Infestation of Oriental House Rat (Rattus tanezumi) with Chigger Mites Varies along Environmental Gradients across Five Provincial Regions of Southwest China
Abstract
1. Introduction
2. Materials and Methods
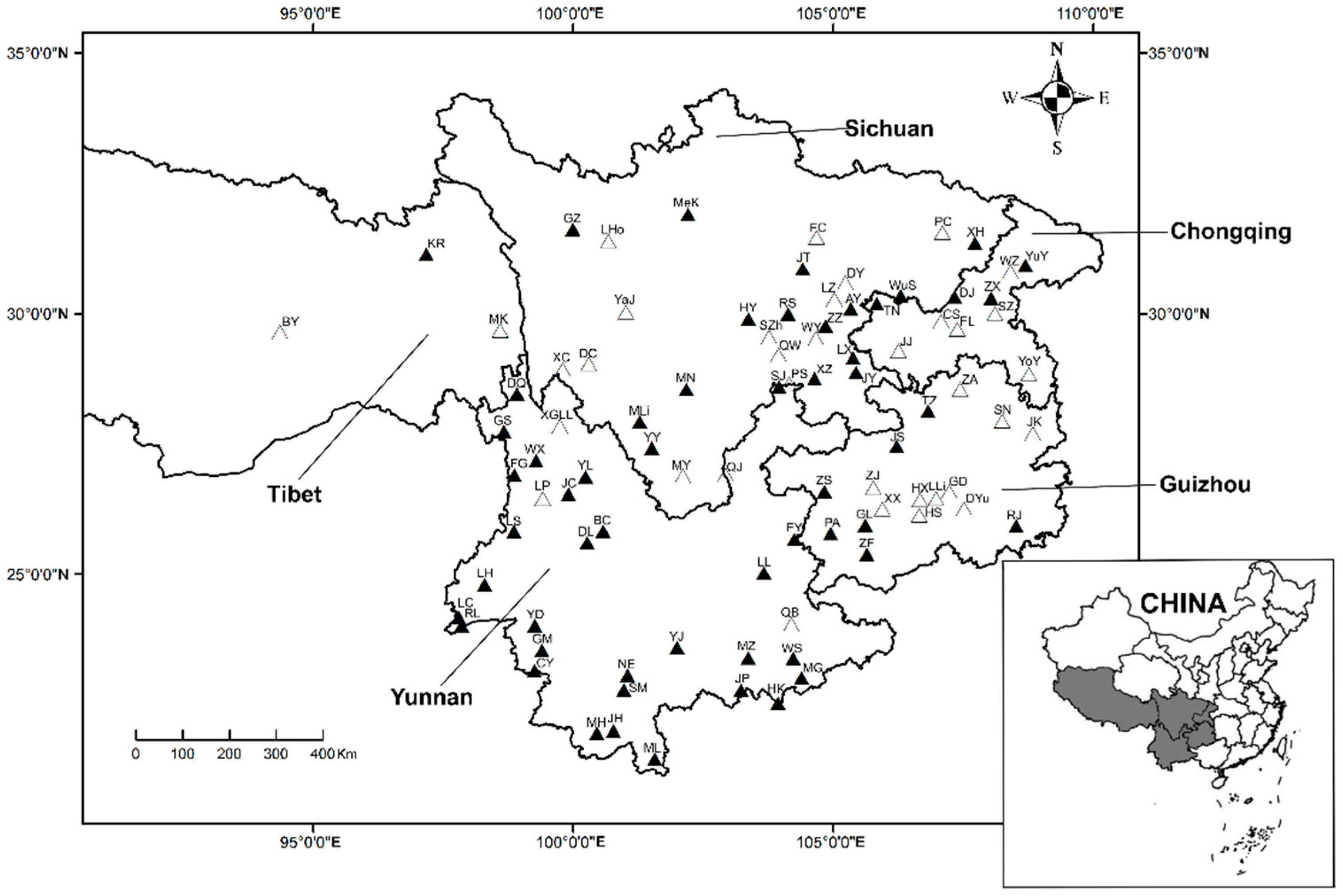
2.1. Field Investigations and Sampling of Chigger Mites
2.2. Statistical Analysis
3. Results
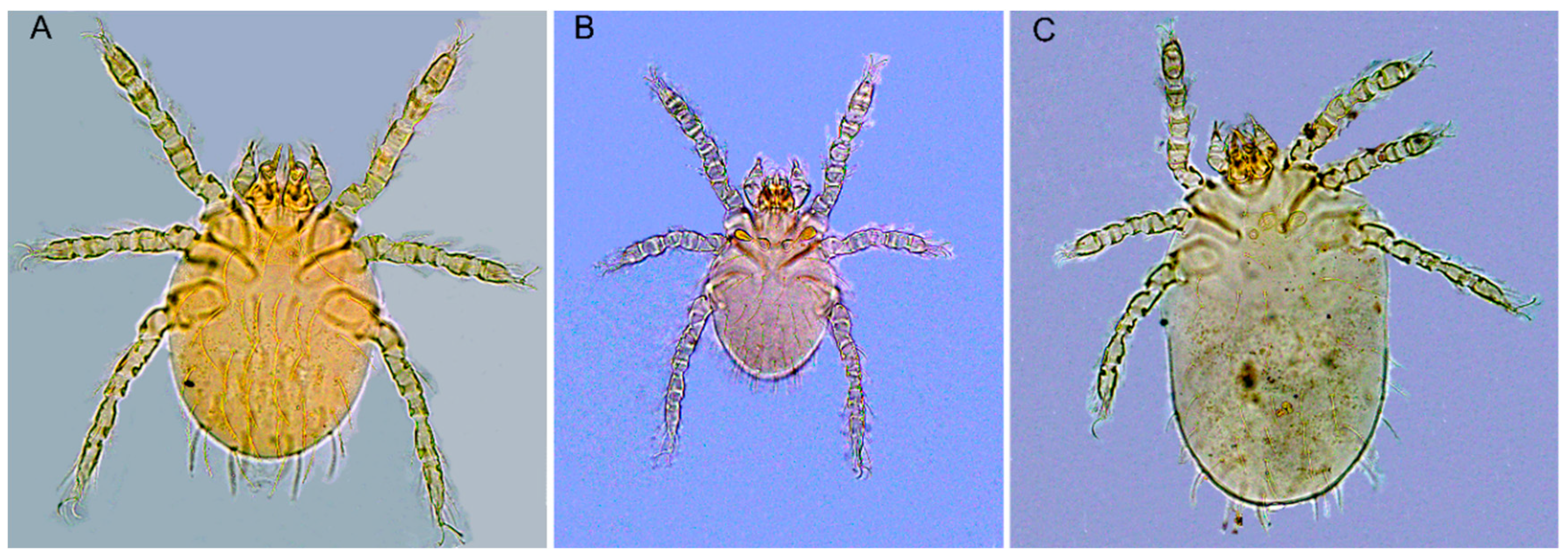
3.1. Sampling and Identification of Chigger Mites on R. tanezumi
3.2. Overall Infestation and Dominant Species of Chiggers on R. tanezumi
3.3. Variation in Chigger Infestation along Environmental Gradients
4. Discussion
4.1. Overall Infestation of Chiggers on R. tanezumi in Southwest China
4.2. Dominant Chigger Species on R. tanezumi in Southwest China
4.3. Variations of Chigger Infestation on R. tanezumi along Different Environmental Gradients
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| The 91 Survey Sites | No. of Rattus tanezumi | No. of Chiggers | The 91 Survey Sites | No. of R. tanezumi | No. of Chiggers | The 91 Survey Sites | No. of Rattus tanezumi | No. of Chiggers | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site No. | Site Abbreviations | Site Names | Site No. | Site Abbreviations | Site Names | Site No. | Site Abbreviations | Site Names | ||||||
| 1 | AY | Anyue | 1 | 0 | 32 | JY | Jiangyang (Luzhou city) | 2 | 0 | 63 | SM | Simao | 32 | 61 |
| 2 | BC | Binchuan | 2 | 0 | 33 | KR | Karuo (Changdu city) | 5 | 0 | 64 | SN | Sinan | 0 | 0 |
| 3 | BY | Bayi (Linzhi city) | 0 | 0 | 34 | LC | Longchuan | 687 | 1317 | 65 | SZ | Shizhu | 0 | 0 |
| 4 | CS | Changshou | 0 | 0 | 35 | LH | Lianghe | 61 | 474 | 66 | SZh | Shizhong (Leshan city) | 0 | 0 |
| 5 | CY | Cangyuan | 2 | 0 | 36 | LHo | Luhuo | 0 | 0 | 67 | TN | Tongnan | 5 | 0 |
| 6 | DC | Daocheng | 0 | 0 | 37 | LL | Luliang | 94 | 16 | 68 | TZ | Tongzhi | 2 | 0 |
| 7 | DJ | Dianjiang | 5 | 0 | 38 | LLi | Longli | 0 | 0 | 69 | WS | Wenshan | 4 | 2 |
| 8 | DL | Dali | 198 | 92 | 39 | LP | Lanping | 0 | 0 | 70 | WuS | Wusheng | 10 | 1 |
| 9 | DQ | Deqin | 52 | 15 | 40 | LS | Lushui | 188 | 1022 | 71 | WX | Weixi | 34 | 189 |
| 10 | DY | Daying | 0 | 0 | 41 | LX | Luxian | 1 | 0 | 72 | WY | Weiyuan | 0 | 0 |
| 11 | DYu | Duyun | 0 | 0 | 42 | LZ | Lezhi | 0 | 0 | 73 | WZ | Wanzhou | 0 | 0 |
| 12 | FC | Fucheng (Mianyang city) | 0 | 0 | 43 | MEK | Maerkang | 7 | 100 | 74 | XC | Xiangcheng | 0 | 0 |
| 13 | FG | Fugong | 25 | 1 | 44 | MG | Maguan | 19 | 0 | 75 | XGLL | Xianggelila | 0 | 0 |
| 14 | FL | Fuling | 0 | 0 | 45 | MH | Menghai | 219 | 40 | 76 | XH | Xuanhan | 1 | 0 |
| 15 | FY | Fuyuan | 59 | 11 | 46 | MK | Mangkang | 0 | 0 | 77 | XX | Xixiu (Anshun city) | 0 | 0 |
| 16 | GD | Guiding | 0 | 0 | 47 | ML | Mengla | 21 | 142 | 78 | XZ | Xuzhou (Yibin city) | 4 | 0 |
| 17 | GL | Guanling | 5 | 0 | 48 | MLi | Muli | 1 | 9 | 79 | YaJ | Yajiang | 0 | 0 |
| 18 | GM | Gengma | 140 | 330 | 49 | MN | Mianning | 1 | 0 | 80 | YD | Yongde | 21 | 9 |
| 19 | GS | Gongshan | 2 | 0 | 50 | MY | Miyi | 0 | 0 | 81 | YJ | Yuanjiang | 143 | 1840 |
| 20 | GZ | Ganzi | 5 | 18 | 51 | MZ | Mengzi | 8 | 0 | 82 | YL | Yulong | 19 | 37 |
| 21 | HK | Hekou | 46 | 568 | 52 | NE | Ninger | 22 | 151 | 83 | YoY | Youyang | 0 | 0 |
| 22 | HS | Huishui | 0 | 0 | 53 | PA | Puan | 1 | 0 | 84 | YuY | Yunyang | 1 | 0 |
| 23 | HX | Huaxi (Guiyang city) | 0 | 0 | 54 | PC | Pingchang | 0 | 0 | 85 | YY | Yanyuan | 15 | 1200 |
| 24 | HY | Hongya | 1 | 0 | 55 | PS | Pingshan | 0 | 0 | 86 | ZA | Zhengan | 0 | 0 |
| 25 | JC | Jianchuan | 12 | 0 | 56 | QB | Qiubei | 0 | 0 | 87 | ZF | Zhenfeng | 1 | 0 |
| 26 | JH | Jinghong | 472 | 10,330 | 57 | QJ | Qiaojia | 0 | 0 | 88 | ZJ | Zhijin | 0 | 0 |
| 27 | JJ | Jiangjin | 0 | 0 | 58 | QW | Qianwei | 0 | 0 | 89 | ZS | Zhongshan (Liupanshui city) | 1 | 0 |
| 28 | JK | Jiangkou | 0 | 0 | 59 | RJ | Rongjiang | 64 | 1906 | 90 | ZX | Zhongxian | 1 | 0 |
| 29 | JP | Jinping | 23 | 168 | 60 | RL | Ruili | 152 | 404 | 91 | ZZ | Zizhong | 1 | 0 |
| 30 | JS | Jinsha | 5 | 0 | 61 | RS | Renshou | 3 | 0 | |||||
| 31 | JT | Jintang | 9 | 0 | 62 | SJ | Suijiang | 4 | 0 | |||||
| Names of Chigger Species | Chigger Individuals | Names of Chigger Species | Chigger Individuals | Names of Chigger Species | Chigger Individuals |
|---|---|---|---|---|---|
| Leptotrombidium scutellare (Nagayo et al., 1921) | 57 | L. saltuosum Yu et al., 1982 | 1 | Lorillatum flagellasensilla Wang and Song, 1992 | 39 |
| L. sinicum Yu et al., 1981 | 11 | L. guzhangense Wang et al., 1985 | 1 | L. tungshihensis Hus and Chen, 1964 | 3 |
| L. eothenomydis Yu and Yang, 1986 | 22 | L. turdicola Vercammen-Grandjean and Langston, 1976 | 152 | Microtrombicula munda (Gater, 1932) | 309 |
| L. hiemale Yu et al., 1982 | 1 | L. shuyui Wen et al., 1984 | 5 | M. vitosa Schluger et al., 1963 | 1 |
| L. cricethrionis Wen et al., 1984 | 115 | L. cangjiangense (Yu et al., 1981) | 7 | Neotrombicula wendai Wen and Wu, 1984 | 1 |
| L. shuqui Wen and Xiang, 1984 | 3 | L. fujianense Liao and Wang, 1983 | 57 | Chiroptella anhuiensis Chen et al., 1980 | 7 |
| L. wangi Yu et al., 1986 | 11 | L. hengdun Wu and Wen, 1984 | 1 | Eutrombicula hirsti (Sambon, 1927) | 49 |
| L. densipunctatum Yu et al., 1982 | 39 | L. sialkotense Vercammen-Grandjean and Langston, 1976 | 59 | E. wichmanni (Oudemans, 1905) | 16 |
| L. yongshengense Yu and Yang, 1986 | 165 | L. kunmingense (Wen and Xiang, 1984) | 2 | Euschoengastia alpina Sasa and Jameson, 1954 | 1 |
| L. yui (Chen and Hsu, 1955) | 3 | L. nyctali Wen and Sun, 1984 | 5 | Walchiella notiala Yu et al., 1981 | 44 |
| L. deliense (Walch, 1922) | 6297 | L. ushi Yu et al., 1986 | 2 | W. yingjiangensis Wen et al., 1984 | 5 |
| L. xiaguanense Yu et al., 1981 | 4 | L. rupestre Traub and Nadchatram, 1967 | 13 | Walchia parapacifica (Chen et al., 1955) | 26 |
| L. rubellum Wang and Liao, 1984 | 663 | L. yunlingense Yu and Zhang, 1981 | 35 | W. micropelta (Traub and Evans, 1957) | 1150 |
| L. imphalum Vercammen-Grandjean and Langston, 1975 | 877 | Trombiculindus yunnanus Wang and Yu, 1965 | 3 | W. chinensis (Chen and Hsu, 1955) | 358 |
| L. gongshanense Yu et al., 1981 | 78 | T. hylomydis Wang and Yu, 1965 | 1 | W. koi (Chen and Hsu, 1957) | 71 |
| L. spicanisetum Yu et al., 1986 | 21 | T. nujiange Wen and Xiang, 1984 | 4 | W. minuscuta Chen, 1978 | 12 |
| L. cuonae Wang et al., 1996 | 5 | Helenicula kohlsi (Philip and Woodward, 1964) | 175 | W. turmalis (Gater, 1932) | 151 |
| L. yuebeiense Zhao, 1982 | 5 | H. abaensis Wang et al., 1984 | 2 | W. nanfangis Wen and Xiang, 1984 | 7 |
| L. deplanoscutum Yu et al., 1981 | 53 | H. comate (Womersley, 1952) | 1 | W. enode Gater, 1932 | 32 |
| L. lianghense Yu et al., 1983 | 8 | H. myospalacis Huang, 1986 | 8 | W. zangnanica Wu and Wen, 1984 | 4 |
| L. quadrifurcatum Xiang and Wen, 1984 | 3 | H. miyagawai (Sasa kunada and Miura, 1951) | 23 | W. chuanica Wen and Song, 1984 | 20 |
| L. wenense Wu et al., 1982 | 6 | H. aulacochaeta Sun et al., 1986 | 1 | W. erana(Traub and Evans, 1957) | 1 |
| L. xiaowei Xiang and Wen, 1984 | 19 | H. rattihaikonga (Hsu and Chen, 1957) | 1 | W. xishaensis Zhao et al., 1986 | 33 |
| L. chuanxi Wen et al., 1984 | 1 | H. globularis (Walch, 1927) | 16 | W. latiscuta Wang et al., 1987 | 1 |
| L. sheshui Xiang and Wen, 1984 | 35 | H. olsufjevi (Schulger, 1955) | 4 | W. rustica (Gater, 1932) | 8 |
| L. qujingense Yu et al., 1981 | 145 | H. simena (Hsu and Chen, 1957) | 228 | W. ewingi (Fuller, 1949) | 1766 |
| L. dianchi Wen and Xiang, 1984 | 4 | H. hsui Zhao, 1990 | 72 | W. jiangxiense Wang and Song, 1981 | 1 |
| L. jinmai Wen and Xiang, 1984 | 1 | H. lanius (Radford, 1946) | 39 | Gahrliepia meridionalis Yu et al., 1980 | 2 |
| L. akamushi (Barumpt, 1910) | 108 | Doloisia. sinensis (Liang and Huang, 1959) | 1 | G. zhongwoi Wen and Xiang, 1984 | 22 |
| L. alpinum Yu and Yang, 1986 | 8 | D. brachypus (Audy and Nadchatram, 1957) | 3 | G. longipedalis Yu and Yang, 1986 | 18 |
| L. pallidum (Nagayo et al., 1919) | 5 | D. manipurensis (Radford, 1946) | 1 | G. silvatica Yu and Yang, 1982 | 3 |
| L. robustisetum Yu et al., 1983 | 4 | D. furcipelta Yu et al., 1983 | 1 | G. banyei Wen and Xiang, 1984 | 2 |
| L. bawangense Zhao, 1982 | 3 | Cheladonta micheneri Wen and Xiang, 1984 | 6 | G radiopunctata Hsu et al., 1965 | 8 |
| L. biluoxueshanense Yu et al., 1982 | 5 | Ascoschoengastia indica (Hirst, 1915) | 4483 | G. octosetosa Chen et al., 1956 | 3 |
| L. kawamurai (Fukuzumi and Obata, 1953) | 2 | A. yunwui Yu et al., 1984 | 136 | G. latiscutata Chen and Fan, 1981 | 3 |
| L. dongluoense Wang et al., 1981 | 1 | A. petauristae Yu et al., 1984 | 23 | G. lengshui Wen and Xiang, 1984 | 1 |
| L. dichotogalium Xiang et Wen, 1986 | 2 | A. yunnanensis Yu et al., 1980 | 94 | G. yangchenensis Chen and Hsu, 1957 | 11 |
| L. hupeicum (Ma and Hsu, 1965) | 1 | A. leechi (Domrow, 1962) | 321 | G. yunnanensis Hsu et al., 1965 | 3 |
| L. apodemi Wen and Sun, 1984 | 1 | A. audyi Womersley, 1952 | 3 | G. agrariusia Hus et al., 1965 | 1 |
| L. kiangsuense Chen, 1975 | 1 | A. montana Yu et al., 1980 | 1 | G. lamella Chen et al., 1980 | 4 |
| L. intermedium (Nagayo et al., 1920) | 1 | A. menghaiensis Yu et al., 1986 | 766 | G. madun Wen and Xiang, 1984 | 1 |
| L. rectanguloscutum (Hsu and Chen, 1964) | 2 | A. sifanga Wen et al., 1984 | 77 | G. fimbriata Traub and Morrow, 1955 | 17 |
| L. longchuanense Yu et al., 1981 | 12 | A. spindalis Wen and Wu, 1984 | 58 | Schoengastiella ligula Radford, 1946 | 362 |
| L. qiui Yu et al., 1986 | 1 | A. latyshevi Schluger, 1955 | 87 | S. himalayana Wu and Wen, 1984 | 2 |
| L. filasensillum Wang and Song, 1982 | 1 | A. minheensis Yang, 1992 | 1 | S. paraconfusiana Wang and Gu, 1983 | 1 |
| L. yigongense Wu and Wen,1984 | 2 | Herpetacarus aristoclavus Yu et al., 1979 | 1 | Chatia maoyi Wen and Xiang, 1984 | 14 |
| L. kitasatoi (Fukuzum and Obata, 1950) | 1 | H. limon (Wen and Xiang, 1984) | 3 | C. acrichela Wen et al., 1984 | 4 |
| L. bishanense Yu et al., 1986 | 36 | H. tengchongensis Yu et al., 1980 | 2 | Odontacarus yosanoi Fukuzumi and Obata, 1953 | 1 |
| L. ejingshanense Yu et al., 1982 | 2 | H. spinosetosus Wang et al., 1980 | 3 | O. tetrasetosus Yu and Yang, 1986 | 12 |
| L. baoshui Wen and Xiang, 1984 | 1 | H. hastoclavus Yu et al., 1979 | 1 | Total: 2 families, 19 Genus, 149 species, and 20,453 individuals | |
References
- Nielsen, D.H.; Robbins, R.G.; Rueda, L.M. Annotated world checklist of the Trombiculidae and Leeuwenhoekiidae (1758–2021) (Acari: Trombiculoidea), with notes on nomenclature, taxonomy, and distribution. Zootaxa 2021, 4967, 1243. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.H., Jr.; McKeever, S.; Pound, J.M. Parasitism of larval Ixodes ticks by chigger mites and fed female Ornithodoros ticks by Ornithodoros males. J. Parasitol. 1986, 72, 811–812. [Google Scholar] [CrossRef] [PubMed]
- Moniuszko, H.; Makol, J. Chigger mites (Actinotrichida: Parasitengona, Trombiculidae) of Poland. An updated distribution and hosts. Ann. Parasitol. 2014, 60, 103–117. [Google Scholar] [PubMed]
- Santibáñez, P.; Palomar, A.M.; Portillo, A.; Santibáñez, S.; Oteo, J.A. The role of chiggers as human pathogens. In An Overview of Tropical Diseases; Samie, A., Ed.; Intechopen: Rijeka, Croatia, 2015; pp. 173–202. [Google Scholar]
- Xu, G.; Walker, D.H.; Jupiter, D.; Melby, P.C.; Arcari, C.M. A review of the global epidemiology of scrub typhus. PLoS Negl. Trop. D 2017, 11, e0006062. [Google Scholar] [CrossRef]
- Bonell, A.; Lubell, Y.; Newton, P.N.; Crump, J.A.; Paris, D.H. Estimating the burden of scrub typhus: A systematic review. PLoS Negl. Trop. D 2017, 11, e0005838. [Google Scholar] [CrossRef]
- Elliott, I.; Pearson, I.; Dahal, P.; Thomas, N.V.; Roberts, T.; Newton, P.N. Scrub typhus ecology: A systematic review of Orientia in vectors and hosts. Parasit. Vectors 2019, 12, 513. [Google Scholar] [CrossRef]
- Li, J.C.; Wang, D.Q.; Chen, X.B. Trombiculid Mites of China (Studies on Vector and Pathogen of Tsutsugamushi Disease); Guangdong Science and Technology Publishing House: Guangzhou, China, 1997. [Google Scholar]
- Wilson, D.E.; Lacher, T.E.; Mittermeier, R.A. Handbook of the Mammals of the World; Volume 7: Rodents II; Lynx Ediciones: Barcelona, Spain, 2017; pp. 1–831. [Google Scholar]
- Guo, S.; Li, G.; Liu, J.; Wang, J.; Lu, L.; Liu, Q. Dispersal route of the Asian house rat (Rattus tanezumi) on mainland China: Insights from microsatellite and mitochondrial DNA. BMC Genet. 2019, 20, 11. [Google Scholar] [CrossRef]
- Lin, F.H.; Chou, Y.C.; Chien, W.C.; Chung, C.H.; Hsieh, C.J.; Yu, C.P. Epidemiology and Risk Factors for Notifiable Scrub Typhus in Taiwan during the Period 2010–2019. Healthcare 2021, 9, 1619. [Google Scholar] [CrossRef]
- Htwe, N.M.; Singleton, G.R.; Hinds, L.A.; Propper, C.R.; Sluydts, V. Breeding ecology of rice field rats, Rattus argentiventer and R. tanezumi in lowland irrigated rice systems in the Philippines. Agr. Ecosyst. Environ. 2012, 161, 39–45. [Google Scholar] [CrossRef]
- Luo, Y.Y.; Yin, J.X. Progress in research of Orientia tsutsugamushi and its host and vector. Dis. Surveill. 2019, 34, 920–923. [Google Scholar]
- Julius, R.S.; Brettschneider, H.; Chimimba, C.T.; Bastos, A. Prevalence and Diversity of the Streptobacillus Rat-bite Fever Agent, in Three Invasive, Commensal Rattus Species from South Africa. Yale J. Biol. Med. 2021, 94, 217–226. [Google Scholar]
- Zhang, M.; Li, Q.; Wu, F.; Ou, Z.; Li, Y.; You, F.; Chen, Q. Epidemiology, Genetic Characterization, and Evolution of Hunnivirus Carried by Rattus norvegicus and Rattus tanezumi: The First Epidemiological Evidence from Southern China. Pathogens 2021, 10, 661. [Google Scholar] [CrossRef]
- Barbara, K.A.; Farzeli, A.; Ibrahim, I.N.; Antonjaya, U.; Yunianto, A.; Winoto, I.; Ester; Perwitasari, D.; Widjaya, S.; Richards, A.L.; et al. Rickettsial infections of fleas collected from small mammals on four islands in Indonesia. J. Med. Entomol. 2010, 47, 1173–1178. [Google Scholar] [CrossRef]
- Kuo, C.C.; Lee, P.L.; Chen, C.H.; Wang, H.C. Surveillance of potential hosts and vectors of scrub typhus in Taiwan. Parasit. Vectors 2015, 8, 611. [Google Scholar] [CrossRef]
- Ding, F.; Jiang, W.L.; Guo, X.G.; Fan, R.; Zhao, C.F.; Zhang, Z.W.; Mao, K.Y.; Xiang, R. Infestation and Related Ecology of Chigger Mites on the Asian House Rat (Rattus tanezumi) in Yunnan Province, Southwest China. Korean J. Parasitol. 2021, 59, 377–392. [Google Scholar] [CrossRef]
- Vercammen-Grandjean, P.H.; Langston, R.L. The Chigger Mites of the World (Acarina: Trombiculidae et Leeuwenhoekiidae). III. Leptotrombidium Complex; George Williams Hooper Foundation, University of California: San Francisco, CA, USA, 1976; pp. 1–1061. [Google Scholar]
- Ding, F.; Guo, X.G.; Song, W.Y.; Fan, R.; Zhao, C.F.; Mao, K.Y.; Zhang, Z.W.; Peng, P.Y.; Lin, H.; Dong, W.G.; et al. Infestation and distribution of chigger mites on brown rat (Rattus norvegicus) in Yunnan province, southwest China. Trop. Biomed. 2021, 38, 111–121. [Google Scholar]
- Yin, P.W.; Guo, X.G.; Jin, D.C.; Song, W.Y.; Zhang, L.; Zhao, C.F.; Fan, R.; Zhang, Z.W.; Mao, K.Y. Infestation and Seasonal Fluctuation of Gamasid Mites (Parasitiformes: Gamasida) on Indochinese Forest Rat, Rattus andamanensis (Rodentia: Muridae) in Southern Yunnan of China. Biology 2021, 10, 1297. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, Y. Species diversity of butterflies in Changbai Mountain in China. Acta Ecol. Sin. 2012, 32, 279–284. [Google Scholar] [CrossRef]
- Li, Z.X.; He, Y.Q.; Wang, P.Y.; Theakstone, W.H.; An, W.L.; Wang, X.F.; Lu, A.G.; Zhang, W.; Cao, W.H. Changes of daily climate extremes in southwestern China during 1961–2008. Glob. Planet Change 2012, 80, 255–272. [Google Scholar]
- Ma, Z.F.; Liu, J.; Zhang, S.Q.; Chen, W.X.; Yang, S.Q. Observed climate changes in southwest China during 1961–2010. Adv. Clim. Chang. Res. 2013, 4, 30–40. [Google Scholar] [CrossRef]
- Chen, Y.L.; Guo, X.G.; Ren, T.G.; Zhang, L.; Fan, R.; Zhao, C.F.; Zhang, Z.W.; Mao, K.Y.; Huang, X.B.; Qian, T.J. Infestation and distribution of chigger mites on Chevrieri’s field mouse (Apodemus chevrieri) in Southwest China. Int. J. Parasitol. 2022, 17, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Guo, X.G.; Ren, T.G.; Zhang, L.; Fan, R.; Zhao, C.F.; Zhang, Z.W.; Mao, K.Y.; Huang, X.B.; Qian, T.J. A Report of Chigger Mites on the Striped Field Mouse, Apodemus agrarius, in Southwest China. Korean J. Parasitol. 2021, 59, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Su, J.J.; Wang, Y.; Zhou, J.; Bin, Y.; Yang, Z.Q. Advances in research of tsutsugamushi disease epidemiology in China in recent years. Chin. J. Hyg. Insect Equip. 2012, 18, 160–163. [Google Scholar]
- Lv, Y.; Guo, X.G.; Jin, D.C.; Fan, R.; Zhao, C.F.; Zhang, Z.W.; Mao, K.Y.; Song, W.Y.; Dong, W.G.; Qian, T.J.; et al. Distribution and host selection of the chigger mite vector of scrub typhus, Leptotrombidium deliense, in southwest China. Int. J. Acarol. 2021, 47, 233–241. [Google Scholar] [CrossRef]
- Li, B.; Guo, X.G.; Zhao, C.F.; Zhang, Z.W.; Fan, R.; Peng, P.Y.; Song, W.Y.; Ren, T.G.; Zhang, L.; Qian, T.J. Infestation of chigger mites on Chinese mole shrew, Anourosorex squamipes, in Southwest China and ecological analysis. Parasite 2022, 29, 39. [Google Scholar] [CrossRef]
- Peng, P.Y.; Guo, X.G.; Jin, D.C.; Dong, W.G.; Qian, T.J.; Qin, F.; Yang, Z.H.; Fan, R. Landscapes with different biodiversity influence distribution of small mammals and their ectoparasitic chigger mites: A comparative study from southwest China. PLoS ONE 2018, 13, e0189987. [Google Scholar] [CrossRef]
- Xiang, R.; Guo, X.G.; Ren, T.G.; Zhao, C.F.; Fan, R.; Zhang, Z.W.; Mao, K.Y.; Peng, P.Y.; Huang, X.B.; Qian, T.J. Infestation and distribution of mites on the Yunnan red-backed vole (Eothenomys miletus) in Yunnan Province of southwest China between 2001 and 2015. Biologia 2022, 77, 61–68. [Google Scholar] [CrossRef]
- Chaisiri, K.; Gill, A.C.; Stekolnikov, A.A.; Hinjoy, S.; McGarry, J.W.; Darby, A.C.; Morand, S.; Makepeace, B.L. Ecological and microbiological diversity of chigger mites, including vectors of scrub typhus, on small mammals across stratified habitats in Thailand. Anim. Microbiome 2019, 1, 18. [Google Scholar] [CrossRef]
- Rahbek, C.; Borregaard, M.K.; Colwell, R.K.; Dalsgaard, B.; Holt, B.G.; Morueta-Holme, N.; Nogues-Bravo, D.; Whittaker, R.J.; Fjeldså, J. Humboldt’s enigma: What causes global patterns of mountain biodiversity? Science 2019, 365, 1108–1113. [Google Scholar] [CrossRef]
- Lv, Y.; Guo, X.G.; Jin, D.C. Research Progress on Leptotrombidium deliense. Korean J. Parasitol. 2018, 56, 313–324. [Google Scholar] [CrossRef]
- Ming, Q.Z.; Shi, Z.T.; Deng, Y.J.; Dong, M. High Gradient Effects of Mountain Regions-Taking the Effects of Nature-Human Landscape in the Hengduan Mountains for Example. J. Glaciol. Geocryol. 2006, 28, 925–930. [Google Scholar]
- McCain, C.M.; Grytnes, J.A. Elevational gradients in species richness. In Encyclopedia of Life Sciences (ELS); John Wiley & Sons, Ltd.: Chichester, UK, 2010. [Google Scholar]
- Wu, Q.; Richard, M.; Rutschmann, A.; Miles, D.B.; Clobert, J. Environmental variation mediates the prevalence and co-occurrence of parasites in the common lizard, Zootoca vivipara. BMC Ecol. 2019, 19, 44. [Google Scholar] [CrossRef]
- Zhan, Y.Z.; Guo, X.G.; Speakman, J.R.; Zuo, X.H.; Wu, D.; Wang, Q.H.; Yang, Z.H. Abundances and host relationships of chigger mites in Yunnan Province, China. Med. Vet. Entomol. 2013, 27, 194–202. [Google Scholar] [CrossRef]
- Peng, P.Y.; Guo, X.G.; Jin, D.C.; Dong, W.G.; Qian, T.J.; Qin, F.; Yang, Z.H. Species abundance distribution and ecological niches of chigger mites on small mammals in Yunnan Province, Southwest China. Biologia 2017, 72, 1031–1040. [Google Scholar] [CrossRef]
- Yang, Y.; Tian, K.; Hao, J.; Pei, S.; Yang, Y. Biodiversity and biodiversity conservation in Yunnan, China. Biodivers. Conserv. 2004, 13, 813–826. [Google Scholar] [CrossRef]
- Wermelinger, B.; Candolfi, M.P.; Baumgärtner, J. A model of the European red mite (Acari, Tetranychidae) population dynamics and its linkage to grapevine growth and development. J. Appl. Entomol. 1992, 114, 155–166. [Google Scholar] [CrossRef]
- Hohenberger, M.E.; Elston, D.M. What’s eating you? chiggers. Cutis 2017, 99, 386–388. [Google Scholar]
- Barry, R.G. Mountain Weather and Climate, 3rd ed.; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
| Dominant Chigger Species | Constituent Ratios of Chiggers | Chigger Infestations on Rattus tanezumi | |||
|---|---|---|---|---|---|
| No. | Cr (%) | PM (%) | MA | MI | |
| Leptotrombidium deliense | 6297 | 30.79 | 6.75 | 2.16 | 31.96 |
| Ascoschoengastia indica | 4483 | 21.92 | 4.32 | 1.54 | 35.58 |
| Walchia ewingi | 1766 | 8.63 | 2.77 | 0.61 | 21.80 |
| All 149 identified chigger species | 20,453 | 100.00 | 21.10 | 7.01 | 33.20 |
| Different Environments | Number of Examined Rattus tanezumi | Community Parameters of Chiggers | Constituent Ratios of Chiggers | Chigger Infestation on Rattus tanezumi | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S | R | H’ | No. | Cr (%) | PM (%) | MA | MI | |||
| Landscapes | Mountainous | 969 | 113 | 12.37 | 2.65 | 8566 | 41.88 | 28.69 | 8.84 | 30.81 |
| Flatland | 1950 | 87 | 9.17 | 2.12 | 11,887 | 58.12 | 17.33 | 6.10 | 35.17 | |
| Altitudes | ≤500 m | 259 | 31 | 3.59 | 1.90 | 4301 | 21.03 | 34.36 | 16.61 | 48.33 |
| 501–1000 m | 1465 | 76 | 7.92 | 2.07 | 12,956 | 63.35 | 23.62 | 8.84 | 37.45 | |
| 1001–1500 m | 575 | 58 | 8.33 | 2.82 | 939 | 4.59 | 13.83 | 1.63 | 12.04 | |
| 1501–3350 m | 620 | 80 | 10.23 | 2.92 | 2257 | 11.04 | 16.61 | 3.64 | 21.91 | |
| Latitudes | <24° N | 1223 | 89 | 9.23 | 2.22 | 13,761 | 67.28 | 32.54 | 11.25 | 34.58 |
| 24–26° N | 1461 | 90 | 10.42 | 1.96 | 5122 | 25.04 | 12.53 | 3.51 | 27.99 | |
| >26° N | 235 | 44 | 5.84 | 2.81 | 1570 | 7.68 | 14.89 | 6.68 | 44.86 | |
| Longitudes | <98° E | 842 | 65 | 8.59 | 2.23 | 1719 | 8.40 | 15.56 | 2.04 | 13.12 |
| 98–100° E | 563 | 55 | 7.06 | 2.19 | 2097 | 10.25 | 17.76 | 3.72 | 20.97 | |
| 100–102° E | 1093 | 87 | 9.02 | 2.26 | 13,766 | 67.31 | 29.00 | 12.59 | 43.43 | |
| >102° E | 421 | 38 | 4.65 | 1.84 | 2871 | 14.04 | 16.15 | 6.82 | 42.22 | |
| Precipitations | <50 mm | 895 | 99 | 11.78 | 2.33 | 4090 | 20.00 | 16.54 | 4.57 | 27.64 |
| 50–100 mm | 515 | 75 | 8.74 | 2.22 | 4771 | 23.33 | 29.51 | 9.26 | 31.39 | |
| 100–200 mm | 728 | 60 | 6.60 | 2.06 | 7655 | 37.43 | 21.98 | 10.52 | 47.84 | |
| >200 mm | 781 | 40 | 4.71 | 1.91 | 3937 | 19.25 | 19.97 | 5.04 | 25.24 | |
| Temperature | <20 °C | 1120 | 115 | 13.53 | 2.85 | 4556 | 22.28 | 15.63 | 4.07 | 26.03 |
| 20–25 °C | 1091 | 74 | 7.81 | 2.21 | 11,402 | 55.34 | 27.50 | 10.45 | 38.01 | |
| >25 °C | 708 | 45 | 5.23 | 2.05 | 4495 | 21.82 | 19.92 | 6.35 | 31.88 | |
| Relative humidity | ≤60% | 153 | 16 | 3.85 | 1.96 | 49 | 0.28 | 7.19 | 0.32 | 4.45 |
| 60–70% | 1006 | 84 | 9.32 | 1.91 | 7395 | 36.16 | 17.30 | 7.35 | 42.50 | |
| 70–80% | 1211 | 97 | 10.89 | 2.82 | 6735 | 32.93 | 22.13 | 5.56 | 25.13 | |
| ≥80% | 549 | 39 | 4.35 | 1.65 | 6274 | 30.68 | 29.69 | 11.43 | 38.49 | |
| NDVI | ≤4000 | 932 | 84 | 10.39 | 2.53 | 2936 | 14.35 | 13.95 | 3.15 | 22.58 |
| 4000–5000 | 708 | 68 | 7.26 | 1.91 | 10,209 | 49.91 | 28.25 | 14.42 | 51.05 | |
| 5000–6000 | 1046 | 72 | 8.15 | 2.44 | 6063 | 29.64 | 21.32 | 5.80 | 27.19 | |
| ≥6000 | 233 | 36 | 4.91 | 2.42 | 1245 | 6.09 | 27.04 | 5.34 | 19.76 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-L.; Guo, X.-G.; Ding, F.; Lv, Y.; Yin, P.-W.; Song, W.-Y.; Zhao, C.-F.; Zhang, Z.-W.; Fan, R.; Peng, P.-Y.; et al. Infestation of Oriental House Rat (Rattus tanezumi) with Chigger Mites Varies along Environmental Gradients across Five Provincial Regions of Southwest China. Int. J. Environ. Res. Public Health 2023, 20, 2203. https://doi.org/10.3390/ijerph20032203
Chen Y-L, Guo X-G, Ding F, Lv Y, Yin P-W, Song W-Y, Zhao C-F, Zhang Z-W, Fan R, Peng P-Y, et al. Infestation of Oriental House Rat (Rattus tanezumi) with Chigger Mites Varies along Environmental Gradients across Five Provincial Regions of Southwest China. International Journal of Environmental Research and Public Health. 2023; 20(3):2203. https://doi.org/10.3390/ijerph20032203
Chicago/Turabian StyleChen, Yan-Ling, Xian-Guo Guo, Fan Ding, Yan Lv, Peng-Wu Yin, Wen-Yu Song, Cheng-Fu Zhao, Zhi-Wei Zhang, Rong Fan, Pei-Ying Peng, and et al. 2023. "Infestation of Oriental House Rat (Rattus tanezumi) with Chigger Mites Varies along Environmental Gradients across Five Provincial Regions of Southwest China" International Journal of Environmental Research and Public Health 20, no. 3: 2203. https://doi.org/10.3390/ijerph20032203
APA StyleChen, Y.-L., Guo, X.-G., Ding, F., Lv, Y., Yin, P.-W., Song, W.-Y., Zhao, C.-F., Zhang, Z.-W., Fan, R., Peng, P.-Y., Li, B., Chen, T., & Jin, D.-C. (2023). Infestation of Oriental House Rat (Rattus tanezumi) with Chigger Mites Varies along Environmental Gradients across Five Provincial Regions of Southwest China. International Journal of Environmental Research and Public Health, 20(3), 2203. https://doi.org/10.3390/ijerph20032203







