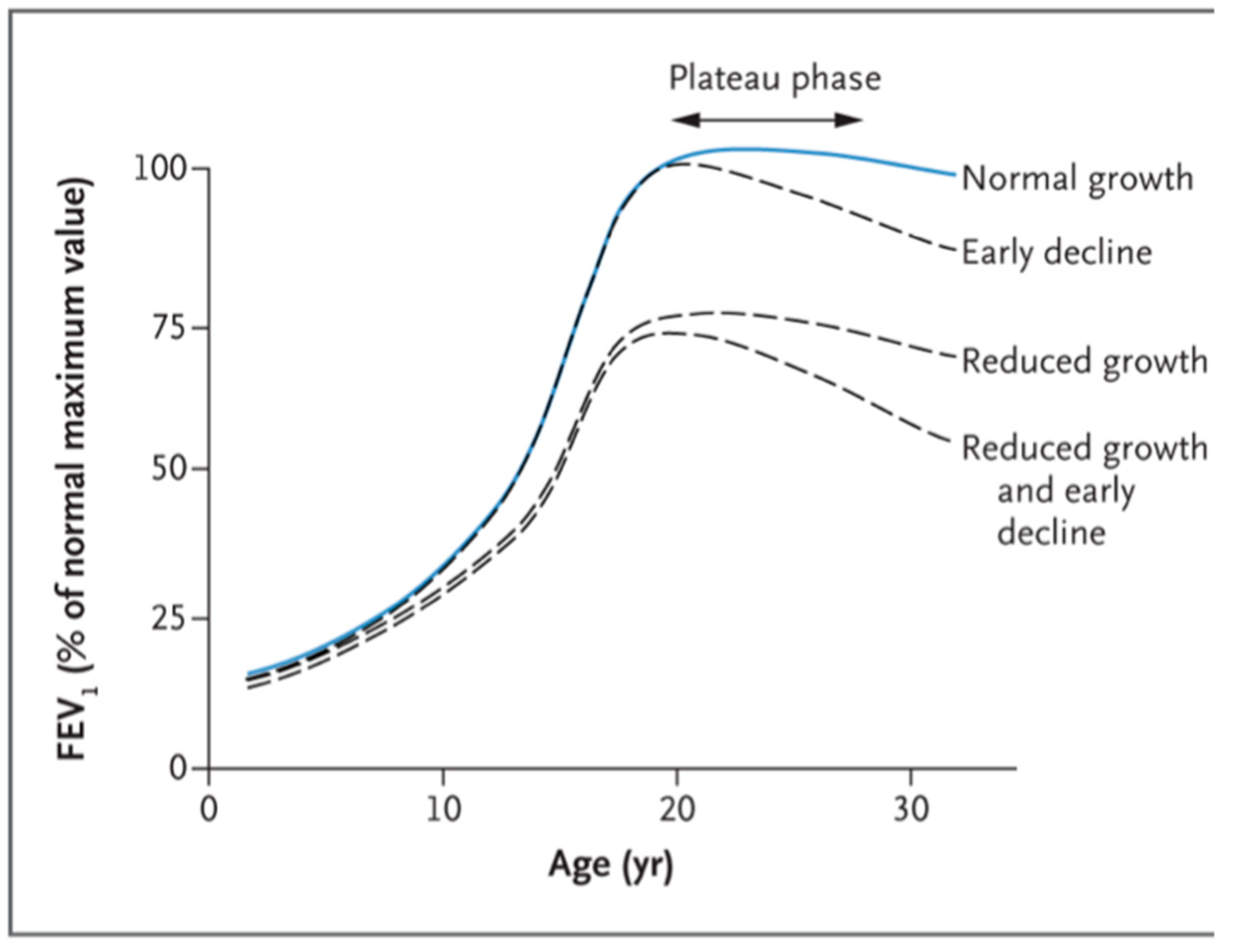
1.3. Lung Function Trajectory and Spirometric Patterns
1.3.1. Stage 1, Pre-Birth Period
1.3.2. Stage 2, from Birth to Young Adult Age
1.3.3. Stage 3, Adult Life
1.4. Dysanapsis
The “Window of Susceptibility” That Increases the Risk of COPD
1.5. Intrauterine Growth Restriction (IUGR), Preterm Birth, and Low BirthWeight
1.6. Bronchopulmonary Dysplasia
2. Environmental Exposures
2.1. Tobacco: In Utero and Early Life Exposure
2.2. Air Pollution
3. Respiratory Infections in Early Life
4. Asthma in Childhood
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COPD | chronic obstructive pulmonary disease |
| FEV1 | forced expiratory volume in 1 s |
| FVC | forced vital capacity |
| FEV1/FVC | ratio (Tiffenau index) |
| RARB | retinoic acid receptor-beta |
| MMP | matrix metalloproteinase |
| TIMPs | tissue inhibitors of MMPs |
| AAT | alpha-1 antitrypsin |
| CI | confidence interval |
| GWAS | genome-wide association studies |
| HIP | Hedgehog-interacting protein |
| VEGF | vascular endothelial growth factor |
| BPD | bronchopulmonary dyspalsia |
| CNVs | copy-number variants |
| WNT | wingless/integrated pathway |
| GLI | Global Lung Initiative |
| IUGR | Intrauterine growth restriction |
| SGA | small for gestational age |
| FEF25–75 | forced mid expiratory flow between 25 and 75% of vital capacity |
| WG | weeks of gestation |
| VmaxFRC | maximal expiratory flow at functional residual capacity |
| NO: | nitrogen oxides |
| SO2 | sulfur dioxide |
| CO | carbon monoxide |
| VOC | volatile organic compounds |
| PM | particulate matter |
| TRAP | Air pollution associated with traffic |
| OR | odds ratio |
| aOR | adjusted odds ratio |
References
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease [Updated 2020]. Available online: https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf (accessed on 1 November 2022).
- Forum of International Respiratory Societies. The Global Impact of Respiratory Disease. Third Edition. European Respiratory Society, 2021. Available online: Firsnet.org/images/publications/FIRS_Master_09202021.pdf (accessed on 1 November 2022).
- Lamprecht, B.; McBurnie, M.A.; Vollmer, W.M.; Gudmundsson, G.; Welte, T.; Nizankowska-Mogilnicka, E.; Studnicka, M.; Bateman, E.; Anto, J.M.; Burney, P.; et al. COPD in never smokers: Results from the population-based burden of obstructive lung disease study. Chest 2011, 139, 752–763. [Google Scholar] [CrossRef]
- Moreno Villares, J.M.; Collado, M.C.; Larqué, E.; Leis Trabazo, R.; Saenz De Pipaón, M.; Moreno Aznar, L.A. The first 1000 days: An opportunity to reduce the burden of noncommunicable diseases. Nutr. Hosp. 2019, 36, 218–232. [Google Scholar]
- Matonti, L.; Blasetti, A.; Chiarelli, F. Nutrition and growth in children. Minerva Pediatr. 2020, 72, 462–471. [Google Scholar] [CrossRef]
- Duan, P.; Wang, Y.; Lin, R.; Zeng, Y.; Chen, C.; Yang, L.; Yue, M.; Zhong, S.; Wang, Y.; Zhang, Q. Impact of early life exposures on COPD in adulthood: A systematic review and meta-analysis. Respirology 2021, 26, 1131–1151. [Google Scholar] [CrossRef]
- Okyere, D.O.; Bui, D.S.; Washko, G.R.; Lodge, C.J.; Lowe, A.J.; Cassim, R.; Perret, J.L.; Abramson, M.J.; Walters, E.H.; Waidyatillake, N.T.; et al. Predictors of lung function trajectories in population-based studies: A systematic review. Respirology 2021, 26, 938–959. [Google Scholar] [CrossRef]
- Calogero, C.; Sly, P.D. Developmental physiology: Lung function during growth and development from birth to old age. In Paediatric Lung Function; European Respiratory Society: Shieffield, UK, 2010; pp. 1–15. [Google Scholar]
- Narayanan, M.; Owers-Bradley, J.; Beardsmore, C.S.; Mada, M.; Ball, I.; Garipov, R.; Panesar, K.S.; Kuehni, C.E.; Spycher, B.D.; Williams, S.E.; et al. Alveolarization continues during childhood and adolescence: New evidence from helium-3 magnetic resonance. Care Med. 2012, 185, 186–191. [Google Scholar] [CrossRef]
- Klimentidis, Y.C.; Vazquez, A.I.; de Los Campos, G.; Allison, D.B.; Dransfield, M.T.; Thannickal, V.J. Heritability of pulmonary function estimated from pedigree and whole-genome markers. Front. Genet. 2013, 4, 174. [Google Scholar] [CrossRef]
- Huang, X.; Mu, X.; Deng, L.; Fu, A.; Pu, E.; Tang, T.; Kong, X. The etiologic origins for chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 1139–1158. [Google Scholar] [CrossRef] [PubMed]
- Postma, D.S.; Bush, A.; van den Berge, M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet 2015, 385, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Janciauskiene, S.M.; Bals, R.; Koczulla, R.; Vogelmeier, C.; Köhnlein, T.; Welte, T. The discovery of α1-antitrypsin and its role in health and disease. Respir. Med. 2011, 105, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Torres-Durán, M.; Lopez-Campos, J.L.; Barrecheguren, M.; Miravitlles, M.; Martinez-Delgado, B.; Castillo, S.; Escribano, A.; Baloira, A.; Navarro-Garcia, M.M.; Pellicer, D.; et al. Alpha-1 antitrypsin deficiency: Outstanding questions and future directions. Orphanet J. Rare Dis. 2018, 13, 114. [Google Scholar] [CrossRef]
- Pini, L.; Tiberio, L.; Arici, M.; Corda, L.; Giordani, J.; Bargagli, E.; Tantucci, C. Z-alpha1-antitrypsin polymers and small airways disease: A new paradigm in alfa-1 anti-trypsin deficiency-related COPD development? Monaldi Arch. Chest Dis. 2021, 91. [Google Scholar] [CrossRef]
- Menga, G.; Fernandez Acquier, M.; Echazarreta, A.L.; Sorroche, P.B.; Lorenzon, M.V.; Fernández, M.E.; Saez, M.S.; grupo de estudio DAAT.AR. Prevalence of Alpha-1 Antitrypsin Deficiency in COPD Patients in Argentina. The DAAT.AR Study. Arch. Bronconeumol. 2020, 56, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Matamala, N.; Lara, B.; Gómez-Mariano, G.; Martínez, S.; Vázquez-Domínguez, I.; Otero-Sobrino, Á.; Muñoz-Callejas, A.; Sánchez, E.; Esquinas, C.; Bustamante, A.; et al. miR-320c Regulates SERPINA1 Expression and Is Induced in Patients with Pulmonary Disease. Arch. Bronconeumol. 2021, 57, 457–463. [Google Scholar] [CrossRef]
- Hall, R.; Hall, I.P.; Sayers, I. Genetic risk factors for the development of pulmonary disease identified by genome-wide association. Respirology 2019, 24, 204–214. [Google Scholar] [CrossRef]
- Van Durme, Y.M.; Eijgelsheim, M.; Joos, G.F.; Hofman, A.; Uitterlinden, A.G.; Brusselle, G.G.; Stricker, B.H. Hedgehog-interacting protein is a COPD susceptibility gene: The Rotterdam Study. Eur. Respir. J. 2010, 36, 89–95. [Google Scholar] [CrossRef]
- Farkas, L.; Farkas, D.; Warburton, D.; Gauldie, J.; Shi, W.; Stampfli, M.R.; Voelkel, N.F.; Kolb, M. Cigarette smoke exposure aggravates air space enlargement and alveolar cell apoptosis in Smad3 knockout mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L391–L401. [Google Scholar] [CrossRef] [PubMed]
- Van Diemen, C.C.; Postma, D.S.; Vonk, J.M.; Bruinenberg, M.; Schouten, J.P.; Boezen, H.M. A disintegrin and metalloprotease 33 polymorphisms and lung function decline in the general population. Am. J. Respir. Crit. Care Med. 2005, 172, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, S.W.; Chang, H.K.; Kim, H.Y.; Rhim, T.; Lee, J.H.; Jang, A.S.; Koh, E.S.; Park, C.S. A disintegrin and metalloproteinase 33 protein in patients with asthma: Relevance to airflow limitation. Am. J. Respir. Crit. Care Med. 2006, 173, 729–735. [Google Scholar] [CrossRef]
- Ghosh, R.; Topinka, J.; Joad, J.P.; Dostal, M.; Sram, R.J.; Hertz-Picciotto, I. Air pollutants, genes and early childhood acute bronchitis. Mutat. Res. 2013, 749, 80–86. [Google Scholar] [CrossRef]
- Vibhuti, A.; Arif, E.; Mishra, A.; Deepak, D.; Singh, B.; Rahman, I.; Mohammad, G.; Pasha, M.A. CYP1A1, CYP1A2 and CYBA gene polymorphisms associated with oxidative stress in COPD. Clin. Chim. Acta 2010, 411, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Lodovici, M.; Luceri, C.; Guglielmi, F.; Bacci, C.; Akpan, V.; Fonnesu, M.L.; Boddi, V.; Dolara, P. Benzo(a)pyrene diolepoxide (BPDE)-DNA adduct levels in leukocytes of smokers in relation to polymorphism of CYP1A1, GSTM1, GSTP1, GSTT1, and mEH. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1342–1348. [Google Scholar] [CrossRef]
- Lakhdar, R.; Denden, S.; Knani, J.; Leban, N.; Daimi, H.; Hassine, M.; Lefranc, G.; Chibani, J.B.; Khelil, A.H. Combined analysis of EPHX1, GSTP1, GSTM1 and GSTT1 gene polymorphisms in relation to chronic obstructive pulmonary disease risk and lung function impairment. Dis. Mark. 2011, 30, 253–263. [Google Scholar] [CrossRef]
- Hadchouel, A.; Durrmeyer, X.; Bouzigon, E.; Incitti, R.; Huusko, J.; Jarreau, P.H.; Lenclen, R.; Demenais, F.; Franco-Montoya, M.L.; Layouni, I.; et al. Identification of SPOCK2 as a susceptibility gene for bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2011, 184, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Ambalavanan, N.; Cotten, C.M.; Page, G.P.; Carlo, W.A.; Murray, J.C.; Bhattacharya, S.; Mariani, T.J.; Cuna, A.C.; Faye-Petersen, O.M.; Kelly, D.; et al. Genomics and Cytokine Subcommittees of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Integrated genomic analyses in bronchopulmonary dysplasia. J. Pediatr. 2015, 166, 531–537.e13. [Google Scholar] [CrossRef]
- Wang, H.; St Julien, K.R.; Stevenson, D.K.; Hoffmann, T.J.; Witte, J.S.; Lazzeroni, L.C.; Krasnow, M.A.; Quaintance, C.C.; Oehlert, J.W.; Jelliffe-Pawlowski, L.L.; et al. A genome-wide association study (GWAS) for bronchopulmonary dysplasia. Pediatrics 2013, 132, 290–297. [Google Scholar] [CrossRef]
- Bhandari, V.; Bizzarro, M.J.; Shetty, A.; Zhong, X.; Page, G.P.; Zhang, H.; Ment, L.R.; Gruen, J.R.; Neonatal Genetics Study Group. Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics 2006, 117, 1901–1906. [Google Scholar] [CrossRef]
- Hoffmann, T.J.; Shaw, G.M.; Stevenson, D.K.; Wang, H.; Quaintance, C.C.; Oehlert, J.; Jelliffe-Pawlowski, L.L.; Gould, J.B.; Witte, J.S.; O’Brodovich, H.M. Copy number variation in bronchopulmonary dysplasia. Am. J. Med. Genet. A 2014, 164, 2672–2675. [Google Scholar] [CrossRef]
- Carrera, P.; Di Resta, C.; Volonteri, C.; Castiglioni, E.; Bonfiglio, S.; Lazarevic, D.; Cittaro, D.; Stupka, E.; Ferrari, M.; Somaschini, M.; et al. Exome sequencing and pathway analysis for identification of genetic variability relevant for bronchopulmonary dysplasia (BPD) in preterm newborns: A pilot study. Clin. Chim. Acta 2015, 451 Pt A, 39–45. [Google Scholar] [CrossRef]
- Li, J.; Yu, K.H.; Oehlert, J.; Jeliffe-Pawlowski, L.L.; Gould, J.B.; Stevenson, D.K.; Snyder, M.; Shaw, G.M.; O’Brodovich, H.M. Exome Sequencing of Neonatal Blood Spots and the Identification of Genes Implicated in Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2015, 192, 589–596. [Google Scholar] [CrossRef]
- Henckel, E.; Svenson, U.; Nordlund, B.; Berggren Broström, E.; Hedlin, G.; Degerman, S.; Bohlin, K. Telomere length was similar in school-age children with bronchopulmonary dysplasia and allergic asthma. Acta Paediatr. 2018, 107, 1395–1401. [Google Scholar] [CrossRef]
- Cuna, A.; Halloran, B.; Faye-Petersen, O.; Kelly, D.; Crossman, D.K.; Cui, X.; Pandit, K.; Kaminski, N.; Bhattacharya, S.; Ahmad, A.; et al. Alterations in gene expression and DNA methylation during murine and human lung alveolar septation. Am. J. Respir. Cell Mol. Biol. 2015, 53, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Van Marter, L.J.; Sun, Y.; Allred, E.; Leviton, A.; Kohane, I.S. Perturbation of gene expression of the chromatin remodeling pathway in premature newborns at risk for bronchopulmonary dysplasia. Genome Biol. 2007, 8, R210. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Schmidt, P.; Dodd, J.; Jeppesen, D.L. Bronchopulmonary dysplasia: A review. Arch. Gynecol. Obstet. 2013, 288, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.M.; Feng, R.; Bazacliu, C.; Ferkol, T.W.; Ren, C.L.; Mariani, T.J.; Poindexter, B.B.; Wang, F.; Moore, P.E.; Prematurity and Respiratory Outcome Program (PROP) Investigators. Black Race Is Associated with a Lower Risk of Bronchopulmonary Dysplasia. J. Pediatr. 2019, 207, 130–135.e2. [Google Scholar] [CrossRef]
- Wai, K.C.; Hibbs, A.M.; Steurer, M.A.; Black, D.M.; Asselin, J.M.; Eichenwald, E.C.; Ballard, P.L.; Ballard, R.A.; Keller, R.L.; Trial of Late Surfactant (TOLSURF) Study Group. Maternal Black Race and Persistent Wheezing Illness in Former Extremely Low Gestational Age Newborns: Secondary Analysis of a Randomized Trial. J. Pediatr. 2018, 198, 201–208.e3. [Google Scholar] [CrossRef] [PubMed]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.; Zheng, J.; et al. ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3-95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Mocelin, H.T.; Fischer, G.B.; Bush, A. Adverse early-life environmental exposures and their repercussions on adult respiratory health. J. Pediatr. 2022, 98 (Suppl. S1), S86–S95. [Google Scholar] [CrossRef]
- Bush, A. Health effects of passive smoking in children. In The Tobacco Epidemic, 2nd ed.; Progress in respiratory research; Loddenkemper, R., Kreuter, M., Eds.; Karger: Basel, Switzerland, 2015; Volume 42, pp. 97–109. [Google Scholar]
- Brauer, M.; Hoek, G.; Smit, H.A.; de Jongste, J.C.; Gerritsen, J.; Postma, D.S.; Kerkhof, M.; Brunekreef, B. Air pollution and development of asthma, allergy and infections in a birth cohort. Eur. Respir. J. 2007, 29, 879–888. [Google Scholar] [CrossRef]
- Jackson, D.J.; Gangnon, R.E.; Evans, M.D.; Roberg, K.A.; Anderson, E.L.; Pappas, T.E.; Printz, M.C.; Lee, W.M.; Shult, P.A.; Reisdorf, E.; et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am. J. Respir. Crit. Care Med. 2008, 178, 667–672. [Google Scholar] [CrossRef]
- Turner, S.W.; Young, S.; Landau, L.I.; Le Souëf, P.N. Reduced lung function both before bronchiolitis and at 11 years. Arch. Dis. Child. 2002, 87, 417–420. [Google Scholar] [CrossRef]
- Illi, S.; von Mutius, E.; Lau, S.; Niggemann, B.; Grüber, C.; Wahn, U.; Multicentre Allergy Study (MAS) group. Perennial allergen sensitisation early in life and chronic asthma in children: A birth cohort study. Lancet 2006, 368, 763–770. [Google Scholar] [CrossRef]
- Covar, R.A.; Spahn, J.D.; Murphy, J.R.; Szefler, S.J.; Childhood Asthma Management Program Research Group. Progression of asthma measured by lung function in the childhood asthma management program. Am. J. Respir. Crit. Care Med. 2004, 170, 234–241. [Google Scholar] [CrossRef]
- Lowe, L.A.; Simpson, A.; Woodcock, A.; Morris, J.; Murray, C.S.; Custovic, A.; NAC Manchester Asthma and Allergy Study Group. Wheeze phenotypes and lung function in preschool children. Am. J. Respir. Crit. Care Med. 2005, 171, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Morgan, W.J.; Stern, D.A.; Sherrill, D.L.; Guerra, S.; Holberg, C.J.; Guilbert, T.W.; Taussig, L.M.; Wright, A.L.; Martinez, F.D. Outcome of asthma and wheezing in the first 6 years of life: Follow-up through adolescence. Am. J. Respir. Crit. Care Med. 2005, 172, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Sears, M.R.; Greene, J.M.; Willan, A.R.; Wiecek, E.M.; Taylor, D.R.; Flannery, E.M.; Cowan, J.O.; Herbison, G.P.; Silva, P.A.; Poulton, R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N. Engl. J. Med. 2003, 349, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Oswald, H.; Phelan, P.D.; Lanigan, A.; Hibbert, M.; Carlin, J.B.; Bowes, G.; Olinsky, A. Childhood asthma and lung function in mid-adult life. Pediatr. Pulmonol. 1997, 23, 14–20. [Google Scholar] [CrossRef]
- Koefoed, H.; Zwitserloot, A.M.; Vonk, J.M.; Koppelman, G.H. Asthma, bronchial hyperresponsiveness, allergy and lung function development until early adulthood: A systematic literature review. Pediatr. Allergy Immunol. 2021, 32, 1238–1254. [Google Scholar] [CrossRef]
- Tsuneyoshi, S.; Kawayama, T.; Sasaki, J.; Kinoshita, T.; Yano, C.; Tokunaga, Y.; Matsuoka, M.; Imaoka, H.; Matsunaga, K.; Furukawa, K.; et al. Poor Asthma Control in Schoolchildren May Lead to Lower Lung Function Trajectory from Childhood to Early Adulthood: A Japanese Cohort Study. J. Asthma Allergy 2022, 15, 885–896. [Google Scholar] [CrossRef]
- Melén, E.; Guerra, S.; Hallberg, J.; Jarvis, D.; Stanojevic, S. Linking COPD epidemiology with pediatric asthma care: Implications for the patient and the physician. Pediatr. Allergy Immunol. 2019, 30, 589–597. [Google Scholar] [CrossRef]
- Godden, D.J.; Ross, S.; Abdalla, M.; McMurray, D.; Douglas, A.; Oldman, D.; Friend, J.A.; Legge, J.S.; Douglas, J.G. Outcome of wheeze in childhood. Symptoms and pulmonary function 25 years later. Am. J. Respir. Crit. Care Med. 1994, 149, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Nève, V.; Girard, F.; Flahault, A.; Boulé, M. Lung and thorax development during adolescence: Relationship with pubertal status. Eur. Respir. J. 2002, 20, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Satrell, E.; Clemm, H.; Røksund, O.D.; Hufthammer, K.O.; Thorsen, E.; Halvorsen, T.; Vollsæter, M. Development of lung diffusion to adulthood following extremely preterm birth. Eur. Respir. J. 2022, 59, 2004103. [Google Scholar] [CrossRef] [PubMed]
- Vissing, N.H.; Chawes, B.L.; Bisgaard, H. Increased risk of pneumonia and bronchiolitis after bacterial colonization of the airways as neonates. Am. J. Respir. Crit. Care Med. 2013, 188, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Følsgaard, N.V.; Schjørring, S.; Chawes, B.L.; Rasmussen, M.A.; Krogfelt, K.A.; Brix, S.; Bisgaard, H. Pathogenic bacteria colonizing the airways in asymptomatic neonates stimulates topical inflammatory mediator release. Am. J. Respir. Crit. Care Med. 2013, 187, 589–595. [Google Scholar] [CrossRef]
- Thorsen, J.; Rasmussen, M.A.; Waage, J.; Mortensen, M.; Brejnrod, A.; Bønnelykke, K.; Chawes, B.L.; Brix, S.; Sørensen, S.J.; Stokholm, J.; et al. Infant airway microbiota and topical immune perturbations in the origins of childhood asthma. Nat. Commun. 2019, 10, 5001. [Google Scholar] [CrossRef]
- Powell, E.A.; Fontanella, S.; Boakes, E.; Belgrave, D.; Shaw, A.G.; Cornwell, E.; Fernandez-Crespo, R.; Fink, C.G.; Custovic, A.; Kroll, J.S. Temporal association of the development of oropharyngeal microbiota with early life wheeze in a population-based birth cohort. EBioMedicine 2019, 46, 486–498. [Google Scholar] [CrossRef]
- Ege, M.J.; Mayer, M.; Normand, A.C.; Genuneit, J.; Cookson, W.O.; Braun-Fahrländer, C.; Heederik, D.; Piarroux, R.; von Mutius, E. Exposure to environmental microorganisms and childhood asthma. N. Engl. J. Med. 2011, 364, 701–709. [Google Scholar] [CrossRef]
- Cardenas, P.A.; Cooper, P.J.; Cox, M.J.; Chico, M.; Arias, C.; Moffatt, M.F.; Cookson, W.O. Upper airways microbiota in antibiotic-naïve wheezing and healthy infants from the tropics of rural Ecuador. PLoS ONE 2012, 7, e46803. [Google Scholar] [CrossRef]
- Teo, S.M.; Tang, H.; Mok, D.; Judd, L.M.; Watts, S.C.; Pham, K.; Holt, B.J.; Kusel, M.; Serralha, M.; Troy, N.; et al. Airway Microbiota Dynamics Uncover a Critical Window for Interplay of Pathogenic Bacteria and Allergy in Childhood Respiratory Disease. Cell Host Microbe 2018, 24, 341–352.e5. [Google Scholar] [CrossRef]
- Ta, L.; Yap, G.C.; Tay, C.; Lim, A.; Huang, C.H.; Chu, C.W.; De Sessions, P.F.; Shek, L.P.; Goh, A.; Van Bever, H.; et al. Establishment of the nasal microbiota in the first 18 months of life: Correlation with early-onset rhinitis and wheezing. J. Allergy Clin. Immunol. 2018, 142, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Lang, A.; Teo, S.M.; Judd, L.M.; Gangnon, R.; Evans, M.D.; Lee, K.E.; Vrtis, R.; Holt, P.G.; Lemanske, R.F., Jr.; et al. Developmental patterns in the nasopharyngeal microbiome during infancy are associated with asthma risk. J. Allergy Clin. Immunol. 2021, 147, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, P.M.; Pedersen, S.; Lamm, C.J.; Tan, W.C.; Busse, W.W.; START Investigators Group. Severe exacerbations and decline in lung function in asthma. Am. J. Respir. Crit. Care Med. 2009, 179, 19–24. [Google Scholar] [CrossRef]
- Grol, M.H.; Gerritsen, J.; Vonk, J.M.; Schouten, J.P.; Koëter, G.H.; Rijcken, B.; Postma, D.S. Risk factors for growth and decline of lung function in asthmatic individuals up to age 42 years. A 30-year follow-up study. Am. J. Respir. Crit. Care Med. 1999, 160, 1830–1837. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.L. Airway function throughout the lifespan: Pediatric origins of adult respiratory disease. Pediatr. Investig. 2019, 3, 236–244. [Google Scholar] [CrossRef]
- McGeachie, M.J.; Yates, K.P.; Zhou, X.; Guo, F.; Sternberg, A.L.; Van Natta, M.L.; Wise, R.A.; Szefler, S.J.; Sharma, S.; Kho, A.T.; et al. Patterns of Growth and Decline in Lung Function in Persistent Childhood Asthma. N. Engl. J. Med. 2016, 374, 1842–1852. [Google Scholar] [CrossRef]
- Lange, P.; Celli, B.; Agustí, A.; Boje Jensen, G.; Divo, M.; Faner, R.; Guerra, S.; Marott, J.L.; Martinez, F.D.; Martinez-Camblor, P.; et al. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2015, 373, 111–122. [Google Scholar] [CrossRef]
- Wang, G.; Kull, I.; Bergström, A.; Hallberg, J.; Bergström, P.U.; Guerra, S.; Pershagen, G.; Gruzieva, O.; van Hage, M.; Georgelis, A.; et al. Early-life risk factors for reversible and irreversible airflow limitation in young adults: Findings from the BAMSE birth cohort. Thorax 2021, 76, 503–507. [Google Scholar] [CrossRef]
- Forno, E.; Weiner, D.J.; Mullen, J.; Sawicki, G.; Kurland, G.; Han, Y.Y.; Cloutier, M.M.; Canino, G.; Weiss, S.T.; Litonjua, A.A.; et al. Obesity and Airway Dysanapsis in Children with and without Asthma. Am. J. Respir. Crit. Care Med. 2017, 195, 314–323. [Google Scholar] [CrossRef]
- Mortola, J.P. Dysanaptic lung growth: An experimental and allometric approach. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 54, 1236–1241. [Google Scholar] [CrossRef]
- Kennedy, J.D. Lung function outcome in children of premature birth. J. Paediatr. Child. Health 1999, 35, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Ioan, I.; Gemble, A.; Hamon, I.; Schweitzer, C.; Metche, S.; Bonabel, C.; Nguyen-Thi, P.L.; Hascoet, J.M.; Demoulin-Alexikova, S.; Marchal, F. Expiratory Flow–Vital Capacity: Airway–Lung Dysanapsis in 7 Year Olds Born Very Preterm? Front. Physiol. 2018, 9, 650. [Google Scholar] [CrossRef]
- Sonnenschein-van der Voort, A.M.; Howe, L.D.; Granell, R.; Duijts, L.; Sterne, J.A.; Tilling, K.; Henderson, A.J. Influence of childhood growth on asthma and lung function in adolescence. J. Allergy Clin. Immunol. 2015, 135, 1435–1443.e7. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Hallberg, J.; Charalampopoulos, D.; Sanahuja, M.C.; Breyer-Kohansal, R.; Langhammer, A.; Granell, R.; Vonk, J.M.; Mian, A.; Olvera, N.; et al. Spirometric phenotypes from early childhood to young adulthood: A Chronic Airway Disease Early Stratification study. ERJ Open Res. 2021, 7, 00457–2021. [Google Scholar] [CrossRef] [PubMed]
- Peralta, G.P.; Abellan, A.; Montazeri, P.; Basterrechea, M.; Esplugues, A.; González-Palacios, S.; Roda, C.; Santa-Marina, L.; Sunyer, J.; Vrijheid, M.; et al. Early childhood growth is associated with lung function at 7 years: A prospective population-based study. Eur. Respir. J. 2020, 56, 2000157. [Google Scholar] [CrossRef]
- Sly, P.; Bush, A. Environmental contributions to respiratory disease in children. In Kendig’s Disorders of the Respiratory Tract in Children; Wilmott, R., Deterding, R., Li, A., Ratjen, F., Sly, P., Zar, H.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Stern, D.A.; Morgan, W.J.; Wright, A.L.; Guerra, S.; Martinez, F.D. Poor airway function in early infancy and lung function by age 22 years: A non-selective longitudinal cohort study. Lancet 2007, 370, 758–764. [Google Scholar] [CrossRef]
- Fletcher, C.; Peto, R. The natural history of chronic airflow obstruction. Br. Med. J. 1977, 1, 1645–1648. [Google Scholar] [CrossRef]
- Martinez, F.D. Early-Life Origins of Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2016, 375, 871–878. [Google Scholar] [CrossRef]
- Edwards, C.A.; Osman, L.M.; Godden, D.J.; Campbell, D.M.; Douglas, J.G. Relationship between birth weight and adult lung function: Controlling for maternal factors. Thorax 2003, 58, 1061–1065. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Ebrahim, S.; Davey Smith, G. Association of birth weight with adult lung function: Findings from the British Women’s Heart and Health Study and a meta-analysis. Thorax 2005, 60, 851–858. [Google Scholar] [CrossRef]
- Boezen, H.M.; Vonk, J.M.; van Aalderen, W.M.; Brand, P.L.; Gerritsen, J.; Schouten, J.P.; Boersma, E.R. Perinatal predictors of respiratory symptoms and lung function at a young adult age. Eur. Respir. J. 2002, 20, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Narang, I.; Rosenthal, M.; Cremonesini, D.; Silverman, M.; Bush, A. Longitudinal evaluation of airway function 21 years after preterm birth. Am. J. Respir. Crit. Care Med. 2008, 178, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Bush, A. Lung Development and Aging. Ann. Am. Thorac Soc. 2016, 13 (Suppl. S5), S438–S446. [Google Scholar] [CrossRef]
- Broström, E.B.; Akre, O.; Katz-Salamon, M.; Jaraj, D.; Kaijser, M. Obstructive pulmonary disease in old age among individuals born preterm. Eur. J. Epidemiol. 2013, 28, 79–85. [Google Scholar] [CrossRef]
- Barker, D.J.; Godfrey, K.M.; Fall, C.; Osmond, C.; Winter, P.D.; Shaheen, S.O. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ 1991, 303, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Rona, R.J.; Gulliford, M.C.; Chinn, S. Effects of prematurity and intrauterine growth on respiratory health and lung function in childhood. BMJ 1993, 306, 817–820. [Google Scholar] [CrossRef]
- Kotecha, S.J.; Watkins, W.J.; Heron, J.; Henderson, J.; Dunstan, F.D.; Kotecha, S. Spirometric lung function in school-age children: Effect of intrauterine growth retardation and catch-up growth. Am. J. Respir. Crit. Care Med. 2010, 181, 969–974. [Google Scholar] [CrossRef]
- Lombardi, E.; Fainardi, V.; Calogero, C.; Puglia, M.; Voller, F.; Cuttini, M.; Rusconi, F. Lung function in a cohort of 5-year-old children born very preterm. Pediatr. Pulmonol. 2018, 53, 1633–1639. [Google Scholar] [CrossRef]
- Pelkonen, A.S.; Hakulinen, A.L.; Turpeinen, M. Bronchial lability and responsiveness in school children born very preterm. Am. J. Respir. Crit. Care Med. 1997, 156 Pt 1, 1178–1184. [Google Scholar] [CrossRef]
- Kotecha, S.J.; Watkins, W.J.; Paranjothy, S.; Dunstan, F.D.; Henderson, A.J.; Kotecha, S. Effect of late preterm birth on longitudinal lung spirometry in school age children and adolescents. Thorax 2012, 67, 54–61. [Google Scholar] [CrossRef]
- Du Berry, C.; Nesci, C.; Cheong, J.; FitzGerald, T.; Mainzer, R.; Ranganathan, S.; Doyle, L.W.; Vrijlandt, E.; Welsh, L. Long-term expiratory airflow of infants born moderate-late preterm: A systematic review and meta-analysis. EClinicalMedicine 2022, 52, 101597. [Google Scholar] [CrossRef]
- Den Dekker, H.T.; Sonnenschein-van der Voort, A.; de Jongste, J.C.; Anessi-Maesano, I.; Arshad, S.H.; Barros, H.; Beardsmore, C.S.; Bisgaard, H.; Phar, S.C.; Craig, L.; et al. Early growth characteristics and the risk of reduced lung function and asthma: A meta-analysis of 25,000 children. J. Allergy Clin. Immunol. 2016, 137, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, E.; Filippone, M. Chronic lung disease after premature birth. N. Engl. J. Med. 2007, 357, 1946–1955. [Google Scholar] [CrossRef]
- Talmaciu, I.; Ren, C.L.; Kolb, S.M.; Hickey, E.; Panitch, H.B. Pulmonary function in technology-dependent children 2 years and older with bronchopulmonary dysplasia. Pediatr. Pulmonol. 2002, 33, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Thunqvist, P.; Gustafsson, P.; Norman, M.; Wickman, M.; Hallberg, J. Lung function at 6 and 18 months after preterm birth in relation to severity of bronchopulmonary dysplasia. Pediatr. Pulmonol. 2015, 50, 978–986. [Google Scholar] [CrossRef]
- Gerhardt, T.; Hehre, D.; Feller, R.; Reifenberg, L.; Bancalari, E. Serial determination of pulmonary function in infants with chronic lung disease. J. Pediatr. 1987, 110, 448–456. [Google Scholar] [CrossRef]
- Robin, B.; Kim, Y.J.; Huth, J.; Klocksieben, J.; Torres, M.; Tepper, R.S.; Castile, R.G.; Solway, J.; Hershenson, M.B.; Goldstein-Filbrun, A. Pulmonary function in bronchopulmonary dysplasia. Pediatr. Pulmonol. 2004, 37, 236–242. [Google Scholar] [CrossRef]
- Abman, S.H.; Bancalari, E.; Jobe, A. The Evolution of Bronchopulmonary Dysplasia after 50 Years. Am. J. Respir. Crit. Care Med. 2017, 195, 421–424. [Google Scholar] [CrossRef]
- Higgins, R.D.; Jobe, A.H.; Koso-Thomas, M.; Bancalari, E.; Viscardi, R.M.; Hartert, T.V.; Ryan, R.M.; Kallapur, S.G.; Steinhorn, R.H.; Konduri, G.G.; et al. Bronchopulmonary Dysplasia: Executive Summary of a Workshop. J. Pediatr. 2018, 197, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Isayama, T.; Lee, S.K.; Yang, J.; Lee, D.; Daspal, S.; Dunn, M.; Shah, P.S.; Canadian Neonatal Network and Canadian Neonatal Follow-Up Network Investigators. Revisiting the Definition of Bronchopulmonary Dysplasia: Effect of Changing Panoply of Respiratory Support for Preterm Neonates. JAMA Pediatr. 2017, 171, 271–279. [Google Scholar] [CrossRef]
- Siffel, C.; Kistler, K.D.; Lewis, J.; Sarda, S.P. Global incidence of bronchopulmonary dysplasia among extremely preterm infants: A systematic literature review. J. Matern. Fetal Neonatal Med. 2021, 34, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Baveja, R.; Liang, O.D.; Fernandez-Gonzalez, A.; Lee, C.; Mitsialis, S.A.; Kourembanas, S. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am. J. Respir. Crit. Care Med. 2009, 180, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Northway, W.H.; Rosan, R.C., Jr.; Porter, D.Y. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N. Engl. J. Med. 1967, 276, 357–368. [Google Scholar] [CrossRef]
- Jobe, A.H. Mechanisms of Lung Injury and Bronchopulmonary Dysplasia. Am. J. Perinatol. 2016, 33, 1076–1078. [Google Scholar] [CrossRef]
- Thébaud, B.; Goss, K.N.; Laughon, M.; Whitsett, J.A.; Abman, S.H.; Steinhorn, R.H.; Aschner, J.L.; Davis, P.G.; McGrath-Morrow, S.A.; Soll, R.F.; et al. Bronchopulmonary dysplasia. Nat. Rev. Dis. Primers 2019, 5, 78. [Google Scholar] [CrossRef]
- Kair, L.R.; Leonard, D.T.; Anderson, J.M. Bronchopulmonary dysplasia. Pediatr. Rev. 2012, 33, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, A.; Bhandari, V. Pitfalls, problems, and progress in bronchopulmonary dysplasia. Pediatrics 2009, 123, 1562–1573. [Google Scholar] [CrossRef]
- Surate Solaligue, D.E.; Rodríguez-Castillo, J.A.; Ahlbrecht, K.; Morty, R.E. Recent advances in our understanding of the mechanisms of late lung development and bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L1101–L1153. [Google Scholar] [CrossRef]
- Auten, R.L.; Mason, S.N.; Auten, K.M.; Brahmajothi, M. Hyperoxia impairs postnatal alveolar epithelial development via NADPH oxidase in newborn mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L134–L142. [Google Scholar] [CrossRef]
- Balasubramaniam, V.; Mervis, C.F.; Maxey, A.M.; Markham, N.E.; Abman, S.H. Hyperoxia reduces bone marrow, circulating, and lung endothelial progenitor cells in the developing lung: Implications for the pathogenesis of bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L1073–L1084. [Google Scholar] [CrossRef]
- Tanswell, A.K.; Freeman, B.A. Pulmonary antioxidant enzyme maturation in the fetal and neonatal rat. I. Developmental profiles. Pediatr. Res. 1984, 18, 584–587. [Google Scholar] [CrossRef]
- Morrow, L.A.; Wagner, B.D.; Ingram, D.A.; Poindexter, B.B.; Schibler, K.; Cotten, C.M.; Dagle, J.; Sontag, M.K.; Mourani, P.M.; Abman, S.H. Antenatal Determinants of Bronchopulmonary Dysplasia and Late Respiratory Disease in Preterm Infants. Am. J. Respir. Crit. Care Med. 2017, 196, 364–374. [Google Scholar] [CrossRef]
- Hartling, L.; Liang, Y.; Lacaze-Masmonteil, T. Chorioamnionitis as a risk factor for bronchopulmonary dysplasia: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 97, F8–F17. [Google Scholar] [CrossRef] [PubMed]
- Lapcharoensap, W.; Kan, P.; Powers, R.J.; Shaw, G.M.; Stevenson, D.K.; Gould, J.B.; Wirtschafter, D.D.; Lee, H.C. The Relationship of Nosocomial Infection Reduction to Changes in Neonatal Intensive Care Unit Rates of Bronchopulmonary Dysplasia. J. Pediatr. 2017, 180, 105–109.e1. [Google Scholar] [CrossRef] [PubMed]
- Ballard, A.R.; Mallett, L.H.; Pruszynski, J.E.; Cantey, J.B. Chorioamnionitis and subsequent bronchopulmonary dysplasia in very-low-birth weight infants: A 25-year cohort. J. Perinatol. 2016, 36, 1045–1048. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Haglund, B.; Odlind, V.; Altman, M.; Ewald, U.; Kieler, H. Perinatal conditions related to growth restriction and inflammation are associated with an increased risk of bronchopulmonary dysplasia. Acta Paediatr. 2015, 104, 259–263. [Google Scholar] [CrossRef]
- Poindexter, B.B.; Martin, C.R. Impact of Nutrition on Bronchopulmonary Dysplasia. Clin. Perinatol. 2015, 42, 797–806. [Google Scholar] [CrossRef]
- Delacourt, C.; Hadchouel, A. Regulation of alveolarization. In Fetal and Neonatal Physiology, 5th ed.; Polin, R., Abman, S., Rowitch, D., Benitz, W., Fox, W., Eds.; Elsevier: Philadelphia, PA, USA, 2017; pp. 642–646. [Google Scholar]
- Shojaie, S.; Post, M. Molecular mechanisms of lung development and lung branching morphogenesis. In Fetal and Neonatal Physiology, 5th ed.; Polin, R., Abman, S., Rowitch, D., Benitz, W., Fox, W., Eds.; Elsevier: Philadelphia, PA, USA, 2017; pp. 658–666. [Google Scholar]
- Resch, B.; Kurath-Koller, S.; Eibisberger, M.; Zenz, W. Prematurity and the burden of influenza and respiratory syncytial virus disease. World J. Pediatr. 2016, 12, 8–18. [Google Scholar] [CrossRef]
- Ralser, E.; Mueller, W.; Haberland, C.; Fink, F.M.; Gutenberger, K.H.; Strobl, R.; Kiechl-Kohlendorfer, U. Rehospitalization in the first 2 years of life in children born preterm. Acta Paediatr. 2012, 101, e1–e5. [Google Scholar] [CrossRef]
- Townsi, N.; Laing, I.A.; Hall, G.L.; Simpson, S.J. The impact of respiratory viruses on lung health after preterm birth. Eur. Clin. Respir. J. 2018, 5, 1487214. [Google Scholar] [CrossRef]
- Greenough, A. Long term respiratory outcomes of very premature birth (<32 weeks). Semin. Fetal Neonatal Med. 2012, 17, 73–76. [Google Scholar] [PubMed]
- Greenough, A.; Alexander, J.; Boit, P.; Boorman, J.; Burgess, S.; Burke, A.; Chetcuti, P.A.; Cliff, I.; Lenney, W.; Lytle, T.; et al. School age outcome of hospitalisation with respiratory syncytial virus infection of prematurely born infants. Thorax 2009, 64, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Praprotnik, M.; Stucin Gantar, I.; Lučovnik, M.; Avčin, T.; Krivec, U. Respiratory morbidity, lung function and fitness assessment after bronchopulmonary dysplasia. J. Perinatol. 2015, 35, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Ruf, K.; Thomas, W.; Brunner, M.; Speer, C.P.; Hebestreit, H. Diverging effects of premature birth and bronchopulmonary dysplasia on exercise capacity and physical activity—A case control study. Respir. Res. 2019, 20, 260. [Google Scholar] [CrossRef]
- Shepherd, E.G.; Clouse, B.J.; Hasenstab, K.A.; Sitaram, S.; Malleske, D.T.; Nelin, L.D.; Jadcherla, S.R. Infant Pulmonary Function Testing and Phenotypes in Severe Bronchopulmonary Dysplasia. Pediatrics 2018, 141, e20173350. [Google Scholar] [CrossRef]
- Sanchez-Solis, M.; Perez-Fernandez, V.; Bosch-Gimenez, V.; Quesada, J.J.; Garcia-Marcos, L. Lung function gain in preterm infants with and without bronchopulmonary dysplasia. Pediatr. Pulmonol. 2016, 51, 936–942. [Google Scholar] [CrossRef]
- Islam, J.Y.; Keller, R.L.; Aschner, J.L.; Hartert, T.V.; Moore, P.E. Understanding the Short- and Long-Term Respiratory Outcomes of Prematurity and Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2015, 192, 134–156. [Google Scholar] [CrossRef]
- Doyle, L.W.; Faber, B.; Callanan, C.; Freezer, N.; Ford, G.W.; Davis, N.M. Bronchopulmonary dysplasia in very low birth weight subjects and lung function in late adolescence. Pediatrics 2006, 118, 108–113. [Google Scholar] [CrossRef]
- Kotecha, S.J.; Gibbons, J.; Course, C.W.; Evans, E.E.; Simpson, S.J.; Watkins, W.J.; Kotecha, S. Geographical Differences and Temporal Improvements in Forced Expiratory Volume in 1 Second of Preterm-Born Children: A Systematic Review and Meta-analysis. JAMA Pediatr. 2022, 176, 867–877. [Google Scholar] [CrossRef]
- Simpson, S.J.; Turkovic, L.; Wilson, A.C.; Verheggen, M.; Logie, K.M.; Pillow, J.J.; Hall, G.L. Lung function trajectories throughout childhood in survivors of very preterm birth: A longitudinal cohort study. Lancet Child. Adolesc. Health 2018, 2, 350–359. [Google Scholar] [CrossRef]
- Um-Bergström, P.; Hallberg, J.; Thunqvist, P.; Berggren-Broström, E.; Anderson, M.; Adenfelt, G.; Lilja, G.; Ferrara, G.; Sköld, C.M.; Melén, E. Lung function development after preterm birth in relation to severity of Bronchopulmonary dysplasia. BMC Pulm Med. 2017, 17, 97. [Google Scholar] [CrossRef]
- Hirata, K.; Nishihara, M.; Kimura, T.; Shiraishi, J.; Hirano, S.; Kitajima, H.; Fujimura, M. Longitudinal impairment of lung function in school-age children with extremely low birth weights. Pediatr. Pulmonol. 2017, 52, 779–786. [Google Scholar] [CrossRef]
- Vom Hove, M.; Prenzel, F.; Uhlig, H.H.; Robel-Tillig, E. Pulmonary outcome in former preterm, very low birth weight children with bronchopulmonary dysplasia: A case-control follow-up at school age. J. Pediatr. 2014, 164, 40–45.e4. [Google Scholar] [CrossRef] [PubMed]
- Carraro, S.; Giordano, G.; Pirillo, P.; Maretti, M.; Reniero, F.; Cogo, P.E.; Perilongo, G.; Stocchero, M.; Baraldi, E. Airway metabolic anomalies in adolescents with bronchopulmonary dysplasia: New insights from the metabolomic approach. J. Pediatr. 2015, 166, 234–239.e1. [Google Scholar] [CrossRef]
- Bartal, M. COPD and tobacco smoke. Monaldi Arch. Chest Dis. 2005, 63, 213–225. [Google Scholar] [CrossRef]
- Perret, J.L.; Walters, H.; Johns, D.; Gurrin, L.; Burgess, J.; Lowe, A.; Thompson, B.; Markos, J.; Morrison, S.; Thomas, P.; et al. Mother’s smoking and complex lung function of offspring in middle age: A cohort study from childhood. Respirology 2016, 21, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Stick, S.M.; Burton, P.R.; Gurrin, L.; Sly, P.D.; LeSouëf, P.N. Effects of maternal smoking during pregnancy and a family history of asthma on respiratory function in newborn infants. Lancet 1996, 348, 1060–1064. [Google Scholar] [CrossRef]
- Jaakkola, J.J.; Kosheleva, A.A.; Katsnelson, B.A.; Kuzmin, S.V.; Privalova, L.I.; Spengler, J.D. Prenatal and postnatal tobacco smoke exposure and respiratory health in Russian children. Respir. Res. 2006, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Beyer, D.; Mitfessel, H.; Gillissen, A. Maternal smoking promotes chronic obstructive lung disease in the offspring as adults. Eur. J. Med. Res. 2009, 14 (Suppl. S4), 27–31. [Google Scholar] [CrossRef]
- Svanes, C.; Omenaas, E.; Jarvis, D.; Chinn, S.; Gulsvik, A.; Burney, P. Parental smoking in childhood and adult obstructive lung disease: Results from the European Community Respiratory Health Survey. Thorax 2004, 59, 295–302. [Google Scholar] [CrossRef]
- Gilliland, F.D.; Li, Y.F.; Peters, J.M. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. Am. J. Respir. Crit. Care Med. 2001, 163, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Neuman, Å.; Hohmann, C.; Orsini, N.; Pershagen, G.; Eller, E.; Kjaer, H.F.; Gehring, U.; Granell, R.; Henderson, J.; Heinrich, J.; et al. Maternal smoking in pregnancy and asthma in preschool children: A pooled analysis of eight birth cohorts. Am. J. Respir. Crit. Care Med. 2012, 186, 1037–1043. [Google Scholar] [CrossRef]
- Magnus, M.C.; Henderson, J.; Tilling, K.; Howe, L.D.; Fraser, A. Independent and combined associations of maternal and own smoking with adult lung function and COPD. Int. J. Epidemiol. 2018, 47, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Håberg, S.E.; Stigum, H.; Nystad, W.; Nafstad, P. Effects of pre- and postnatal exposure to parental smoking on early childhood respiratory health. Am. J. Epidemiol. 2007, 166, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Hylkema, M.N.; Blacquière, M.J. Intrauterine effects of maternal smoking on sensitization, asthma, and chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2009, 6, 660–662. [Google Scholar] [CrossRef] [PubMed]
- Markunas, C.A.; Xu, Z.; Harlid, S.; Wade, P.A.; Lie, R.T.; Taylor, J.A.; Wilcox, A.J. Identification of DNA methylation changes in newborns related to maternal smoking during pregnancy. Environ. Health Perspect. 2014, 122, 1147–1153. [Google Scholar] [CrossRef]
- Lee, K.W.; Richmond, R.; Hu, P.; French, L.; Shin, J.; Bourdon, C.; Reischl, E.; Waldenberger, M.; Zeilinger, S.; Gaunt, T.; et al. Prenatal exposure to maternal cigarette smoking and DNA methylation: Epigenome-wide association in a discovery sample of adolescents and replication in an independent cohort at birth through 17 years of age. Environ. Health Perspect. 2015, 123, 193–199. [Google Scholar] [CrossRef]
- Joubert, B.R.; Felix, J.F.; Yousefi, P.; Bakulski, K.M.; Just, A.C.; Breton, C.; Reese, S.E.; Markunas, C.A.; Richmond, R.C.; Xu, C.J.; et al. DNA Methylation in Newborns and Maternal Smoking in Pregnancy: Genome-wide Consortium Meta-analysis. Am. J. Hum. Genet. 2016, 98, 680–696. [Google Scholar] [CrossRef]
- Richmond, R.C.; Simpkin, A.J.; Woodward, G.; Gaunt, T.R.; Lyttleton, O.; McArdle, W.L.; Ring, S.M.; Smith, A.D.; Timpson, N.J.; Tilling, K.; et al. Prenatal exposure to maternal smoking and offspring DNA methylation across the lifecourse: Findings from the Avon Longitudinal Study of Parents and Children (ALSPAC). Hum. Mol. Genet. 2015, 24, 2201–2217. [Google Scholar] [CrossRef]
- Joubert, B.R.; Håberg, S.E.; Nilsen, R.M.; Wang, X.; Vollset, S.E.; Murphy, S.K.; Huang, Z.; Hoyo, C.; Midttun, Ø.; Cupul-Uicab, L.A.; et al. 450K epigenome-wide scan identifies differential DNA methylation in newborns related to maternal smoking during pregnancy. Environ. Health Perspect. 2012, 120, 1425–1431. [Google Scholar] [CrossRef]
- Pattenden, S.; Antova, T.; Neuberger, M.; Nikiforov, B.; De Sario, M.; Grize, L.; Heinrich, J.; Hruba, F.; Janssen, N.; Luttmann-Gibson, H.; et al. Parental smoking and children’s respiratory health: Independent effects of prenatal and postnatal exposure. Tob. Control 2006, 15, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Lovasi, G.S.; Diez Roux, A.V.; Hoffman, E.A.; Kawut, S.M.; Jacobs, D.R.; Barr, R.G., Jr. Association of environmental tobacco smoke exposure in childhood with early emphysema in adulthood among nonsmokers: The MESA-lung study. Am. J. Epidemiol. 2010, 171, 54–62. [Google Scholar] [CrossRef]
- Johannessen, A.; Bakke, P.S.; Hardie, J.A.; Eagan, T.M. Association of exposure to environmental tobacco smoke in childhood with chronic obstructive pulmonary disease and respiratory symptoms in adults. Respirology 2012, 17, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Baccarelli, A.; Wright, R.O.; Bollati, V.; Tarantini, L.; Litonjua, A.A.; Suh, H.H.; Zanobetti, A.; Sparrow, D.; Vokonas, P.S.; Schwartz, J. Rapid DNA methylation changes after exposure to traffic particles. Am. J. Respir. Crit. Care Med. 2009, 179, 572–578. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, M.G.; Park, M.K.; Seo, Y.R. Predictive and Prognostic Biomarkers of Respiratory Diseases due to Particulate Matter Exposure. J. Cancer Prev. 2017, 22, 6–15. [Google Scholar] [CrossRef]
- Cai, J.; Zhao, Y.; Liu, P.; Xia, B.; Zhu, Q.; Wang, X.; Song, Q.; Kan, H.; Zhang, Y. Exposure to particulate air pollution during early pregnancy is associated with placental DNA methylation. Sci. Total Environ. 2017, 607–608, 1103–1108. [Google Scholar] [CrossRef]
- Kravitz-Wirtz, N.; Teixeira, S.; Hajat, A.; Woo, B.; Crowder, K.; Takeuchi, D. Early-Life Air Pollution Exposure, Neighborhood Poverty, and Childhood Asthma in the United States, 1990–2014. Int. J. Environ. Res. Public Health 2018, 15, 1114. [Google Scholar] [CrossRef]
- Salvi, S. Tobacco smoking and environmental risk factors for chronic obstructive pulmonary disease. Clin. Chest Med. 2014, 35, 17–27. [Google Scholar] [CrossRef]
- Smith, R.B.; Fecht, D.; Gulliver, J.; Beevers, S.D.; Dajnak, D.; Blangiardo, M.; Ghosh, R.E.; Hansell, A.L.; Kelly, F.J.; Anderson, H.R.; et al. Impact of London’s road traffic air and noise pollution on birth weight: Retrospective population based cohort study. BMJ 2017, 359, j5299. [Google Scholar] [CrossRef] [PubMed]
- Morales, E.; Garcia-Esteban, R.; de la Cruz, O.A.; Basterrechea, M.; Lertxundi, A.; de Dicastillo, M.D.; Zabaleta, C.; Sunyer, J. Intrauterine and early postnatal exposure to outdoor air pollution and lung function at preschool age. Thorax 2015, 70, 64–73. [Google Scholar] [CrossRef]
- Lee, A.; Leon Hsu, H.H.; Mathilda Chiu, Y.H.; Bose, S.; Rosa, M.J.; Kloog, I.; Wilson, A.; Schwartz, J.; Cohen, S.; Coull, B.A.; et al. Prenatal fine particulate exposure and early childhood asthma: Effect of maternal stress and fetal sex. J. Allergy Clin. Immunol. 2018, 141, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Glinianaia, S.V.; Rankin, J.; Bell, R.; Pless-Mulloli, T.; Howel, D. Particulate air pollution and fetal health: A systematic review of the epidemiologic evidence. Epidemiology 2004, 15, 36–45. [Google Scholar] [CrossRef]
- Gauderman, W.J.; Avol, E.; Gilliland, F.; Vora, H.; Thomas, D.; Berhane, K.; McConnell, R.; Kuenzli, N.; Lurmann, F.; Rappaport, E.; et al. The effect of air pollution on lung development from 10 to 18 years of age. N. Engl. J. Med. 2004, 351, 1057–1067. [Google Scholar] [CrossRef]
- Morgenstern, V.; Zutavern, A.; Cyrys, J.; Brockow, I.; Koletzko, S.; Krämer, U.; Behrendt, H.; Herbarth, O.; von Berg, A.; Bauer, C.P.; et al. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am. J. Respir. Crit. Care Med. 2008, 177, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Qiu, A.Y.; Leng, S.; McCormack, M.; Peden, D.B.; Sood, A. Lung Effects of Household Air Pollution. J. Allergy Clin. Immunol. Pract. 2022, 10, 2807–2819. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.; Osmond, C.; Southall, H.; Aucott, P.; Jones, A.; Holgate, S.T. Evaluating the long-term consequences of air pollution in early life: Geographical correlations between coal consumption in 1951/1952 and current mortality in England and Wales. BMJ Open 2018, 8, e018231. [Google Scholar] [CrossRef]
- Soppa, V.J.; Schins, R.P.; Hennig, F.; Hellack, B.; Quass, U.; Kaminski, H.; Kuhlbusch, T.A.; Hoffmann, B.; Weinmayr, G. Respiratory effects of fine and ultrafine particles from indoor sources—A randomized sham-controlled exposure study of healthy volunteers. Int. J. Environ. Res. Public Health 2014, 11, 6871–6889. [Google Scholar] [CrossRef]
- Trenga, C.A.; Sullivan, J.H.; Schildcrout, J.S.; Shepherd, K.P.; Shapiro, G.G.; Liu, L.J.; Kaufman, J.D.; Koenig, J.Q. Effect of particulate air pollution on lung function in adult and pediatric subjects in a Seattle panel study. Chest 2006, 129, 1614–1622. [Google Scholar] [CrossRef]
- Makrinioti, H.; Hasegawa, K.; Lakoumentas, J.; Xepapadaki, P.; Tsolia, M.; Castro-Rodriguez, J.A.; Feleszko, W.; Jartti, T.; Johnston, S.L.; Bush, A.; et al. The role of respiratory syncytial virus- and rhinovirus-induced bronchiolitis in recurrent wheeze and asthma—A systematic review and meta-analysis. Pediatr. Allergy Immunol. 2022, 33, e13741. [Google Scholar] [CrossRef]
- Calışkan, M.; Bochkov, Y.A.; Kreiner-Møller, E.; Bønnelykke, K.; Stein, M.M.; Du, G.; Bisgaard, H.; Jackson, D.J.; Gern, J.E.; Lemanske, R.F., Jr.; et al. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N. Engl. J. Med. 2013, 368, 1398–1407. [Google Scholar] [CrossRef]
- Copenhaver, C.C.; Gern, J.E.; Li, Z.; Shult, P.A.; Rosenthal, L.A.; Mikus, L.D.; Kirk, C.J.; Roberg, K.A.; Anderson, E.L.; Tisler, C.J.; et al. Cytokine response patterns, exposure to viruses, and respiratory infections in the first year of life. Am. J. Respir. Crit. Care Med. 2004, 170, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.; Tran, H.; Roberts, M.; Clarke, N.; Wilson, J.; Robertson, C.F. The association between childhood asthma and adult chronic obstructive pulmonary disease. Thorax 2014, 69, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Shirtcliffe, P.; Marsh, S.; Travers, J.; Weatherall, M.; Beasley, R. Childhood asthma and GOLD-defined chronic obstructive pulmonary disease. Intern. Med. J. 2012, 42, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Hayden, L.P.; Hobbs, B.D.; Cohen, R.T.; Wise, R.A.; Checkley, W.; Crapo, J.D.; Hersh, C.P.; COPDGene Investigators. Childhood pneumonia increases risk for chronic obstructive pulmonary disease: The COPDGene study. Respir. Res. 2015, 16, 115. [Google Scholar] [CrossRef]
- Tagiyeva, N.; Devereux, G.; Fielding, S.; Turner, S.; Douglas, G. Outcomes of Childhood Asthma and Wheezy Bronchitis. A 50-Year Cohort Study. Am. J. Respir. Crit. Care Med. 2016, 193, 23–30. [Google Scholar] [CrossRef]
- Chan, J.Y.; Stern, D.A.; Guerra, S.; Wright, A.L.; Morgan, W.J.; Martinez, F.D. Pneumonia in childhood and impaired lung function in adults: A longitudinal study. Pediatrics 2015, 135, 607–616. [Google Scholar] [CrossRef]
- Parker, A.L.; Abu-Hijleh, M.; McCool, F.D. Ratio between forced expiratory flow between 25% and 75% of vital capacity and FVC is a determinant of airway reactivity and sensitivity to methacholine. Chest 2003, 124, 63–69. [Google Scholar] [CrossRef]
- Hirayama, F.; Lee, A.H. Association between childhood asthma and chronic obstructive pulmonary disease in later life. Asia Pac. J. Public Health 2015, 27, NP1273–NP1279. [Google Scholar] [CrossRef]
- Denguezli, M.; Daldoul, H.; Harrabi, I.; Gnatiuc, L.; Coton, S.; Burney, P.; Tabka, Z. COPD in Nonsmokers: Reports from the Tunisian Population-Based Burden of Obstructive Lung Disease Study. PLoS ONE 2016, 11, e0151981. [Google Scholar] [CrossRef]
- Leung, C.; Bourbeau, J.; Sin, D.D.; Aaron, S.D.; FitzGerald, J.M.; Maltais, F.; Marciniuk, D.D.; O’Donnell, D.; Hernandez, P.; Chapman, K.R.; et al. The Prevalence of Chronic Obstructive Pulmonary Disease (COPD) and the Heterogeneity of Risk Factors in the Canadian Population: Results from the Canadian Obstructive Lung Disease (COLD) Study. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 305–320. [Google Scholar] [CrossRef]
- Davies, D.E.; Wicks, J.; Powell, R.M.; Puddicombe, S.M.; Holgate, S.T. Airway remodeling in asthma: New insights. J. Allergy Clin. Immunol. 2003, 111, 215–226. [Google Scholar] [PubMed]
- Omori, K.; Iwamoto, H.; Yamane, T.; Nakashima, T.; Haruta, Y.; Hattori, N.; Yokoyama, A.; Kohno, N. Clinically remitted childhood asthma is associated with airflow obstruction in middle-aged adults. Respirology 2017, 22, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, H.; Nørgaard, S.; Sevelsted, A.; Chawes, B.L.; Stokholm, J.; Mortensen, E.L.; Ulrik, C.S.; Bønnelykke, K. Asthma-like symptoms in young children increase the risk of COPD. J. Allergy Clin. Immunol. 2021, 147, 569–576.e9. [Google Scholar] [CrossRef]
- Bui, D.S.; Lodge, C.J.; Perret, J.L.; Lowe, A.; Hamilton, G.S.; Thompson, B.; Giles, G.; Tan, D.; Erbas, B.; Pirkis, J.; et al. Trajectories of asthma and allergies from 7 years to 53 years and associations with lung function and extrapulmonary comorbidity profiles: A prospective cohort study. Lancet Respir. Med. 2021, 9, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.R.; Vonk, J.M.; Postma, D.S.; Boezen, H.M. Severe exacerbations predict excess lung function decline in asthma. Eur. Respir. J. 2007, 30, 452–456. [Google Scholar] [CrossRef]
- Bui, D.S.; Burgess, J.A.; Lowe, A.J.; Perret, J.L.; Lodge, C.J.; Bui, M.; Morrison, S.; Thompson, B.R.; Thomas, P.S.; Giles, G.G.; et al. Childhood Lung Function Predicts Adult Chronic Obstructive Pulmonary Disease and Asthma-Chronic Obstructive Pulmonary Disease Overlap Syndrome. Am. J. Respir. Crit. Care Med. 2017, 196, 39–46. [Google Scholar] [CrossRef]
| Risk Factors Associated with BPD |
|---|
| Gestational age, birthweight [98,99,100,101,102] |
| Mechanical trauma and oxygen toxicity related to ventilation [107,114,115,116] |
| Maternal smoking, hypertension, preeclampsia [117] |
| Prenatal infections [118] |
| Postnatal infections [119,120] |
| IUGR [121] |
| Poor nutrition [122] |
| Genetics [27,29,30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deolmi, M.; Decarolis, N.M.; Motta, M.; Makrinioti, H.; Fainardi, V.; Pisi, G.; Esposito, S. Early Origins of Chronic Obstructive Pulmonary Disease: Prenatal and Early Life Risk Factors. Int. J. Environ. Res. Public Health 2023, 20, 2294. https://doi.org/10.3390/ijerph20032294
Deolmi M, Decarolis NM, Motta M, Makrinioti H, Fainardi V, Pisi G, Esposito S. Early Origins of Chronic Obstructive Pulmonary Disease: Prenatal and Early Life Risk Factors. International Journal of Environmental Research and Public Health. 2023; 20(3):2294. https://doi.org/10.3390/ijerph20032294
Chicago/Turabian StyleDeolmi, Michela, Nicola Mattia Decarolis, Matteo Motta, Heidi Makrinioti, Valentina Fainardi, Giovanna Pisi, and Susanna Esposito. 2023. "Early Origins of Chronic Obstructive Pulmonary Disease: Prenatal and Early Life Risk Factors" International Journal of Environmental Research and Public Health 20, no. 3: 2294. https://doi.org/10.3390/ijerph20032294
APA StyleDeolmi, M., Decarolis, N. M., Motta, M., Makrinioti, H., Fainardi, V., Pisi, G., & Esposito, S. (2023). Early Origins of Chronic Obstructive Pulmonary Disease: Prenatal and Early Life Risk Factors. International Journal of Environmental Research and Public Health, 20(3), 2294. https://doi.org/10.3390/ijerph20032294







