Is It Feasible to Predict Cardiovascular Risk among Healthy Vegans, Lacto-/Ovo-Vegetarians, Pescatarians, and Omnivores under Forty?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethics
2.3. Subjects
2.4. Biochemical Blood Tests
2.5. Blood Pressure Measurement
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics and the Biochemical Blood Tests
3.2. Results of Blood Pressure Measurement
3.3. Relationships between the Biochemical Analyses, Behavioral Factors, and Body Composition Parameters
3.4. Prediction of CV Risk
4. Discussion
4.1. Cardiovascular Risk Factors
4.1.1. Apolipoproteins
4.1.2. Lipoprotein (a)
4.1.3. Blood Pressure
4.1.4. HCY
4.2. Inflammatory Markers: hsCRP, IL-6, TNF-alpha
4.2.1. hsCRP
4.2.2. TNF-Alpha
4.2.3. IL-6
4.3. Assessment of Possible Cardiovascular Risk by Creating Research Metrics
5. Strengths and Limitations of the Study
5.1. Strengths of the Study
- To the best of our knowledge, this is the first study comparing lipids, lipoproteins, and other CV parameters and numerous inflammatory markers among healthy people <40 being on different diets.
- The study was conducted during the COVID-19 pandemic. However, compared to other studies, the number of respondents is relatively high, considering how unique groups were recruited.
- The results of this study confirm the possibly beneficial consequences on cardiovascular risk of using a well-balanced vegan diet and maybe another argument “in favor” of recommending this diet. Specialists in various fields of medicine may consider recommending their use in some instances.
- The study participants did not show differences in average age, which proves the samples’ reliability. It was initially planned to divide the study participants into age categories, but due to the above, such division was abandoned.
5.2. Limitations of the Study
- The number of women and men in the study groups is random; the respondents are mainly women.
- The narrow age group does not provide a complete cross-section of possible outcomes.
- Due to the subjects’ young age and health status, comparisons were not made concerning CV risk using the standard recommended CV risk factors tools, which mainly consider age 40 and above. The lack of direct differences concerning diagnostic indications may result from a young age and targeted selection of healthy people.
- The blood sampling assessment as a diagnostic part of the study was performed once. This is a single sample study due to the timing of the pandemic.
- Possible mistakes may have resulted from the incorrect preparation of the participants, or other errors from the pre-analytical stage, as well as during the analyses performed, and these may have led to unintentional biases.
- The criterion of vaccination against SARS-CoV-2 (severe acute respiratory syndrome coronavirus-2) was not a necessary condition for entering the study. The study was conducted during the COVID-19 pandemic; there is a risk that some of the subjects may have been infected (without symptoms), which could have affected the obtained results; however, the obtained results (within the normal range) exclude the influence of the disease.
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Joseph, P.G.; McKee, M.; Anand, S.S.; Teo, K.K.; Schwalm, J.-D.; Yusuf, S. Reducing the Global Burden of Cardiovascular Disease, Part 2: Prevention and Treatment of Cardiovascular Disease. Circ. Res. 2017, 121, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Nations, U. LIFESTYLE DISEASES: An Economic Burden on the Health Services. Available online: https://www.un.org/en/chronicle/article/lifestyle-diseases-economic-burden-health-services (accessed on 1 December 2022).
- Livingstone, K.M.; Abbott, G.; Ward, J.; Bowe, S.J. Unhealthy Lifestyle, Genetics and Risk of Cardiovascular Disease and Mortality in 76,958 Individuals from the UK Biobank Cohort Study. Nutrients 2021, 13, 4283. [Google Scholar] [CrossRef] [PubMed]
- Schwalm, J.-D.; McKee, M.; Huffman, M.D.; Yusuf, S. Resource Effective Strategies to Prevent and Treat Cardiovascular Disease. Circ. 2016, 133, 742–755. [Google Scholar] [CrossRef]
- D’Agostino, R.B.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General Cardiovascular Risk Profile for Use in Primary Care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef]
- SCORE2 working group and ESC Cardiovascular risk collaboration SCORE2 Risk Prediction Algorithms: New Models to Estimate 10-Year Risk of Cardiovascular Disease in Europe. Eur. Heart J. 2021, 42, 2439–2454. [CrossRef]
- Piepoli, M.; Hoes, A.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.; Cooney, M.-T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Romanens, M.; Szucs, T.; Sudano, I.; Adams, A. Agreement of PROCAM and SCORE to Assess Cardiovascular Risk in Two Different Low Risk European Populations. Prev. Med. Rep. 2018, 13, 113–117. [Google Scholar] [CrossRef]
- McClelland, R.L.; Jorgensen, N.W.; Budoff, M.; Blaha, M.J.; Post, W.S.; Kronmal, R.A.; Bild, D.E.; Shea, S.; Liu, K.; Watson, K.E.; et al. 10-Year Coronary Heart Disease Risk Prediction Using Coronary Artery Calcium and Traditional Risk Factors: Derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) With Validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). J. Am. Coll. Cardiol. 2015, 66, 1643–1653. [Google Scholar] [CrossRef]
- Khera, A.; Budoff, M.J.; O’Donnell, C.J.; Ayers, C.A.; Locke, J.; de Lemos, J.A.; Massaro, J.M.; McClelland, R.L.; Taylor, A.; Levine, B.D. Astronaut Cardiovascular Health and Risk Modification (Astro-CHARM) Coronary Calcium Atherosclerotic Cardiovascular Disease Risk Calculator. Circulation 2018, 138, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Hajar, R. Risk Factors for Coronary Artery Disease: Historical Perspectives. Heart Views 2017, 18, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.L.; Jones, J.; Bolleddu, S.I.; Vanthenapalli, S.; Rodgers, L.E.; Shah, K.; Karia, K.; Panguluri, S.K. Cardiovascular Risks Associated with Gender and Aging. J. Cardiovasc. Dev. Dis. 2019, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- GBD Compare|IHME Viz Hub. Available online: http://vizhub.healthdata.org/gbd-compare (accessed on 3 November 2022).
- World Health Organization; Food and Agriculture Organization of the United Nations. Sustainable Healthy Diets: Guiding Principles; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-151664-8. [Google Scholar]
- Healthy Diet. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 3 November 2022).
- Urgent Health Challenges for the Next Decade. Available online: https://www.who.int/news-room/photo-story/photo-story-detail/urgent-health-challenges-for-the-next-decade (accessed on 3 November 2022).
- Scott, P. Global Panel on Agriculture and Food Systems for Nutrition: Food Systems and Diets: Facing the Challenges of the 21st Century. Available online: http://glopan.org/sites/default/files/ForesightReport.pdf (accessed on 3 November 2022).
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on Healthy Diets from Sustainable Food Systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
- Romanello, M.; McGushin, A.; Napoli, C.D.; Drummond, P.; Hughes, N.; Jamart, L.; Kennard, H.; Lampard, P.; Rodriguez, B.S.; Arnell, N.; et al. The 2021 Report of the Lancet Countdown on Health and Climate Change: Code Red for a Healthy Future. Lancet 2021, 398, 1619–1662. [Google Scholar] [CrossRef]
- Burlingame, B.; Nishida, C.; Uauy, R.; Weisell, R. (Eds.) Fats and Fatty Acids in Human Nutrition. Report of an Expert Consultation; FAO Food and Nutrition Paper; S. Karger: Basel, Switzerland, 2010; Volume 91, pp. 1–166. [Google Scholar]
- Li, J.; Sun, Q. Consumption of Saturated Fatty Acids and Coronary Heart Disease Risk. Int. J. Cardiol. 2019, 279, 27–28. [Google Scholar] [CrossRef]
- Islam, M.A.; Amin, M.N.; Siddiqui, S.A.; Hossain, M.P.; Sultana, F.; Kabir, M.R. Trans Fatty Acids and Lipid Profile: A Serious Risk Factor to Cardiovascular Disease, Cancer and Diabetes. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1643–1647. [Google Scholar] [CrossRef]
- De Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of Saturated and Trans Unsaturated Fatty Acids and Risk of All Cause Mortality, Cardiovascular Disease, and Type 2 Diabetes: Systematic Review and Meta-Analysis of Observational Studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. 2015–2020DietaryGuidelinesforAmericans, 8th ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2015. Available online: https://health.gov/our-work/nutrition-physical-activity/dietary-guidelines/previous-dietary-guidelines/2015 (accessed on 3 November 2022).
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef]
- Bargieł, W.; Cierpiszewska, K.; Maruszczak, K.; Pakuła, A.; Szwankowska, D.; Wrzesińska, A.; Gutowski, Ł.; Formanowicz, D. Recognized and Potentially New Biomarkers—Their Role in Diagnosis and Prognosis of Cardiovascular Disease. Medicina 2021, 57, 701. [Google Scholar] [CrossRef]
- Romero-Cabrera, J.L.; Ankeny, J.; Fernández-Montero, A.; Kales, S.N.; Smith, D.L. A Systematic Review and Meta-Analysis of Advanced Biomarkers for Predicting Incident Cardiovascular Disease among Asymptomatic Middle-Aged Adults. Int. J. Mol. Sci. 2022, 23, 13540. [Google Scholar] [CrossRef]
- Kaptoge, S.; Di Angelantonio, E.; Pennells, L.; Wood, A.M.; White, I.R.; Gao, P.; Walker, M.; Thompson, A.; Sarwar, N.; Caslake, M.; et al. C-Reactive Protein, Fibrinogen, and Cardiovascular Disease Prediction. N. Engl. J. Med. 2012, 367, 1310–1320. [Google Scholar] [CrossRef]
- De Ruijter, W.; Westendorp, R.G.J.; Assendelft, W.J.J.; den Elzen, W.P.J.; de Craen, A.J.M.; le Cessie, S.; Gussekloo, J. Use of Framingham Risk Score and New Biomarkers to Predict Cardiovascular Mortality in Older People: Population Based Observational Cohort Study. BMJ 2009, 338, a3083. [Google Scholar] [CrossRef] [PubMed]
- Santilli, F.; Guagnano, M.T.; Vazzana, N.; La Barba, S.; Davi, G. Oxidative Stress Drivers and Modulators in Obesity and Cardiovascular Disease: From Biomarkers to Therapeutic Approach. Curr. Med. Chem. 2015, 22, 582–595. [Google Scholar] [CrossRef]
- Balta, S. Endothelial Dysfunction and Inflammatory Markers of Vascular Disease. Curr. Vasc. Pharmacol. 2021, 19, 243–249. [Google Scholar] [CrossRef]
- Menzel, J.; Biemann, R.; Longree, A.; Isermann, B.; Mai, K.; Schulze, M.B.; Abraham, K.; Weikert, C. Associations of a Vegan Diet with Inflammatory Biomarkers. Sci. Rep. 2020, 10, 1933. [Google Scholar] [CrossRef]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O.; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: A Statement for Healthcare Professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef]
- Il’yasova, D.; Colbert, L.H.; Harris, T.B.; Newman, A.B.; Bauer, D.C.; Satterfield, S.; Kritchevsky, S.B. Circulating Levels of Inflammatory Markers and Cancer Risk in the Health Aging and Body Composition Cohort. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2413–2418. [Google Scholar] [CrossRef]
- Kwiatkowska, I.; Olszak, J.; Formanowicz, P.; Formanowicz, D. Nutritional Status and Habits among People on Vegan, Lacto/Ovo-Vegetarian, Pescatarian and Traditional Diets. Nutrients 2022, 14, 4591. [Google Scholar] [CrossRef]
- Cui, Q.; Xia, Y.; Wu, Q.; Chang, Q.; Niu, K.; Zhao, Y. A Meta-Analysis of the Reproducibility of Food Frequency Questionnaires in Nutritional Epidemiological Studies. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 12. [Google Scholar] [CrossRef]
- Niedzwiedzka, E.; Wadolowska, L.; Kowalkowska, J. Reproducibility of A Non-Quantitative Food Frequency Questionnaire (62-Item FFQ-6) and PCA-Driven Dietary Pattern Identification in 13-21-Year-Old Females. Nutrients 2019, 11, E2183. [Google Scholar] [CrossRef]
- Bel-Serrat, S.; Mouratidou, T.; Pala, V.; Huybrechts, I.; Börnhorst, C.; Fernández-Alvira, J.M.; Hadjigeorgiou, C.; Eiben, G.; Hebestreit, A.; Lissner, L.; et al. Relative Validity of the Children’s Eating Habits Questionnaire-Food Frequency Section among Young European Children: The IDEFICS Study. Public Health Nutr. 2014, 17, 266–276. [Google Scholar] [CrossRef]
- Saeedi, P.; Skeaff, S.A.; Wong, J.E.; Skidmore, P.M.L. Reproducibility and Relative Validity of a Short Food Frequency Questionnaire in 9–10 Year-Old Children. Nutrients 2016, 8, E271. [Google Scholar] [CrossRef] [PubMed]
- Osadnik, T.; Pawlas, N.; Lonnie, M.; Osadnik, K.; Lejawa, M.; Wądołowska, L.; Bujak, K.; Fronczek, M.; Reguła, R.; Gawlita, M.; et al. Family History of Premature Coronary Artery Disease (P-CAD)-A Non-Modifiable Risk Factor? Dietary Patterns of Young Healthy Offspring of P-CAD Patients: A Case-Control Study (MAGNETIC Project). Nutrients 2018, 10, E1488. [Google Scholar] [CrossRef]
- The Jamovi Project. 2021. Available online: https://www.jamovi.org (accessed on 1 June 2022).
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Desideri, G.; Castaldo, G.; Lombardi, A.; Mussap, M.; Testa, A.; Pontremoli, R.; Punzi, L.; Borghi, C. Is it time to revise the normal range of serum uric acid levels? Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1295–1306. [Google Scholar]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on Diabetes, Pre-Diabetes, and Cardiovascular Diseases Developed in Collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. J. Am. Coll. Cardiol. 2019, 73, e285–e350. [Google Scholar] [CrossRef]
- Understanding Your Cholesterol Test Results. Available online: https://www.heartuk.org.uk/cholesterol/understanding-your-cholesterol-test-results (accessed on 3 November 2022).
- Kuchta, A.; Lebiedzińska, A.; Fijałkowski, M.; Gałąska, R.; Kreft, E.; Totoń, M.; Czaja, K.; Kozłowska, A.; Ćwiklińska, A.; Kortas-Stempak, B.; et al. Impact of Plant-Based Diet on Lipid Risk Factors for Atherosclerosis. Cardiol. J. 2016, 23, 141–148. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Jian, Z.-H.; Chang, H.-C.; Nfor, O.N.; Ko, P.-C.; Lung, C.-C.; Lin, L.-Y.; Ho, C.-C.; Chiang, Y.-C.; Liaw, Y.-P. Vegan Diet and Blood Lipid Profiles: A Cross-Sectional Study of Pre and Postmenopausal Women. BMC Womens Health 2014, 14, 55. [Google Scholar] [CrossRef]
- Saintila, J.; Lozano López, T.E.; Ruiz Mamani, P.G.; White, M.; Huancahuire-Vega, S. Health-Related Quality of Life, Blood Pressure, and Biochemical and Anthropometric Profile in Vegetarians and Nonvegetarians. J. Nutr. Metab. 2020, 2020, 3629742. [Google Scholar] [CrossRef]
- Matsumoto, S.; Beeson, W.L.; Shavlik, D.J.; Siapco, G.; Jaceldo-Siegl, K.; Fraser, G.; Knutsen, S.F. Association between Vegetarian Diets and Cardiovascular Risk Factors in Non-Hispanic White Participants of the Adventist Health Study-2. J. Nutr. Sci. 2019, 8, e6. [Google Scholar] [CrossRef]
- Dawczynski, C.; Weidauer, T.; Richert, C.; Schlattmann, P.; Dawczynski, K.; Kiehntopf, M. Nutrient Intake and Nutrition Status in Vegetarians and Vegans in Comparison to Omnivores—the Nutritional Evaluation (NuEva) Study. Front. Nutr. 2022, 9, 819106. [Google Scholar] [CrossRef]
- Jakše, B.; Jakše, B.; Godnov, U.; Pinter, S. Nutritional, Cardiovascular Health and Lifestyle Status of ‘Health Conscious’ Adult Vegans and Non-Vegans from Slovenia: A Cross-Sectional Self-Reported Survey. Int. J. Environ. Res. Public Health 2021, 18, 5968. [Google Scholar] [CrossRef]
- Najjar, R.S.; Moore, C.E.; Montgomery, B.D. Consumption of a Defined, Plant-Based Diet Reduces Lipoprotein(a), Inflammation, and Other Atherogenic Lipoproteins and Particles within 4 Weeks. Clin. Cardiol. 2018, 41, 1062–1068. [Google Scholar] [CrossRef]
- Yıldız, A.; Seçen, Ö.; Yıldız, C.; Çiçekçi, M. Relationship between Breast Arterial Calcification and Lipid Profile, Plasma Atherogenic Index, Castelli’s Risk Index and Atherogenic Coefficient in Premenopausal Women. IJC Metab. Endocr. 2016, 11, 19–22. [Google Scholar] [CrossRef]
- Wu, J.; Chen, S.; Liu, L.; Gao, X.; Zhou, Y.; Wang, C.; Zhang, Q.; Wang, A.; Hussain, M.; Sun, B.; et al. Non-High-Density Lipoprotein Cholesterol vs Low-Density Lipoprotein Cholesterol as a Risk Factor for Ischemic Stroke: A Result from the Kailuan Study. Neurol. Res. 2013, 35, 505–511. [Google Scholar] [CrossRef]
- Sujatha, R.; Kavitha, S. Atherogenic Indices in Stroke Patients: A Retrospective Study. Iran. J. Neurol. 2017, 16, 78–82. [Google Scholar]
- Bradbury, K.E.; Crowe, F.L.; Appleby, P.N.; Schmidt, J.A.; Travis, R.C.; Key, T.J. Serum Concentrations of Cholesterol, Apolipoprotein A-I and Apolipoprotein B in a Total of 1694 Meat-Eaters, Fish-Eaters, Vegetarians and Vegans. Eur. J. Clin. Nutr. 2014, 68, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.C.A.; Antoniazzi, L.; Oki, A.M.; Bonfim, M.C.; Hong, V.; Bortolotto, L.A.; Acosta-Cardenas, P.; Sandrim, V.; Miname, M.H.; Santos Filho, R.D.D. Prevalence of Metabolic Syndrome and Framingham Risk Score in Apparently Healthy Vegetarian and Omnivorous Men. Arq. Bras. Cardiol. 2018, 110, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, I.; Olszak, J.; Formanowicz, P.; Formanowicz, D. Dietary Habits and Lifestyle, including Cardiovascular Risk among Vegetarians and Omnivores during the COVID-19 Pandemic in the Polish Population. Nutrients 2023, 15, 442. [Google Scholar] [CrossRef] [PubMed]
- Harari, G.; Green, M.S.; Magid, A.; Zelber-Sagi, S. Usefulness of Non-High-Density Lipoprotein Cholesterol as a Predictor of Cardiovascular Disease Mortality in Men in 22-Year Follow-Up. Am. J. Cardiol. 2017, 119, 1193–1198. [Google Scholar] [CrossRef]
- Gu, X.; Yang, X.; Li, Y.; Cao, J.; Li, J.; Liu, X.; Chen, J.; Shen, C.; Yu, L.; Huang, J.; et al. Usefulness of Low-Density Lipoprotein Cholesterol and Non-High-Density Lipoprotein Cholesterol as Predictors of Cardiovascular Disease in Chinese. Am. J. Cardiol. 2015, 116, 1063–1070. [Google Scholar] [CrossRef]
- Van den Berg, M.J.; van der Graaf, Y.; de Borst, G.J.; Kappelle, L.J.; Nathoe, H.M.; Visseren, F.L.J.; SMART Study Group. Low-Density Lipoprotein Cholesterol, Non-High-Density Lipoprotein Cholesterol, Triglycerides, and Apolipoprotein B and Cardiovascular Risk in Patients with Manifest Arterial Disease. Am. J. Cardiol. 2016, 118, 804–810. [Google Scholar] [CrossRef]
- Walldius, G. The apoB/apoA-I Ratio is a Strong Predictor of Cardiovascular Risk. In Lipoproteins—Role in Health and Diseases; Frank, S., Kostner, G., Eds.; Intech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Pettersen, B.J.; Anousheh, R.; Fan, J.; Jaceldo-Siegl, K.; Fraser, G.E. Vegetarian Diets and Blood Pressure among White Subjects: Results from the Adventist Health Study-2 (AHS-2). Public Health Nutr. 2012, 15, 1909–1916. [Google Scholar] [CrossRef]
- Fraser, G.; Katuli, S.; Anousheh, R.; Knutsen, S.; Herring, P.; Fan, J. Vegetarian Diets and Cardiovascular Risk Factors in Black Members of the Adventist Health Study-2. Public Health Nutr. 2015, 18, 537–545. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Nishimura, K.; Barnard, N.D.; Takegami, M.; Watanabe, M.; Sekikawa, A.; Okamura, T.; Miyamoto, Y. Vegetarian Diets and Blood Pressure: A Meta-Analysis. JAMA Intern. Med. 2014, 174, 577. [Google Scholar] [CrossRef]
- Gibbs, J.; Gaskin, E.; Ji, C.; Miller, M.A.; Cappuccio, F.P. The Effect of Plant-Based Dietary Patterns on Blood Pressure: A Systematic Review and Meta-Analysis of Controlled Intervention Trials. J. Hypertens. 2021, 39, 23–37. [Google Scholar] [CrossRef]
- Ganguly, P.; Alam, S.F. Role of Homocysteine in the Development of Cardiovascular Disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef]
- Fan, R.; Zhang, A.; Zhong, F. Association between Homocysteine Levels and All-Cause Mortality: A Dose-Response Meta-Analysis of Prospective Studies. Sci. Rep. 2017, 7, 4769. [Google Scholar] [CrossRef] [PubMed]
- Obersby, D.; Chappell, D.C.; Dunnett, A.; Tsiami, A.A. Plasma Total Homocysteine Status of Vegetarians Compared with Omnivores: A Systematic Review and Meta-Analysis. Br. J. Nutr. 2013, 109, 785–794. [Google Scholar] [CrossRef]
- Krajcovicová-Kudlácková, M.; Blazícek, P.; Kopcová, J.; Béderová, A.; Babinská, K. Homocysteine Levels in Vegetarians versus Omnivores. Ann. Nutr. Metab. 2000, 44, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, W.; Schorr, H.; Purschwitz, K.; Rassoul, F.; Richter, V. Total Homocysteine, Vitamin B(12), and Total Antioxidant Status in Vegetarians. Clin. Chem. 2001, 47, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Krivosíková, Z.; Krajcovicová-Kudlácková, M.; Spustová, V.; Stefíková, K.; Valachovicová, M.; Blazícek, P.; Nĕmcová, T. The Association between High Plasma Homocysteine Levels and Lower Bone Mineral Density in Slovak Women: The Impact of Vegetarian Diet. Eur. J. Nutr. 2010, 49, 147–153. [Google Scholar] [CrossRef]
- Su, T.-C.; Jeng, J.-S.; Wang, J.-D.; Torng, P.-L.; Chang, S.-J.; Chen, C.-F.; Liau, C.-S. Homocysteine, Circulating Vascular Cell Adhesion Molecule and Carotid Atherosclerosis in Postmenopausal Vegetarian Women and Omnivores. Atherosclerosis 2006, 184, 356–362. [Google Scholar] [CrossRef]
- Koebnick, C.; Garcia, A.L.; Dagnelie, P.C.; Strassner, C.; Lindemans, J.; Katz, N.; Leitzmann, C.; Hoffmann, I. Long-Term Consumption of a Raw Food Diet Is Associated with Favorable Serum LDL Cholesterol and Triglycerides but Also with Elevated Plasma Homocysteine and Low Serum HDL Cholesterol in Humans. J. Nutr. 2005, 135, 2372–2378. [Google Scholar] [CrossRef]
- Majchrzak, D.; Singer, I.; Männer, M.; Rust, P.; Genser, D.; Wagner, K.-H.; Elmadfa, I. B-Vitamin Status and Concentrations of Homocysteine in Austrian Omnivores, Vegetarians and Vegans. Ann. Nutr. Metab. 2006, 50, 485–491. [Google Scholar] [CrossRef]
- Menzel, J.; Jabakhanji, A.; Biemann, R.; Mai, K.; Abraham, K.; Weikert, C. Systematic Review and Meta-Analysis of the Associations of Vegan and Vegetarian Diets with Inflammatory Biomarkers. Sci. Rep. 2020, 10, 21736. [Google Scholar] [CrossRef]
- Eichelmann, F.; Schwingshackl, L.; Fedirko, V.; Aleksandrova, K. Effect of Plant-Based Diets on Obesity-Related Inflammatory Profiles: A Systematic Review and Meta-Analysis of Intervention Trials. Obes. Rev. 2016, 17, 1067–1079. [Google Scholar] [CrossRef]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nöthlings, U. Dietary Pattern Analysis and Biomarkers of Low-Grade Inflammation: A Systematic Literature Review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Šebeková, K.; Krajčovičová-Kudláčková, M.; Schinzel, R.; Faist, V.; Klvanová, J.; Heidland, A. Plasma Levels of Advanced Glycation End Products in Healthy, Long-Term Vegetarians and Subjects on a Western Mixed Diet. Eur. J. Nutr. 2001, 40, 275–281. [Google Scholar] [CrossRef]
- Franco-de-Moraes, A.C.; de Almeida-Pititto, B.; da Rocha Fernandes, G.; Gomes, E.P.; da Costa Pereira, A.; Ferreira, S.R.G. Worse Inflammatory Profile in Omnivores than in Vegetarians Associates with the Gut Microbiota Composition. Diabetol. Metab. Syndr. 2017, 9, 62. [Google Scholar] [CrossRef]
- Krajcovicova-Kudlackova, M.; Blazicek, P. C-Reactive Protein and Nutrition. Bratisl. Lek. Listy 2005, 106, 345–347. [Google Scholar] [PubMed]
- Sebeková, K.; Boor, P.; Valachovicová, M.; Blazícek, P.; Parrák, V.; Babinská, K.; Heidland, A.; Krajcovicová-Kudlácková, M. Association of metabolic syndrome risk factors with selected markers of oxidative status and microinflammation in healthy omnivores and vegetarians. Mol. Nutr. Food Res. 2006, 50, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-W.; Lin, Y.-L.; Lin, T.-K.; Lin, C.-T.; Chen, B.-C.; Lin, C.-L. Total Cardiovascular Risk Profile of Taiwanese Vegetarians. Eur. J. Clin. Nutr. 2008, 62, 138–144. [Google Scholar] [CrossRef]
- Chen, C.-W.; Lin, C.-T.; Lin, Y.-L.; Lin, T.-K.; Lin, C.-L. Taiwanese Female Vegetarians Have Lower Lipoprotein-Associated Phospholipase A2 Compared with Omnivores. Yonsei Med. J. 2011, 52, 13–19. [Google Scholar] [CrossRef]
- Su, T.-C.; Torng, P.-L.; Jeng, J.-S.; Chen, M.-F.; Liau, C.-S. Arterial Function of Carotid and Brachial Arteries in Postmenopausal Vegetarians. Vasc. Health Risk Manag. 2011, 7, 517–523. [Google Scholar] [CrossRef]
- Lee, C.G.; Hahn, S.J.; Song, M.K.; Lee, J.K.; Kim, J.H.; Lim, Y.J.; Koh, M.-S.; Lee, J.H.; Kang, H.W. Vegetarianism as a Protective Factor for Colorectal Adenoma and Advanced Adenoma in Asians. Dig. Dis. Sci. 2014, 59, 1025–1035. [Google Scholar] [CrossRef]
- Acosta-Navarro, J.; Antoniazzi, L.; Oki, A.M.; Bonfim, M.C.; Hong, V.; Acosta-Cardenas, P.; Strunz, C.; Brunoro, E.; Miname, M.H.; Filho, W.S.; et al. Reduced Subclinical Carotid Vascular Disease and Arterial Stiffness in Vegetarian Men: The CARVOS Study. Int. J. Cardiol. 2017, 230, 562–566. [Google Scholar] [CrossRef]
- Ganie, M.A.; Sahar, T.; Rashid, A.; Wani, I.A.; Nisar, S.; Sathyapalan, T.; Vishnubhatla, S.; Ramakrishnan, L.; Parvez, T.; Geer, I. Comparative Evaluation of Biomarkers of Inflammation Among Indian Women With Polycystic Ovary Syndrome (PCOS) Consuming Vegetarian vs. Non-Vegetarian Diet. Front. Endocrinol. 2019, 10, 699. [Google Scholar] [CrossRef]
- Craddock, J.C.; Neale, E.P.; Peoples, G.E.; Probst, Y.C. Vegetarian-Based Dietary Patterns and Their Relation with Inflammatory and Immune Biomarkers: A Systematic Review and Meta-Analysis. Adv. Nutr. 2019, 10, 433–451. [Google Scholar] [CrossRef]
- Shah, B.; Newman, J.D.; Woolf, K.; Ganguzza, L.; Guo, Y.; Allen, N.; Zhong, J.; Fisher, E.A.; Slater, J. Anti-Inflammatory Effects of a Vegan Diet Versus the American Heart Association–Recommended Diet in Coronary Artery Disease Trial. J. Am. Heart Assoc. 2018, 7, e011367. [Google Scholar] [CrossRef]
- Montalcini, T.; De Bonis, D.; Ferro, Y.; Carè, I.; Mazza, E.; Accattato, F.; Greco, M.; Foti, D.; Romeo, S.; Gulletta, E.; et al. High Vegetable Fats Intake Is Associated with High Resting Energy Expenditure in Vegetarians. Nutrients 2015, 7, 5933–5947. [Google Scholar] [CrossRef] [PubMed]
- Bogdanski, P.; Pupek-Musialik, D.; Dytfeld, J.; Lacinski, M.; Jablecka, A.; Jakubowski, H. Plasma Homocysteine Is a Determinant of Tissue Necrosis Factor-α in Hypertensive Patients. Biomed. Pharmacother. 2008, 62, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Anghel, D.; Sîrbu, C.A.; Hoinoiu, E.-M.; Petrache, O.-G.; Pleșa, C.-F.; Negru, M.M.; Ioniţă-Radu, F. Influence of Anti-TNF Therapy and Homocysteine Level on Carotid Intima-media Thickness in Rheumatoid Arthritis Patients. Exp. Ther. Med. 2022, 23, 59. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J.; van Daalen, K.R.; Thayyil, A.; Cocco, M.T.d.A.R.R.; Caputo, D.; Oliver-Williams, C. A Systematic Review of the Association Between Vegan Diets and Risk of Cardiovascular Disease. J. Nutr. 2021, 151, 1539–1552. [Google Scholar] [CrossRef] [PubMed]
- Walldius, G.; de Faire, U.; Alfredsson, L.; Leander, K.; Westerholm, P.; Malmström, H.; Ivert, T.; Hammar, N. Long-Term Risk of a Major Cardiovascular Event by ApoB, ApoA-1, and the ApoB/ApoA-1 Ratio-Experience from the Swedish AMORIS Cohort: A Cohort Study. PLoS Med. 2021, 18, e1003853. [Google Scholar] [CrossRef]
- Thompson, A.; Danesh, J. Associations between Apolipoprotein B, Apolipoprotein AI, the Apolipoprotein B/AI Ratio and Coronary Heart Disease: A Literature-Based Meta-Analysis of Prospective Studies. J. Intern. Med. 2006, 259, 481–492. [Google Scholar] [CrossRef]
- Huang, T.; Yang, B.; Zheng, J.; Li, G.; Wahlqvist, M.L.; Li, D. Cardiovascular Disease Mortality and Cancer Incidence in Vegetarians: A Meta-Analysis and Systematic Review. Ann. Nutr. Metab. 2012, 60, 233–240. [Google Scholar] [CrossRef]
- Orlich, M.J.; Singh, P.N.; Sabaté, J.; Jaceldo-Siegl, K.; Fan, J.; Knutsen, S.; Beeson, W.L.; Fraser, G.E. Vegetarian Dietary Patterns and Mortality in Adventist Health Study 2. JAMA Intern. Med. 2013, 173, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J.; Mangels, A.R. American Dietetic Association Position of the American Dietetic Association: Vegetarian Diets. J. Am. Diet. Assoc. 2009, 109, 1266–1282. [Google Scholar] [CrossRef] [PubMed]
- Tonstad, S.; Stewart, K.; Oda, K.; Batech, M.; Herring, R.P.; Fraser, G.E. Vegetarian Diets and Incidence of Diabetes in the Adventist Health Study-2. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-Y.; Huang, C.-C.; Hu, F.B.; Chavarro, J.E. Vegetarian Diets and Weight Reduction: A Meta-Analysis of Randomized Controlled Trials. J. Gen. Intern. Med. 2016, 31, 109–116. [Google Scholar] [CrossRef]
- Rosi, A.; Mena, P.; Pellegrini, N.; Turroni, S.; Neviani, E.; Ferrocino, I.; Di Cagno, R.; Ruini, L.; Ciati, R.; Angelino, D.; et al. Environmental Impact of Omnivorous, Ovo-Lacto-Vegetarian, and Vegan Diet. Sci. Rep. 2017, 7, 6105. [Google Scholar] [CrossRef]
- Appleby, P.N.; Key, T.J. The Long-Term Health of Vegetarians and Vegans. Proc. Nutr. Soc. 2016, 75, 287–293. [Google Scholar] [CrossRef]
- Satija, A.; Bhupathiraju, S.N.; Spiegelman, D.; Chiuve, S.E.; Manson, J.E.; Willett, W.; Rexrode, K.M.; Rimm, E.B.; Hu, F.B. Healthful and Unhealthful Plant-Based Diets and the Risk of Coronary Heart Disease in US Adults. J. Am. Coll. Cardiol. 2017, 70, 411–422. [Google Scholar] [CrossRef]
- Romanos-Nanclares, A.; Willett, W.C.; Rosner, B.A.; Collins, L.C.; Hu, F.B.; Toledo, E.; Heather Eliassen, A. Healthful and Unhealthful Plant-Based Diets and Risk of Breast Cancer in U.S. Women: Results from the Nurses’ Health Studies. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1921–1931. [Google Scholar] [CrossRef]
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.E.; Borgi, L.; Willett, W.C.; Manson, J.E.; Sun, Q.; Hu, F.B. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016, 13, e1002039. [Google Scholar] [CrossRef]
- Baden, M.Y.; Shan, Z.; Wang, F.; Li, Y.; Manson, J.E.; Rimm, E.B.; Willett, W.C.; Hu, F.B.; Rexrode, K.M. Quality of Plant-Based Diet and Risk of Total, Ischemic, and Hemorrhagic Stroke. Neurology 2021, 96, e1940–e1953. [Google Scholar] [CrossRef]

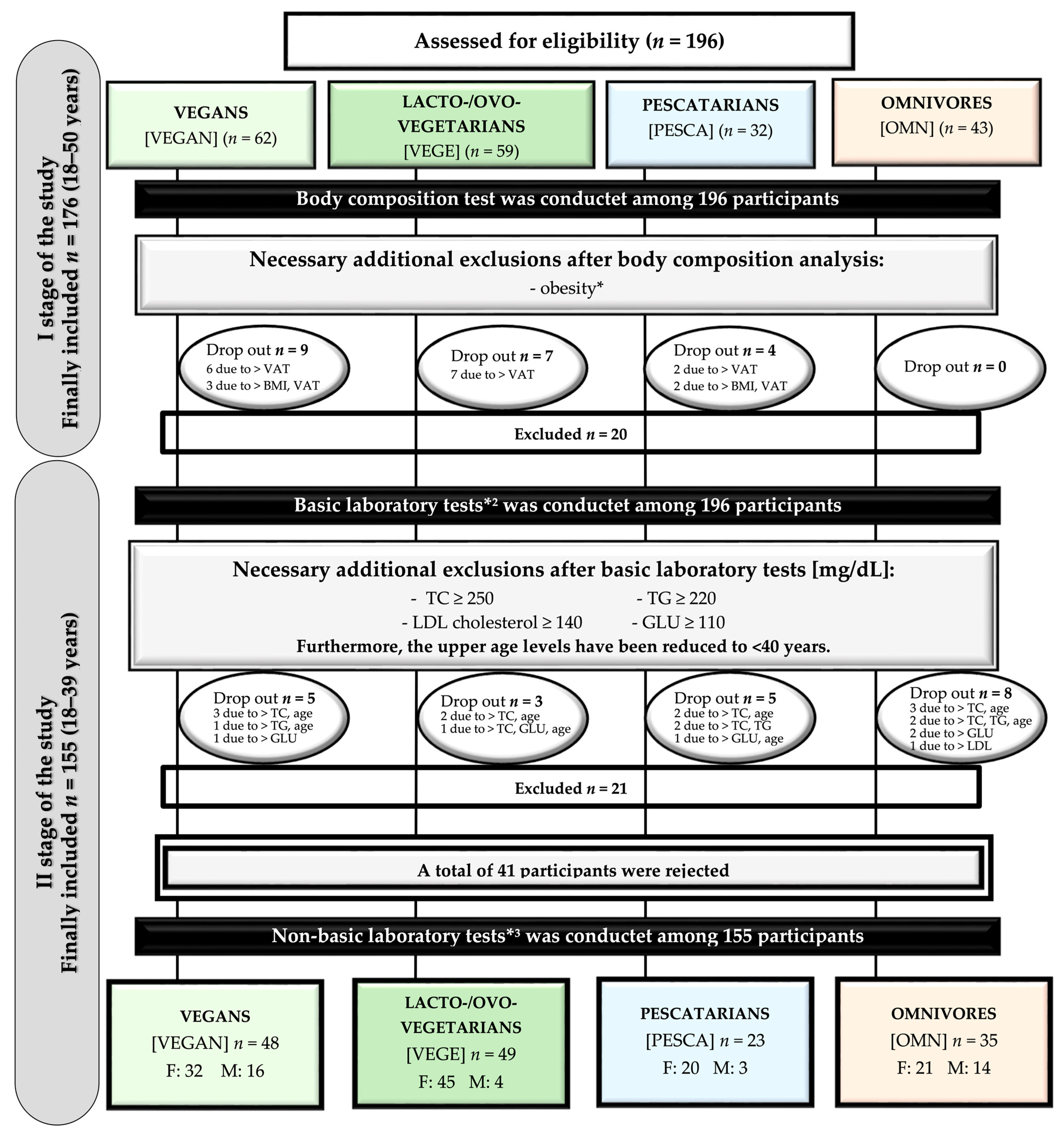
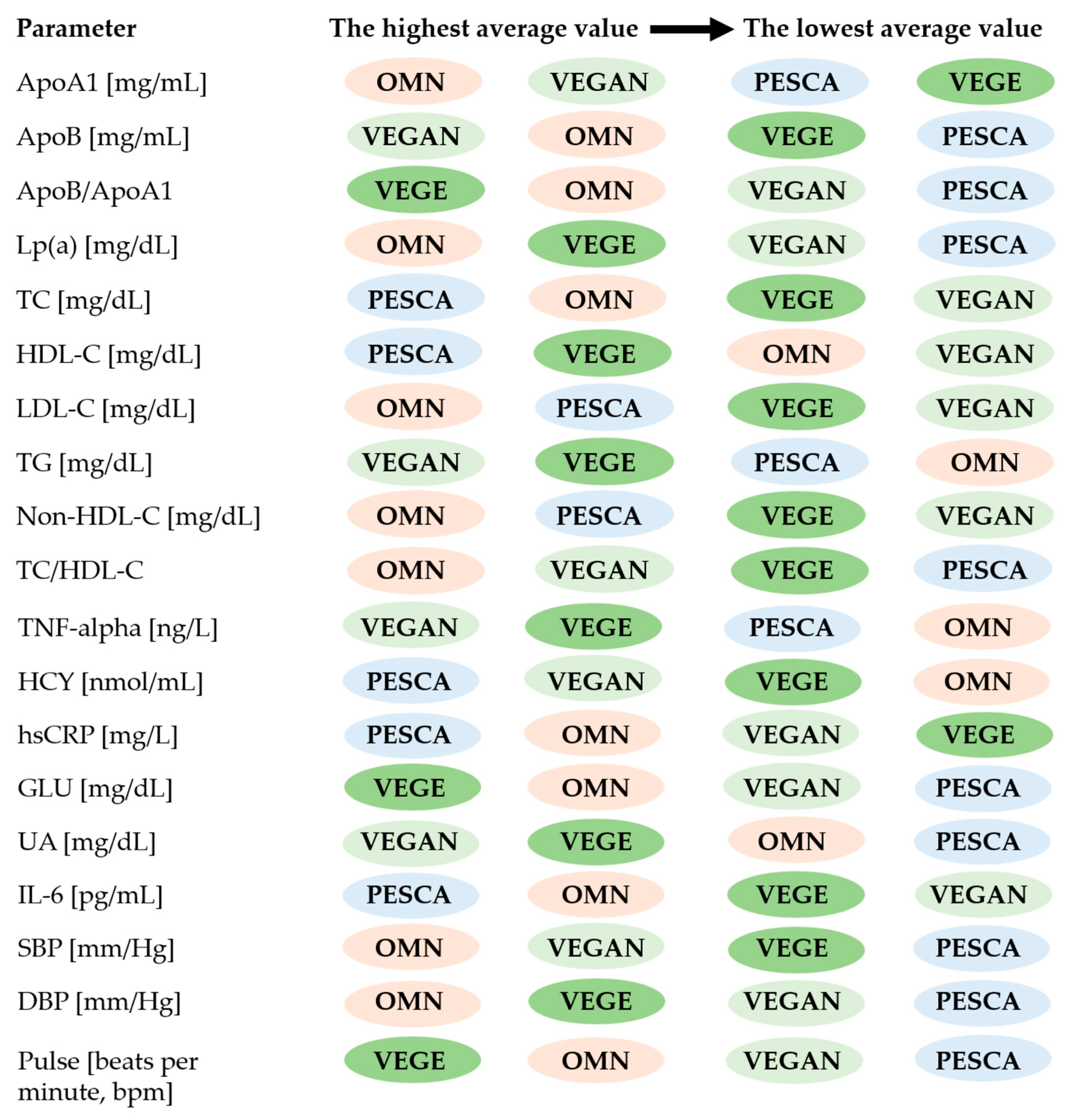
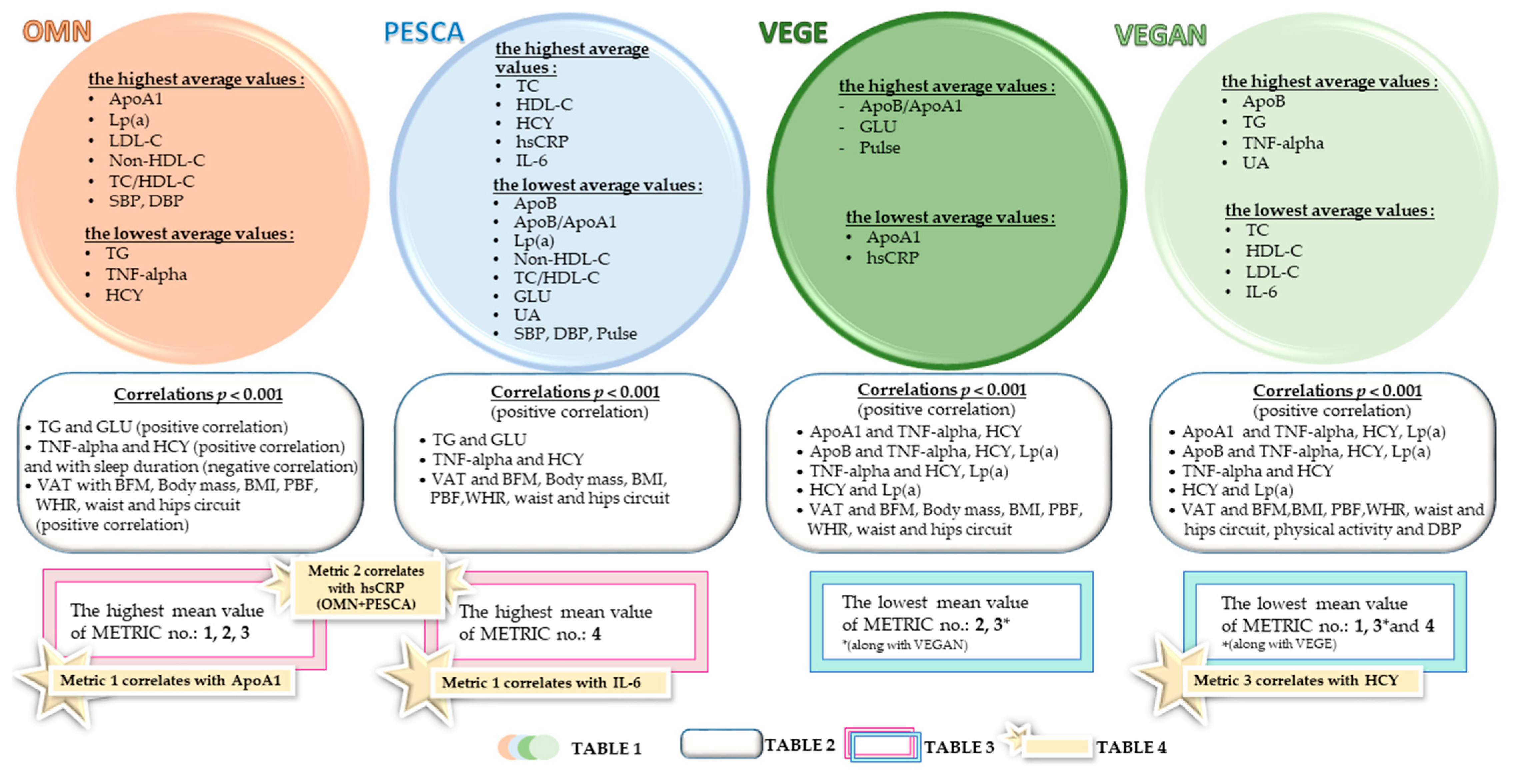
| Variables | Sex | Studied Groups n = 155 | p * | |||
| OMN n = 35 F/M: 21/14 %F 60.0 | PESCA n = 23 F/M: 20/3 %F 86.9 | VEGE n = 49 F/M: 45/4 %F 91.8 | VEGAN n = 48 F/M: 32/16 %F 66.7 | |||
| Blood lipids and lipoproteins mean ± SD (min–max) | ||||||
| ApoA1 [mg/mL] | All | 2.14 ± 1.77 (0.458–6.11) | 1.56 ± 1.09 (0.459–4.26) | 1.30 ± 0.948 (0.248–5.69) | 1.80 ± 1.87 (0.269–7.26) | 0.380 |
| F | 2.17 ± 1.78 (0.548–6.11) | 1.64 ± 1.15 (0.459–4.26) | 1.33 ± 0.980 (0.248–5.69) | 1.92 ± 2.01 (0.459–7.26) | 0.561 | |
| M | 2.09 ± 1.83 (0.458–6.11) | 1.05 ± 0.151 (0.875–1.15) | 0.946 ± 0.317 (0.615–1.35) | 1.55 ± 1.59 (0.269–6.11) | 0.608 | |
| ApoB [mg/mL] | All | 1.05 ± 0.744 (0.252–3.36) | 0.957 ± 0.495 (0.381–2.04) | 1.04 ± 0.700 (0.252–3.96) | 1.15 ± 0.983 (0.252–3.96) | 0.983 |
| ApoB/ApoA1 | F | 0.914 ± 1.28 (0.0656–7.34) | 0.871 ± 0.811 (0.115– 4.05) | 0.944 ± 0.684 (0.186–4.01) | 0.900 ± 1.06 (0.0730–6.85) | 0.107 |
| M | 0.763 ± 0.680 (0.0656–2.82) | 0.873 ± 0.859 (0.115–4.05) | 0.975 ± 0.702 (0.207–4.01) | 0.805 ± 0.669 (0.0730–4.07) | 0.172 | |
| All | 1.14 ± 1.87 (0.0667–7.34) | 0.852 ± 0.461 (0.545–1.38) | 0.600 ± 0.290 (0.186–0.862) | 1.09 ± 1.60 (0.337–6.85) | 0.820 | |
| Lp(a) [mg/dL] | All | 23.8 ± 18.0 (5.80–74.1) | 19.1 ± 16.1 (6.62–79.2) | 23.6 ± 17.4 (2.70–81.1) | 22.5 ± 17.6 (9.30–71.4) | 0.404 |
| TC [mg/dL] | All | 158 ± 30.3 (86–207) | 160 ± 34.7 (93–218) | 157 ± 22.8 (113–220) | 142 ± 29.2 (71–206) | 0.032 |
| HDL–C [mg/dL] | All | 55.8 ± 14.3 (36–92) | 60.6 ± 13.0 (41–94) | 59.3 ± 13.4 (26–94) | 51.7 ± 10.1 (31–72) | 0.006 |
| F | 60.8 ± 15.6 (36–92) | 61.9 ± 13.0 (44–94) | 60.7 ± 12.9 (26–94) | 53.4 ± 10.6 (31–72) | 0.055 | |
| M | 48.4 ± 8.04 (37–62) | 52.0 ± 11.0 (41–63) | 43.8 ± 6.99 (37–52) | 48.3 ± 8.43 (35–70) | 0.607 | |
| LDL–C [mg/dL] | All | 80.1 ± 20.9 (30–113) | 75.1 ± 24.8 (39.6–136) | 74.1 ± 18.2 (32–139) | 70.0 ± 21.0 (21–111) | 0.133 |
| TG [mg/dL] | All | 77.9 ± 34.7 (33–202) | 92.4 ± 45.9 (44–217) | 94.0 ± 27.2 (49–153) | 95.1 ± 26.4 (41–165) | 0.005 |
| Non–HDL–C [mg/dL] | All | 102 ± 27.9 (47–153) | 99.6 ± 35.0 (39–155) | 98.1 ± 19.9 (64–158) | 90.1 ± 25.9 (28–149) | 0.297 |
| TC/HDL–C | All | 2.94 ± 0.778 (1.74–5.03) | 2.73 ± 0.698 (1.54–3.78) | 2.76 ± 0.613 (1.83–4.96) | 2.79 ± 0.596 (1.65–4.37) | 0.746 |
| Other blood parameters mean ± SD (min–max) | ||||||
| HCY [nmol/mL] | All | 10.0 ± 5.53 (4.10–33.7) | 15.3 ± 8.78 (5.87–33.7) | 12.8 ± 8.17 (5.07–37.9) | 14.3 ± 10.9 (4.06–37.9) | 0.096 |
| TNF–alpha [ng/L] | All | 89.1 ± 39.2 (52.8–193 | 111 ± 49.1 (54.1–203) | 121 ± 83.8 (51.5–368) | 128 ± 90.6 (52.8–368) | 0.149 |
| hsCRP [mg/L] | All | 5.19 ± 3.01 (0.564–11.2) | 5.47 ± 3.45 (1.12–15.7) | 3.83 ± 2.50 (0.675–9.65) | 4.76 ± 3.07 (0.962–13.7) | 0.100 |
| GLU [mg/dL] | All | 88.1 ± 10.3 (60.1–107) | 85.4 ± 9.49 (65.8–98) | 88.4 ± 6.04 (75.2–101) | 87.7 ± 8.19 (59.3–105) | 0.658 |
| UA [mg/dL] | All | 4.41 ± 1.40 (1.46–6.77) | 4.21 ± 1.52 (1.29–8.64) | 4.54 ± 0.877 (2.76–7.22) | 4.85 ± 0.986 (2.57–7.28) | 0.057 |
| IL–6 [pg/mL] | All | 3.05 ± 1.87 (0.367–6.91) | 3.16 ± 2.40 (0.131–7.09) | 2.87 ± 2.31 (0.341–8.54) | 2.79 ± 2.27 (0.341–8.12) | 0.833 |
| Blood pressure and pulse mean ± SD (min–max) | ||||||
| SBP [mm/Hg] | All | 122 ± 14.5 (97–153) | 116 ± 12.2 (95–143) | 118 ± 9.68 (99–140) | 119 ± 11.1 (96–153) | 0.439 |
| F | 117 ± 12.3 (97–151) | 115 ± 12.6 (95–143) | 118 ± 9.47 (99–140) | 116 ± 9.99 (96–136) | 0.490 | |
| M | 129 ± 14.9 (107–153) | 122 ± 6.03 (116–128) | 121 ± 13.2 (104–136) | 125 ± 11.6 (104–153) | 0.788 | |
| DBP [mm/Hg] | All | 76 ± 8.27 (60–96) | 71.9 ± 7.83 (57–82) | 75.4 ± 6.62 (60–92) | 74.5 ± 8.51 (52–89) | 0.376 |
| F | 75.4 ± 8.81 (60–96) | 71.0 ± 7.89 (57–82) | 75.3 ± 6.73 (60–92) | 74.5 ± 8.45 (52–86) | 0.303 | |
| M | 76.9 ± 7.63 (66–89) | 77.7 ± 5.13 (72–82) | 75.8 ± 6.13 (70–82) | 74.4 ± 8.90 (62–89) | 0.727 | |
| Pulse [bpm] | All | 78.0 ± 15.7 (46–121) | 74.6 ± 14.5 (56–104) | 82.5 ± 13.5 (52–117) | 77.0 ± 14.0 (40–114) | 0.053 |
| F | 80.8 ± 13.7 (51–112) | 75.0 ± 14.8 (56–104) | 82.6 ± 13.6 (52–117) | 80.3 ± 15.1 (40–114) | 0.229 | |
| M | 73.8 ± 17.9 (46–121) | 71.3 ± 15.5 (56–87) | 81.5 ± 13.7 (61–89) | 70.7 ± 9.00 (53–83) | 0.359 | |
| Group | The Strongest Correlations between the Studied Parameters p < 0.001 |
|---|---|
| OMN (for detailed data, see Table S2) |
|
| PESCA (for detailed data, see Table S3) |
|
| VEGE (for detailed data, see Table S4) |
|
| VEGAN (for detailed data, see Table S5) |
|
| METRIC 1 | METRIC 2 | METRIC 3 | METRIC 4 | |
|---|---|---|---|---|
| Parameters included in metrics |
|
|
|
|
| GROUP | Mean ± SD | |||
| p-value | ||||
| OMN | 1.71 ± 1.23 | 0.571 ± 0.698 | 1.97 ± 1.40 | 1.86 ± 1.29 |
| PESCA | 1.57 ± 1.24 | 0.435 ± 0.590 | 1.91 ± 1.62 | 1.87 ± 1.52 |
| VEGE | 1.45 ± 1.17 | 0.327 ± 0.474 | 1.69 ± 1.19 | 1.61 ± 1.20 |
| VEGAN | 1.42 ± 1.16 | 0.354 ±0.483 | 1.69 ± 1.24 | 1.54 ± 1.05 |
| p *1 | 0.723 | 0.388 | 0.757 | 0.619 |
| OMN + PESCA | 1.66 ± 1.22 | 0.517 ± 0.655 | 1.95 ± 1.48 | 1.86 ± 1.37 |
| VEGE + VEGAN | 1.43 ± 1.16 | 0.340 ± 0.476 | 1.69 ± 1.21 | 1.58 ± 1.13 |
| p *2 | 0.155 | 0.061 | 0.170 | 0.103 |
| Group | ||
|---|---|---|
| Metric | Parameter | p ** |
| OMN | ||
| METRIC 1 (group L = 0–1 group H > 1) * | ApoA1 [mg/mL] H 1.809 vs. L 2.632 median: H 0.945 vs. L 1.797 | 0.028 |
| PESCA | ||
| METRIC 1 (group L = 0–1 group H > 1) * | IL-6 [pg/mL] H 3.988 vs. L 2.401 median: H 4.098 vs. L 1.888 | 0.046 |
| VEGAN | ||
| METRIC 3 (group L = 0–1 group H > 1) * | HCY [nmol/mL] H 16.444 vs. L 12.009 median: H 10.624 vs. L 8.611 | 0.050 |
| OMN + PESCA | ||
| METRIC 2 (group L = 0 group H > 0) * | hsCRP [mg/L] H 6.269 H vs. L 4.509 median: H 5.942 vs. L 4.216 | 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiatkowska, I.; Olszak, J.; Brożek, A.; Blacha, A.; Nowicki, M.; Maćkowiak, K.; Formanowicz, P.; Formanowicz, D. Is It Feasible to Predict Cardiovascular Risk among Healthy Vegans, Lacto-/Ovo-Vegetarians, Pescatarians, and Omnivores under Forty? Int. J. Environ. Res. Public Health 2023, 20, 2237. https://doi.org/10.3390/ijerph20032237
Kwiatkowska I, Olszak J, Brożek A, Blacha A, Nowicki M, Maćkowiak K, Formanowicz P, Formanowicz D. Is It Feasible to Predict Cardiovascular Risk among Healthy Vegans, Lacto-/Ovo-Vegetarians, Pescatarians, and Omnivores under Forty? International Journal of Environmental Research and Public Health. 2023; 20(3):2237. https://doi.org/10.3390/ijerph20032237
Chicago/Turabian StyleKwiatkowska, Izabela, Jakub Olszak, Alicja Brożek, Anna Blacha, Marcin Nowicki, Kalina Maćkowiak, Piotr Formanowicz, and Dorota Formanowicz. 2023. "Is It Feasible to Predict Cardiovascular Risk among Healthy Vegans, Lacto-/Ovo-Vegetarians, Pescatarians, and Omnivores under Forty?" International Journal of Environmental Research and Public Health 20, no. 3: 2237. https://doi.org/10.3390/ijerph20032237
APA StyleKwiatkowska, I., Olszak, J., Brożek, A., Blacha, A., Nowicki, M., Maćkowiak, K., Formanowicz, P., & Formanowicz, D. (2023). Is It Feasible to Predict Cardiovascular Risk among Healthy Vegans, Lacto-/Ovo-Vegetarians, Pescatarians, and Omnivores under Forty? International Journal of Environmental Research and Public Health, 20(3), 2237. https://doi.org/10.3390/ijerph20032237







