Oral Health-Related Quality of Life in Different Subtypes of Ehlers-Danlos Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Recruitment and Study Design
2.2. Eligibility Criteria
2.3. Data Collection
2.4. Statistical Methods
- Primary outcome
- OHIP score
- Co-factors
- General patient information
- ■
- Gender
- ■
- Age
- ■
- Country
- ■
- Membership in a support group
- ■
- Comorbidities
- Diagnosis
- ■
- Subtype
- ■
- Age at the time of diagnosis
- ■
- The time between the first symptoms and diagnosis
- Oral health data
- ■
- The annual number of dentists visits
- ■
- Involvement of the oral cavity
- ■
- Number of lost teeth
- ■
- Effectiveness of local anesthetics
3. Results
3.1. Participants
3.2. EDS-Type
3.3. OHRQoL
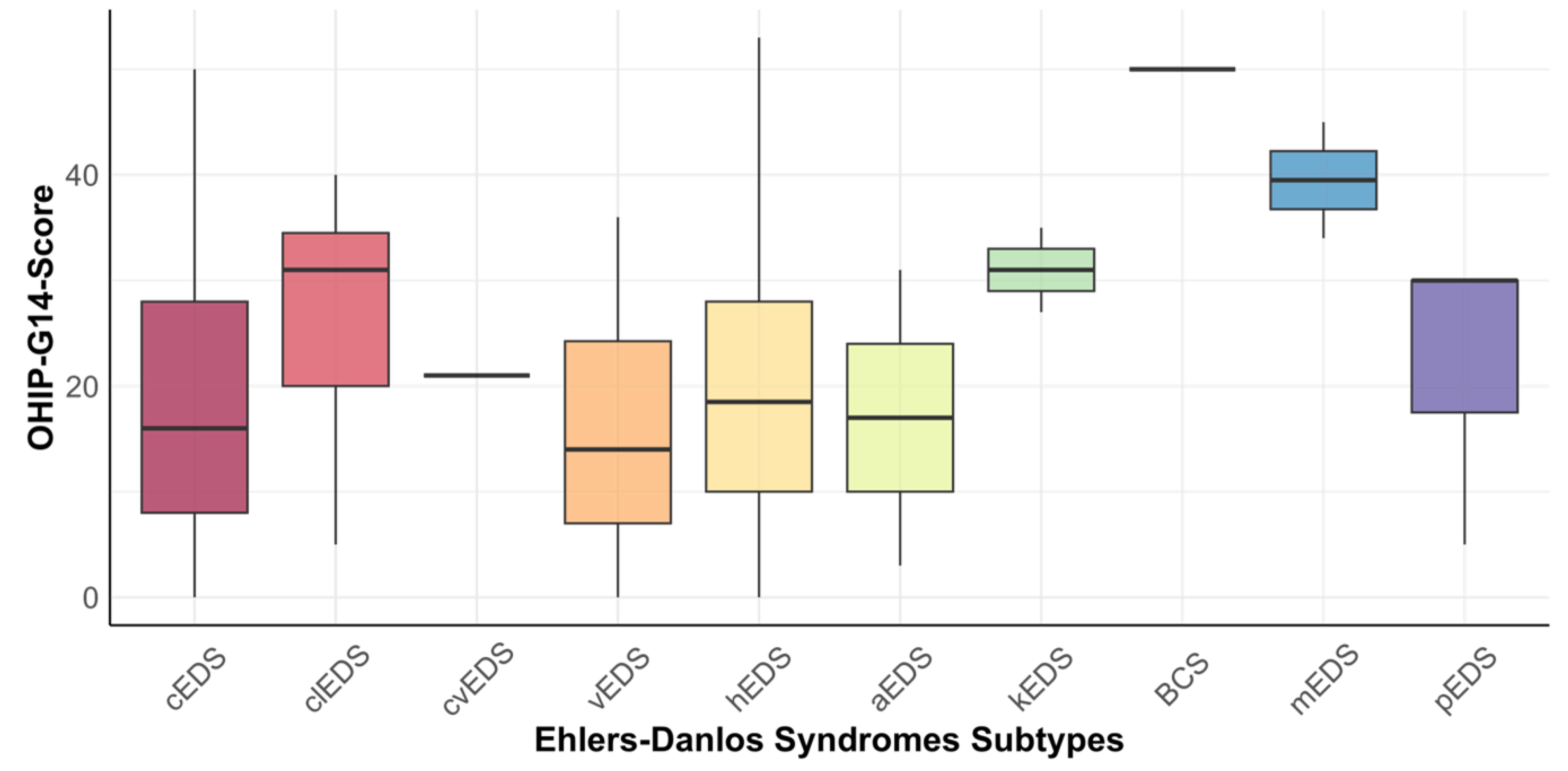
3.4. Relationship between OHIP-14 and EDS Subtypes
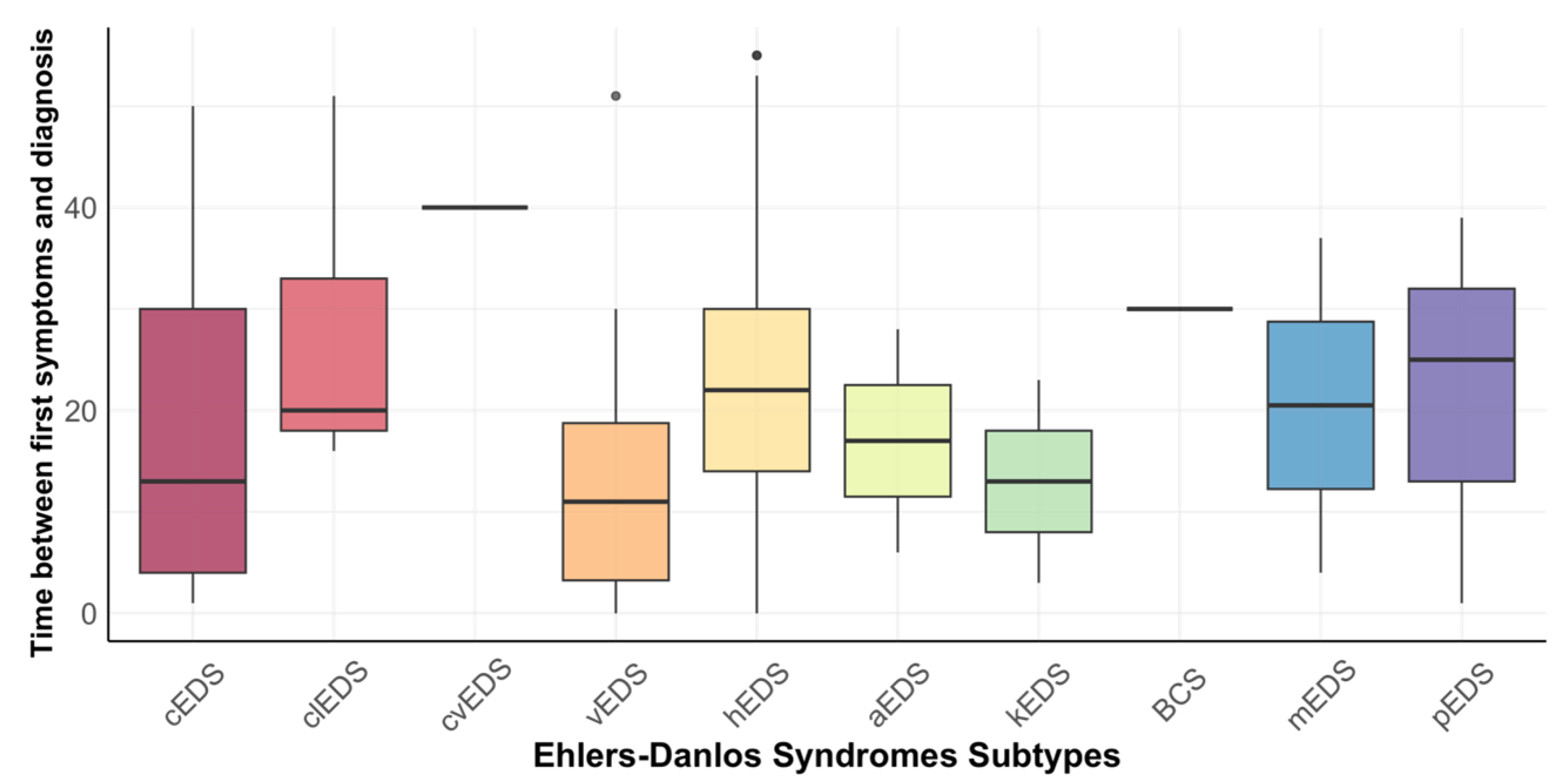
3.5. Time of Diagnosis and Involvement of the Oral Cavity
3.6. Membership of a Support Group
3.7. Is There a Difference in the Frequency of Dental Visits between Subtypes?
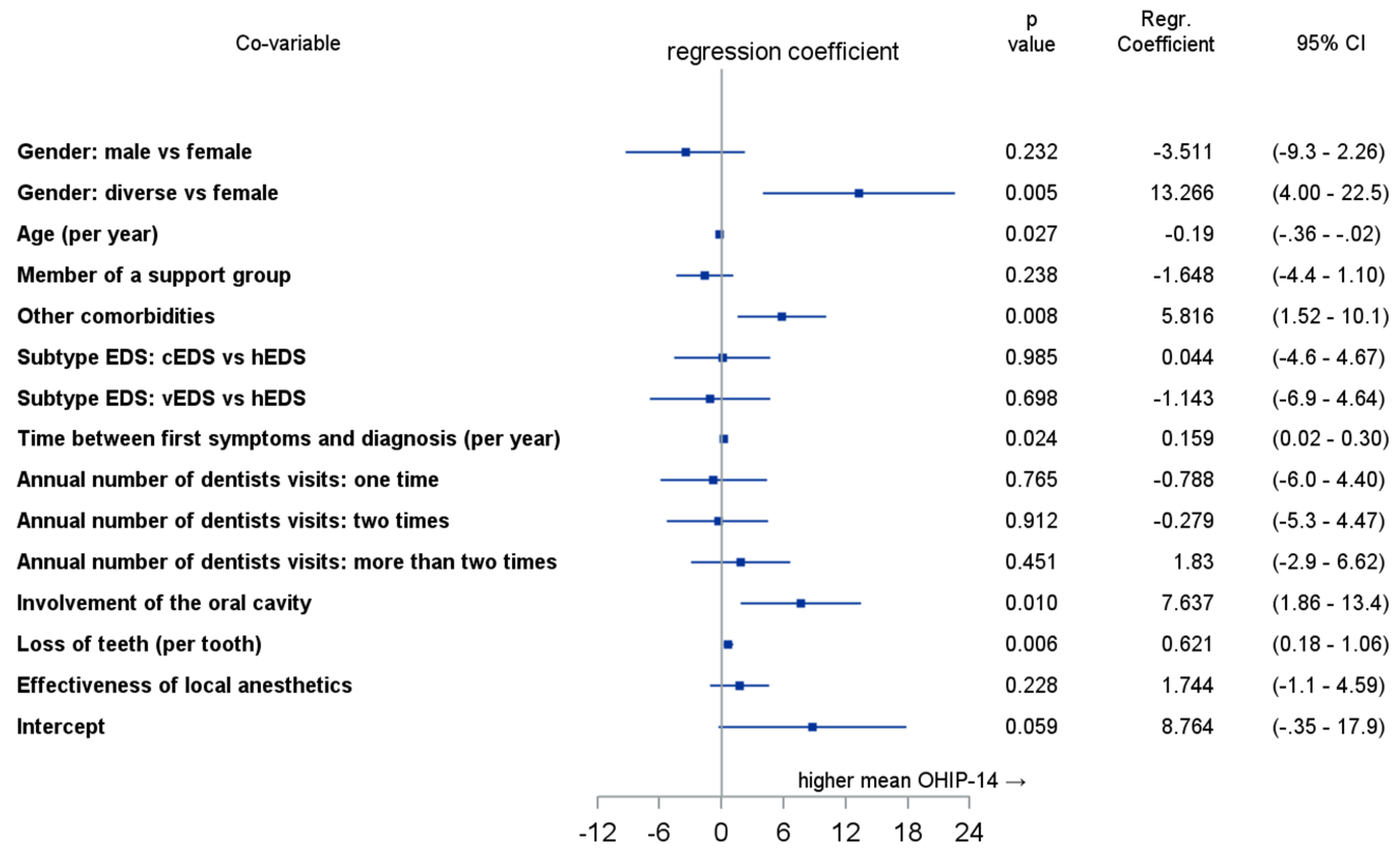
3.8. Multivariate Analysis
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vanakker, O.; Callewaert, B.; Malfait, F.; Coucke, P. The Genetics of Soft Connective Tissue Disorders. Annu. Rev. Genom. Hum. Genet. 2015, 16, 229–255. [Google Scholar] [CrossRef] [PubMed]
- Commission of the European Communities Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions on Rare Diseases: Europe’s Challenges. Available online: https://ec.europa.eu/health/ph_threats/non_com/docs/rare_com_en.pdf (accessed on 16 August 2022).
- Malfait, F.; Francomano, C.; Byers, P.; Belmont, J.; Berglund, B.; Black, J.; Bloom, L.; Bowen, J.M.; Brady, A.F.; Burrows, N.P.; et al. The 2017 International Classification of the Ehlers-Danlos Syndromes. Am. J. Med. Genet. 2017, 175, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Beighton, P.; de Paepe, A.; Danks, D.; Finidori, G.; Gedde-Dahl, T.; Goodman, R.; Hall, J.G.; Hollister, D.W.; Horton, W.; McKusick, V.A.; et al. International Nosology of Heritable Disorders of Connective Tissue, Berlin, 1986. Am. J. Med. Genet. 1988, 29, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, C.; Berglund, B.; Korpe, L.; Andersson-Norinder, J. Ehlers-Danlos Syndrome (EDS) Focusing on Oral Symptoms: A Questionnaire Study. Orthod. Craniofac. Res. 2004, 7, 178–185. [Google Scholar] [CrossRef]
- Demmler, J.C.; Atkinson, M.D.; Reinhold, E.J.; Choy, E.; Lyons, R.A.; Brophy, S.T. Diagnosed Prevalence of Ehlers-Danlos Syndrome and Hypermobility Spectrum Disorder in Wales, UK: A National Electronic Cohort Study and Case–Control Comparison. BMJ Open 2019, 9, e031365. [Google Scholar] [CrossRef]
- Brady, A.F.; Demirdas, S.; Fournel-Gigleux, S.; Ghali, N.; Giunta, C.; Kapferer-Seebacher, I.; Kosho, T.; Mendoza-Londono, R.; Pope, M.F.; Rohrbach, M.; et al. The Ehlers-Danlos Syndromes, Rare Types. Am. J. Med. Genet. 2017, 175, 70–115. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, B.; Royce, P.M.; Superti-Furga, A. The Ehlers-Danlos Syndrome. In Connective Tissue and Its Heritable Disorders; Royce, P.M., Steinmann, B., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; pp. 431–523. ISBN 978-0-471-25185-9. [Google Scholar]
- Mitakides, J.E. The Effect of Ehlers-Danlos Syndromes on TMJ Function and Craniofacial Pain. CRANIO® 2018, 36, 71–72. [Google Scholar] [CrossRef]
- Kapferer-Seebacher, I.; Schnabl, D.; Zschocke, J.; Pope, F. Dental Manifestations of Ehlers-Danlos Syndromes: A Systematic Review. Acta Derm. Venerol. 2020, 100, 152–160. [Google Scholar] [CrossRef]
- Kapferer-Seebacher, I.; Lundberg, P.; Malfait, F.; Zschocke, J. Periodontal Manifestations of Ehlers-Danlos Syndromes: A Systematic Review. J. Clin. Periodontol. 2017, 44, 1088–1100. [Google Scholar] [CrossRef] [PubMed]
- Lepperdinger, U.; Zschocke, J.; Kapferer-Seebacher, I. Oral Manifestations of Ehlers-Danlos Syndromes. Am. J. Med. Genet. 2021, 187, 520–526. [Google Scholar] [CrossRef]
- Mitakides, J.; Tinkle, B.T. Oral and Mandibular Manifestations in the Ehlers-Danlos Syndromes. Am. J. Med. Genet. 2017, 175, 220–225. [Google Scholar] [CrossRef]
- Berglund, B.; Nordström, G.; Lützén, K. Living a Restricted Life with Ehlers-Danlos Syndrome (EDS). Int. J. Nurs. Stud. 2000, 37, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, M.; Wiemann, S.; Bohner, L.; Kleinheinz, J.; Jung, S. Association between Oral Health-Related Quality of Life in People with Rare Diseases and Their Satisfaction with Dental Care in the Health System of the Federal Republic of Germany. Int. J. Environ. Res. Public Health 2018, 15, 1732. [Google Scholar] [CrossRef]
- Oelerich, O.; Kleinheinz, J.; Reissmann, D.R.; Köppe, J.; Hanisch, M. Correlation between Oral Health-Related Quality of Life and Objectively Measured Oral Health in People with Ehlers–Danlos Syndromes. Int. J. Environ. Res. Public Health 2020, 17, 8243. [Google Scholar] [CrossRef] [PubMed]
- Berglund, B.; Björck, E. Women with Ehlers-Danlos Syndrome Experience Low Oral Health-Related Quality of Life. J. Orofac. Pain 2012, 26, 307–314. [Google Scholar] [PubMed]
- John, M.T.; Miglioretti, D.L.; LeResche, L.; Koepsell, T.D.; Hujoel, P.; Micheelis, W. German Short Forms of the Oral Health Impact Profile. Commun. Dent. Oral. Epidemiol. 2006, 34, 277–288. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Erlbaum Associates: Hillsdale, NJ, USA, 1988; ISBN 978-0-8058-0283-2. [Google Scholar]
- John, M.; Omara, M.; Su, N.; List, T.; Sekulic, S.; Häggman-Henrikson, B.; Visscher, C.; Bekes, K.; Reissmann, D.; Baba, K.; et al. Recommendations for use and scoring of oral health impact profile versions. J. Evid. Based Dent. Pract. 2021, 22, 101619. [Google Scholar] [CrossRef] [PubMed]
- John, M.T.; Micheelis, W.; Biffar, R. Reference values in oral health-related quality of life for the abbreviated version of the Oral Health Impact Profile. Schweiz Mon. Zahnmed 2004, 114, 784–791. [Google Scholar]
- John, M.T.; Micheelis, W. Mundgesundheitsbezogene Lebensqualität in Der Bevölkerung: Grundlagen Und Ergebnisse Des Oral Health Impact Profile (OHIP) Aus Einer Repräsentativen Stichprobe in Deutschland; IDZ-Information 1/2003; IDZ, Institut der Deutschen Zahnärzte: Köln, Germany, 2003; p. 28. [Google Scholar]
- Reissmann, D.R.; Sierwald, I.; Heydecke, G.; John, M.T. Interpreting One Oral Health Impact Profile Point. Health Qual. Life Outcomes 2013, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, M.; Hoffmann, T.; Bohner, L.; Hanisch, L.; Benz, K.; Kleinheinz, J.; Jackowski, J. Rare Diseases with Periodontal Manifestations. Int. J. Environ. Res. Public Health 2019, 16, 867. [Google Scholar] [CrossRef]
- Rinner, A.; Zschocke, J.; Schossig, A.; Gröbner, R.; Strobl, H.; Kapferer-Seebacher, I. High Risk of Peri-Implant Disease in Periodontal Ehlers-Danlos Syndrome. A Case Series. Clin. Oral. Impl. Res. 2018, 29, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Arendt-Nielsen, L.; Kaalund, S.; Bjerring, P.; Høgsaa, B. Insufficient Effect of Local Analgesics in Ehlers Danlos Type III Patients (Connective Tissue Disorder). Acta Anaesthesiol. Scand. 1990, 34, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Hakim, A.J.; Grahame, R.; Norris, P.; Hopper, C. Local Anaesthetic Failure in Joint Hypermobility Syndrome. J. R. Soc. Med. 2005, 98, 84–85. [Google Scholar] [CrossRef] [PubMed]
- Schmitt-Sausen, N. Tag Der Seltenen Erkrankungen: Mehr Aufmerksamkeit Für Waisenkinder Der Medizin. Medizin. Dtsch. Arztebl. 2010, 107, A430. [Google Scholar]
- Kühne, A.; Kleinheinz, J.; Jackowski, J.; Köppe, J.; Hanisch, M. Study to Investigate the Knowledge of Rare Diseases among Dentists, Orthodontists, Periodontists, Oral Surgeons and Craniomaxillofacial Surgeons. Int. J. Environ. Res. Public Health 2020, 18, 139. [Google Scholar] [CrossRef]
- Benz, K.; Trapp, R.; Voss, M.; Hanisch, M.; Geisthoff, U.; Jackowski, J. Awareness and Knowledge of Rare Diseases in German Dentists, Dental Specialists and Oral and Maxillofacial Surgeons: A Country-Wide Survey. Medicina 2022, 58, 1114. [Google Scholar] [CrossRef] [PubMed]
- Osica, P.; Janas, A. Dental Problems in a Patient with the Classic Type of Ehlers-Danlos Syndrome—A Case Report. Dev. Period. Med. 2015, 19, 496–502. [Google Scholar]
- Bohner, L.; Wiemann, S.; Jung, S.; Kleinheinz, J.; Hanisch, M. Oral Health-Related Quality of Life in Rare Diseases Associated with Oral Symptoms, Diagnostic Delay, and Sex. Bundesgesundheitsblatt Gesundh. Gesundh. 2019, 62, 1406–1411. [Google Scholar] [CrossRef]
- Krizek, C.; Roberts, C.; Ragan, R.; Ferrara, J.J.; Lord, B. Gender and Cancer Support Group Participation. Cancer Pract. 1999, 7, 86–92. [Google Scholar] [CrossRef]
- Mo, P.K.H.; Malik, S.H.; Coulson, N.S. Gender Differences in Computer-Mediated Communication: A Systematic Literature Review of Online Health-Related Support Groups. Patient Educ. Couns. 2009, 75, 16–24. [Google Scholar] [CrossRef]
- Cella, D.F.; Yellen, S.B. Cancer Support Groups: The State of the Art. Cancer Pract. 1993, 1, 56–61. [Google Scholar] [PubMed]
- Hanisch, M.; Sielker, S.; Jung, S.; Kleinheinz, J.; Bohner, L. Self-Assessment of Oral Health-Related Quality of Life in People with Ectodermal Dysplasia in Germany. Int. J. Environ. Res. Public Health 2019, 16, 1933. [Google Scholar] [CrossRef] [PubMed]
| Mean (SD) | Range | Classical EDS | Classical-Like EDS | Cardiac-Valvular EDS | Vascular EDS | Hypermobile EDS | Arthrochalasia EDS | Kyphoscoliotic EDS | Brittle Cornea Syndrome | Myopathic EDS | Periodontal EDS | p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Count (%) | 29 (9.8%) | 7 (2.4%) | 1 (0.3%) | 18 (6.1%) | 230 (78.0%) | 2 (0.7%) | 2 (0.7%) | 1 (0.3%) | 2 (0.7%) | 3 (1.0%) | |||
| Age 1 | 39.2 (±11.2) | 18–65 | 43.0 (±12.4) | 45.7 (±11.1) | 53 | 40.1 (±13.0) | 38.2 (±10.7) | 34,0 (±8.5) | 41.5 (±14.8) | 63 | 51.0 (±14.1) | 41.7 (± 1.5) | 0.129 |
| Gender 2 | 0.465 | ||||||||||||
| men | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (5.6%) | 16 (7.0%) | 1 (50.0%) | 1 (50.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |||
| women | 29 (100%) | 7 (100.0%) | 1 (100.0%) | 16 (88.9%) | 209 (90.9%) | 1 (50.0%) | 1 (50.0%) | 1 (100.0%) | 2 (100.0%) | 3 (100.0%) | |||
| diverse | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (5.6%) | 5 (2.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |||
| Country 2 | 0.214 | ||||||||||||
| Germany | 26 (89.7%) | 7 (100.0%) | 1 (100.0%) | 14 (77.8%) | 211 (91.7%) | 2 (100.0%) | 2 (100.0%) | 1 (100.0%) | 2 (100.0%) | 3 (100.0%) | |||
| Austria | 2 (6.9%) | 0 (0.0%) | 0 (0.0%) | 4 (22.2%) | 5 (2.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |||
| Switzerland | 1 (3.4%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 14 (6.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Oral Function | Orofacial Pain | Orofacial Appearance | Psychosocial Impact | OHIP-G14 Score | |
|---|---|---|---|---|---|
| Classical EDS (n = 29) | 2.41 (±2.40) | 3.83 (±2.76) | 3.12 (±2.05) | 1.86 (±2.08) | 18.03 (±12.93) |
| Classik-like EDS (n = 7) | 3.29 (±1.98) | 6.14 (±2.27) | 4.86 (±2.54) | 2.86 (±2.48) | 26.43 (±12.83) |
| Cardiac-valvular EDS (n = 1) | 1.00 | 2.00 | 6.00 | 3.00 | 21.00 |
| Vascular EDS (n = 18) | 1.61 (±2.17) | 3.17 (±2.43) | 2.83 (±2.26) | 2.00 (±1.91) | 15.17 (±11.56) |
| Hypermobile EDS (n = 230) | 2.59 (±2.26) | 4.30 (±2.15) | 3.70 (±2.31) | 1.82 (±1.83) | 19.47 (±11.97) |
| Arthrochalasia EDS (n = 2) | 1.00 (±1.41) | 2.50 (±2.12) | 2.50 (±3.54) | 2.50 (±3.54) | 17.00 (±19.80) |
| Kyphoscoliotic EDS (n = 2) | 4.50 (±3.54) | 6.5 (±2.12) | 4.50 (±0.71) | 3.00 (±0.00) | 31.00 (±5.66) |
| Brittle Cornea Syndrome (n = 1) | 6.00 | 8.00 | 8.00 | 6.00 | 50.00 |
| Myopathic EDS (n = 2) | 7.50 (±0.71) | 7.50 (±0.71) | 8.00 (±0.00) | 5.00 (±2.83) | 39.50 (±7.78) |
| Periodontal EDS (n = 3) | 2.00 (±2.65) | 4.67 (±1.53) | 5.00 (±2.65) | 2.67 (±2.52) | 21.67 (±14.43) |
| Total | 2.56 (±2.31) | 4.26 (±2.28) | 3.72 (±2.32) | 1.92 (±1.91) | 19.56 (±12.29) |
| cEDS | clEDS | cvEDS | vEDS | hEDS | aEDS | kEDS | BCS | mEDS | pEDS | |
|---|---|---|---|---|---|---|---|---|---|---|
| cEDS | – | 8.39 (−2.65–19.44) | 2.97 (−23.97–29.90) | −2.87 (−10.38–4.64) | 1.44 (−3.25–6.12) | −1.03 (−20.81–18.74) | 12.97 (−6.09–32.02) | 31.97 (5.03–58.90) | 21.47 (2.35–40.58) | 3.63 (−12.51–19.78) |
| clEDS | −0.65 (−1.49–0.19) | – | −5.43 (−38.99–28.13) | −11.26 (−22.23–−0.30) | −6.96 (−16.02–2.10) | −9.43 (−36.05–17.19) | 4.57 (−18.31–27.45) | 23.57 (−9.99–57.13) | 13.07 (−10.13–36.27) | −4.76 (−25.85–16.32) |
| cvEDS | −0.23 (−2.22–1.77) | 0.42 (−1.70–2.51) | – | −5.83 (−30.88–19.22) | −1.53 (−25.16–22.10) | −4.00 (−312.11–304.11) | −10.00 (−78.03–98.03) | 29.00 | 18.50 (−102.54–139.54) | 0.67 (−71.04–72.39) |
| vEDS | 0.23 (−0.36–0.82) | 0.95 (0.02–1.85) | 0.51 (−1.52–2.52) | – | 4.30 (−1.45–10.06) | 1.83 (−17.21–20.88) | 15.83 (−1.88–33.54) | 34.83 (9.78–59.88) | 24.33 (6.51–42.15) | 6.50 (−9.02–22.02) |
| hEDS | −0.12 (−0.51–0.27) | 0.58 (−0.17–1.33) | 0.13 (−1.84–2.09) | −0.36 (−0.84–0.12) | – | −2.47 (−19.28–14.34) | 11.53 (−5.19–28.25) | 30.53 (5.90–54.16) | 20.03 (3.31–36.76) | −2.20 (−11.53–15.93) |
| aEDS | 0.08 (−1.36–1.51) | 0.67 (−0.96–2.26) | 0.20 (−2.25–2.57) | −0.15 (−1.61–1.31) | 0.21 (−1.19–1.60) | – | 14.00 (−48.65–76.65) | 33.00 (−275.11–341.11) | 22.50 (−42.22–87.22) | 4.67 (−43.03–52.36) |
| kEDS | −1.02 (−2.47–0.45) | −0.38 (−1.95–1.22) | −1.77 (−4.82–1.56) | −1.40 (−2.91–0.15) | −0.97 (−2.36–0.43) | −0.96 (−3.02–1.27) | – | 19.00 (−69.03–107.03) | 8.50 (−20.76–37.76) | −9.33 (−44.86–26.20) |
| BCS | −2.47 (−4.55–−0.36) | −1.84 (−4.11–0.55) | n.a. | −3.01 (−5.23–−0.73) | −2.55 (−4.53–−0.57) | −1.67 (−4.64–1.59) | −3.36 (−8.01–1.19) | – | −10.50 (−131.54–110.54) | −28.33 (−100.04–43.38) |
| mEDS | −1.68 (−3.16–−0.17) | −1.07 (−2.70–0.63) | −2.38 (−6.00–1.40) | −2.14 (−3.73–−0.50) | −1.68 (−3.07–−0.27) | −1.50 (−3.78–0.99) | −1.25 (−3.42–1.11) | 1.35 (−1.69–4.08) | – | −17.83 (−54.47–18.81) |
| pEDS | −0.28 (−1.47–0.91) | 0.36 (−1.02–1.71) | 0.05 (−2.30–2.22) | −0.55 (−1.77–0.70) | −0.18 (−1.32–0.96) | −0.28 (−2.07–1.54) | 0.76 (−1.17–2.59) | 1.96 (−1.03–4.73) | 1.41 (−0.75–3.43) | – |
| Classical EDS | Classical-Like EDS | Cardiac-Valvular EDS | Vascular EDS | Hypermobile EDS | Arthrochalasia EDS | Kyphoscoliotic EDS | Brittle Cornea Syndrome | Myopathic EDS | Periodontal EDS | p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time of diagnosis 1 | 27.7 (±18.5) | 43.1 (±12.2) | 43 | 33.6 (±12.6) | 34.9 (10.8) | 31.5 (±7.8) | 24.5 (±6.4) | 58 | 48.0 (±15.6) | 27.3 (±21.1) | 0.086 |
| Time between first symptoms and diagnosis 1 | 19.8 (±17.2) | 27.0 (±13.3) | 40 | 13.3 (±13.0) | 22.5 (±11.8) | 17.0. (±15.6)) | 13.0 (±14.1) | 30 | 20.5 (±23.3) | 21.7 (±19.2) | 0.058 |
| Involvement of the oral cavity | 0.004 | ||||||||||
| yes | 24 (82.8%) | 6 (85.7%) | 1 (100.0%) | 13 (72.2%) | 222 (96.5%) | 2 (100.0%) | 2 (100.0%) | 1 (100.0%) | 2 (100.0%) | 3 (100.0%) | |
| no | 5 (17.2%) | 1 (14.3%) | 0 (0.0%) | 5 (27.8%) | 8 (3.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balke, J.; Bohner, L.; Köppe, J.; Jackowski, J.; Oelerich, O.; Hanisch, M. Oral Health-Related Quality of Life in Different Subtypes of Ehlers-Danlos Syndrome. Int. J. Environ. Res. Public Health 2023, 20, 2218. https://doi.org/10.3390/ijerph20032218
Balke J, Bohner L, Köppe J, Jackowski J, Oelerich O, Hanisch M. Oral Health-Related Quality of Life in Different Subtypes of Ehlers-Danlos Syndrome. International Journal of Environmental Research and Public Health. 2023; 20(3):2218. https://doi.org/10.3390/ijerph20032218
Chicago/Turabian StyleBalke, Julius, Lauren Bohner, Jeanette Köppe, Jochen Jackowski, Ole Oelerich, and Marcel Hanisch. 2023. "Oral Health-Related Quality of Life in Different Subtypes of Ehlers-Danlos Syndrome" International Journal of Environmental Research and Public Health 20, no. 3: 2218. https://doi.org/10.3390/ijerph20032218
APA StyleBalke, J., Bohner, L., Köppe, J., Jackowski, J., Oelerich, O., & Hanisch, M. (2023). Oral Health-Related Quality of Life in Different Subtypes of Ehlers-Danlos Syndrome. International Journal of Environmental Research and Public Health, 20(3), 2218. https://doi.org/10.3390/ijerph20032218








