Closing the Nutrient Loop—The New Approaches to Recovering Biomass Minerals during the Biorefinery Processes
Abstract
1. Introduction
2. Recent Approaches for Mineral Recovery That Could Be Used during the First Stages of Biomass Biorefinery
2.1. Dry Separation Processes
2.2. Wet Treatments
2.2.1. Wet Pre-Treatments Based on Biological and Enzymatic Processes
2.2.2. Wet Treatments with Low Transition Temperature Mixtures (LTTMs)—Ionic Liquids (IL)
- ✔
- ✔
- Wood processing technologies (reviewed in [125]);
- ✔
- Biomass pre-treatment using ILs derived from lignin and hemicellulose [126];
- ✔
- ✔
- Extraction of nanocellulose [130];
- ✔
- ✔
- In situ hydrolysis of empty fruit bunches combining pre-treatment and enzymatic hydrolysis [133];
- ✔
- Extraction of natural compounds such as alkaloids, flavonoids, terpenoids, lipids, etc., reviewed in [134].
2.2.3. Wet Treatment—(Natural) Deep Eutectic Solvents, (Na)DESs
3. Silicon Species Separation during the Initial Stages of Biorefinery
3.1. Dry Separation Processes
3.2. Wet Separation Processes—Biological and Enzymatic Processes
3.3. Wet Separation Processes—Chemical and Physico-Chemical Processes
3.4. Wet Treatment—(Natural) Deep Eutectic Solvents, (Na)DESs
4. Phosphorus, Nitrogen, and Sulfur Recovery during the Biorefinery Process
4.1. Pyrolysis, Hydrothermal Carbonization, and Combustion
4.2. Reverse Osmosis
4.3. Phosphorous Precipitation
4.4. (Bio)electrochemical Systems
4.5. Nitrogen, Sulfur, and Phosphate Recovery during the Anaerobic Digestion
5. Critical Evaluation of the Methods used to Recover Mineral Nutrients during Biorefinery Processes
6. Agricultural Use of the Recovered Biofertilizers
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chandel, A.K.; Garlapati, V.K.; Singh, A.K.; Antunes, F.A.F.; da Silva, S.S. The path forward for lignocellulose biorefineries: Bottlenecks, solutions, and perspective on commercialization. Bioresour. Technol. 2018, 264, 370–381. [Google Scholar] [CrossRef]
- Kohli, K.; Prajapati, R.; Sharma, B.K. Bio-Based Chemicals from Renewable Biomass for Integrated Biorefineries. Energies 2019, 12, 233. [Google Scholar] [CrossRef]
- Agler, M.T.; Wrenn, B.A.; Zinder, S.H.; Angenent, L.T. Waste to bioproduct conversion with undefined mixed cultures: The carboxylate platform. Trends Biotechnol. 2011, 29, 70–78. [Google Scholar] [CrossRef]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef] [PubMed]
- Sawatdeenarunat, C.; Surendra, K.C.; Takara, D.; Oechsner, H.; Khanal, S.K. Anaerobic digestion of lignocellulosic biomass: Challenges and opportunities. Bioresour. Technol. 2015, 178, 178–186. [Google Scholar] [CrossRef]
- FitzPatrick, M.; Champagne, P.; Cunningham, M.F.; Whitney, R.A. A biorefinery processing perspective: Treatment of lignocellulosic materials for the production of value-added products. Bioresour. Technol. 2010, 101, 8915–8922. [Google Scholar] [CrossRef]
- Singh, N.; Singhania, R.R.; Nigam, P.S.; Dong, C.-D.; Patel, A.K.; Puri, M. Global status of lignocellulosic biorefinery: Challenges and perspectives. Bioresour. Technol. 2022, 344, 126415. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Sharma, A.; Pareek, V.; Zhang, D. Biomass pyrolysis—A review of modelling, process parameters and catalytic studies. Renew. Sustain. Energy Rev. 2015, 50, 1081–1096. [Google Scholar] [CrossRef]
- Kumar, A.; Jones, D.D.; Hanna, M.A. Thermochemical biomass gasification: A review of the current status of the technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef]
- Bichot, A.; Delgenès, J.-P.; Méchin, V.; Carrère, H.; Bernet, N.; García-Bernet, D. Understanding biomass recalcitrance in grasses for their efficient utilization as biorefinery feedstock. Rev. Environ. Sci. Bio/Technol. 2018, 17, 707–748. [Google Scholar] [CrossRef]
- Satlewal, A.; Agrawal, R.; Bhagia, S.; Das, P.; Ragauskas, A.J. Rice straw as a feedstock for biofuels: Availability, recalcitrance, and chemical properties. Biofuels Bioprod. Biorefining 2018, 12, 83–107. [Google Scholar] [CrossRef]
- Capolupo, L.; Faraco, V. Green methods of lignocellulose pretreatment for biorefinery development. Appl. Microbiol. Biotechnol. 2016, 100, 9451–9467. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Lam, S.K.; Suter, H.; Mosier, A.R.; Chen, D. Using nitrification inhibitors to mitigate agricultural N2O emission: A double-edged sword? Glob. Change Biol. 2017, 23, 485–489. [Google Scholar] [CrossRef]
- Carey, D.E.; Yang, Y.; McNamara, P.J.; Mayer, B.K. Recovery of agricultural nutrients from biorefineries. Bioresour. Technol. 2016, 215, 186–198. [Google Scholar] [CrossRef]
- Tonini, D.; Martinez-Sanchez, V.; Astrup, T.F. Material Resources, Energy, and Nutrient Recovery from Waste: Are Waste Refineries the Solution for the Future? Environ. Sci. Technol. 2013, 47, 8962–8969. [Google Scholar] [CrossRef] [PubMed]
- Santamaria-Fernandez, M.; Ytting, N.K.; Lübeck, M.; Uellendahl, H. Potential Nutrient Recovery in a Green Biorefinery for Production of Feed, Fuel and Fertilizer for Organic Farming. Waste Biomass Valorization 2020, 11, 5901–5911. [Google Scholar] [CrossRef]
- Veríssimo, N.V.; Mussagy, C.U.; Oshiro, A.A.; Mendonça, C.M.N.; de Carvalho Santos-Ebinuma, V.; Pessoa, A.; de Souza Oliveira, R.P.; Pereira, J.F.B. From green to blue economy: Marine biorefineries for a sustainable ocean-based economy. Green Chem. 2021, 23, 9377–9400. [Google Scholar] [CrossRef]
- Mekonnen, T.; Mussone, P.; Bressler, D. Valorization of rendering industry wastes and co-products for industrial chemicals, materials and energy. Crit. Rev. Biotechnol. 2016, 36, 120–131. [Google Scholar] [CrossRef]
- Hidalgo, D.; Corona, F.; Martín-Marroquín, J.M. Nutrient recycling: From waste to crop. Biomass Convers. Biorefinery 2021, 11, 207–217. [Google Scholar] [CrossRef]
- Mehta, C.M.; Khunjar, W.O.; Nguyen, V.; Tait, S.; Batstone, D.J. Technologies to Recover Nutrients from Waste Streams: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 385–427. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef]
- Binod, P.; Sindhu, R.; Singhania, R.R.; Vikram, S.; Devi, L.; Nagalakshmi, S.; Kurien, N.; Sukumaran, R.K.; Pandey, A. Bioethanol production from rice straw: An overview. Bioresour. Technol. 2010, 101, 4767–4774. [Google Scholar] [CrossRef]
- Van Soest, P. Rice straw, the role of silica and treatments to improve quality. Anim. Feed Sci. Technol. 2006, 130, 137–171. [Google Scholar] [CrossRef]
- Steven, S.; Restiawaty, E.; Bindar, Y. Routes for energy and bio-silica production from rice husk: A comprehensive review and emerging prospect. Renew. Sustain. Energy Rev. 2021, 149, 111329. [Google Scholar] [CrossRef]
- Pan, M.-Z.; Zhou, D.-G.; Mei, C.-T.; Deng, J.; Wang, X.-M.; Zhang, T.S. Effects of Thermomechanical Refining Conditions on the Morphology and Thermal Properties of Wheat Straw Fibre; De Gruyter: Berlin, Germany, 2008. [Google Scholar]
- Deniz, I.; Kırcı, H.; Ates, S. Optimisation of wheat straw Triticum drum kraft pulping. Ind. Crops Prod. 2004, 19, 237–243. [Google Scholar] [CrossRef]
- Xu, Y.; Porter, N.; Foster, J.L.; Muir, J.P.; Schwab, P.; Burson, B.L.; Jessup, R.W. Silica Production across Candidate Lignocellulosic Biorefinery Feedstocks. Agronomy 2020, 10, 82. [Google Scholar] [CrossRef]
- Ge, X.; Xu, F.; Vasco-Correa, J.; Li, Y. Giant reed: A competitive energy crop in comparison with miscanthus. Renew. Sustain. Energy Rev. 2016, 54, 350–362. [Google Scholar] [CrossRef]
- Corno, L.; Pilu, R.; Adani, F. Arundo donax L.: A non-food crop for bioenergy and bio-compound production. Biotechnol. Adv. 2014, 32, 1535–1549. [Google Scholar] [CrossRef]
- Shakoor, S.A.; Bhat, M.A.; Soodan, A.S. Taxonomic demarcation of Arundo donax L. and Phragmites karka (Retz.) Trin. ex Steud. (Arundinoideae, Poaceae) from phytolith signatures. Flora 2016, 224, 130–153. [Google Scholar] [CrossRef]
- Jørgensen, H.; van Hecke, J.; Zhang, H.; Malik, P.L.; Felby, C.; Schjoerring, J.K. Wheat as a dual crop for biorefining: Straw quality parameters and their interactions with nitrogen supply in modern elite cultivars. GCB Bioenergy 2019, 11, 400–415. [Google Scholar] [CrossRef]
- Yan, C.; Yan, S.-S.; Jia, T.-Y.; Dong, S.-K.; Ma, C.-M.; Gong, Z.-P. Decomposition characteristics of rice straw returned to the soil in northeast China. Nutr. Cycl. Agroecosyst. 2019, 114, 211–224. [Google Scholar] [CrossRef]
- Nguyen, M.N.; Dultz, S.; Picardal, F.; Bui, A.T.K.; Van Pham, Q.; Schieber, J. Release of potassium accompanying the dissolution of rice straw phytolith. Chemosphere 2015, 119, 371–376. [Google Scholar] [CrossRef]
- Tran, C.T.; Mai, N.T.; Nguyen, V.T.; Nguyen, H.X.; Meharg, A.; Carey, M.; Dultz, S.; Marone, F.; Cichy, S.B.; Nguyen, M.N. Phytolith-associated potassium in fern: Characterization, dissolution properties and implications for slash-and-burn agriculture. Soil Use Manag. 2018, 34, 28–36. [Google Scholar] [CrossRef]
- Trinh, T.K.; Nguyen, T.T.; Nguyen, T.N.; Wu, T.Y.; Meharg, A.A.; Nguyen, M.N. Characterization and dissolution properties of phytolith occluded phosphorus in rice straw. Soil Tillage Res. 2017, 171, 19–24. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Nguyen, A.T.Q.; Vu, T.T.T.; Duong, L.T.; Nguyen, M.N. Potassium in silicon-rich biomass wastes: A perspective of slow-release potassium sources. Biofuels Bioprod. Biorefining 2022, 16, 1159–1164. [Google Scholar] [CrossRef]
- Negrão, D.R.; Grandis, A.; Buckeridge, M.S.; Rocha, G.J.M.; Leal, M.R.L.V.; Driemeier, C. Inorganics in sugarcane bagasse and straw and their impacts for bioenergy and biorefining: A review. Renew. Sustain. Energy Rev. 2021, 148, 111268. [Google Scholar] [CrossRef]
- Baghel, R.S.; Suthar, P.; Gajaria, T.K.; Bhattacharya, S.; Anil, A.; Reddy, C.R.K. Seaweed biorefinery: A sustainable process for valorising the biomass of brown seaweed. J. Clean. Prod. 2020, 263, 121359. [Google Scholar] [CrossRef]
- Le, D.M.; Sorensen, H.R.; Knudsen, N.O.; Schjoerring, J.K.; Meyer, A.S. Biorefining of wheat straw: Accounting for the distribution of mineral elements in pretreated biomass by an extended pretreatment-severity equation. Biotechnol. Biofuels 2014, 7, 141. [Google Scholar] [CrossRef]
- Cheng, G.; Chen, L.; Feng, H.; Jiang, B.; Ding, Y. Preliminary Study on Fish Scale Collagen Lamellar Matrix as Artificial Cornea. Membranes 2021, 11, 737. [Google Scholar] [CrossRef]
- Chen, L.; Cheng, G.; Meng, S.; Ding, Y. Collagen Membrane Derived from Fish Scales for Application in Bone Tissue Engineering. Polymers 2022, 14, 2532. [Google Scholar] [CrossRef]
- Iosageanu, A.; Ilie, D.; Craciunescu, O.; Seciu-Grama, A.-M.; Oancea, A.; Zarnescu, O.; Moraru, I.; Oancea, F. Effect of fish bone bioactive peptides on oxidative, inflammatory and pigmentation processes triggered by UVB irradiation in skin cells. Molecules 2021, 26, 2691. [Google Scholar] [CrossRef]
- Ilie, D.; Iosageanu, A.; Craciunescu, O.; Seciu-Grama, A.-M.; Sanda, C.; Oancea, F. Free Radical Scavenging, Redox Balance and Wound Healing Activity of Bioactive Peptides Derived from Proteinase K-Assisted Hydrolysis of Hypophthalmichthys molitrix Skin Collagen. Food Technol. Biotechnol. 2022, 60, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.V.S.; Torquato, L.D.M.; Cruz, G. Potential application of fish scales as feedstock in thermochemical processes for the clean energy generation. Waste Manag. 2019, 100, 91–100. [Google Scholar] [CrossRef]
- Savlak, N.; Çağındı, Ö.; Erk, G.; Öktem, B.; Köse, E. Treatment Method Affects Color, Chemical, and Mineral Composition of Seabream (Sparus aurata) Fish Bone Powder from by-Products of Fish Fillet. J. Aquat. Food Prod. Technol. 2020, 29, 592–602. [Google Scholar] [CrossRef]
- Ahmed, M.; Ahmad, S.; Qadir, G.; Hayat, R.; Shaheen, F.A.; Raza, M.A. Innovative processes and technologies for nutrient recovery from wastes:A comprehensive review. Sustainability 2019, 11, 4938. [Google Scholar] [CrossRef]
- Van Grinsven, H.J.M.; Holland, M.; Jacobsen, B.H.; Klimont, Z.; Sutton, M.A.; Jaap Willems, W. Costs and Benefits of Nitrogen for Europe and Implications for Mitigation. Environ. Sci. Technol. 2013, 47, 3571–3579. [Google Scholar] [CrossRef]
- Lam, S.K.; Wille, U.; Hu, H.-W.; Caruso, F.; Mumford, K.; Liang, X.; Pan, B.; Malcolm, B.; Roessner, U.; Suter, H. Next-generation enhanced-efficiency fertilizers for sustained food security. Nat. Food 2022, 3, 575–580. [Google Scholar] [CrossRef]
- Barbieri, P.; MacDonald, G.K.; Bernard de Raymond, A.; Nesme, T. Food system resilience to phosphorus shortages on a telecoupled planet. Nat. Sustain. 2022, 5, 114–122. [Google Scholar] [CrossRef]
- Guerriero, G.; Hausman, J.-F.; Legay, S. Silicon and the plant extracellular matrix. Front. Plant Sci. 2016, 7, 463. [Google Scholar] [CrossRef]
- ElMekawy, A.; Diels, L.; De Wever, H.; Pant, D. Valorization of cereal based biorefinery byproducts: Reality and expectations. Environ. Sci. Technol. 2013, 47, 9014–9027. [Google Scholar] [CrossRef]
- Hortsch, R.; Corvo, P. The Biorefinery Concept: Producing Cellulosic Ethanol from Agricultural Residues. Chem. Ing. Tech. 2020, 92, 1803–1809. [Google Scholar] [CrossRef]
- EUROSTAT. Agricultural Production—Crops—Statistics Explained. Available online: http://ec.europa.eu/eurostat/statistics-explained/index.php/Agricultural_production_-_crops#Cereals (accessed on 18 December 2022).
- Pu, J.; Wang, L.; Zhang, W.; Ma, J.; Zhang, X.; Putnis, C.V. Organically-bound silicon enhances resistance to enzymatic degradation and nanomechanical properties of rice plant cell walls. Carbohydr. Polym. 2021, 266, 118057. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, W.; Li, Y.; Feng, Y.; Zhang, H.; Wu, Z.; Tu, Y.; Wang, Y.; Cai, X.; Peng, L. Silica distinctively affects cell wall features and lignocellulosic saccharification with large enhancement on biomass production in rice. Plant Sci. 2015, 239, 84–91. [Google Scholar] [CrossRef]
- Le, D.M.; Sørensen, H.R.; Knudsen, N.O.; Meyer, A.S. Implications of silica on biorefineries–interactions with organic material and mineral elements in grasses. Biofuels Bioprod. Biorefining 2015, 9, 109–121. [Google Scholar] [CrossRef]
- Głazowska, S.; Baldwin, L.; Mravec, J.; Bukh, C.; Hansen, T.H.; Jensen, M.M.; Fangel, J.U.; Willats, W.G.; Glasius, M.; Felby, C. The impact of silicon on cell wall composition and enzymatic saccharification of Brachypodium distachyon. Biotechnol. Biofuels 2018, 11, 171. [Google Scholar] [CrossRef]
- Sharma, B.; Kumawat, K.C.; Tiwari, S.; Kumar, A.; Dar, R.A.; Singh, U.; Cardinale, M. Silicon and plant nutrition: Dynamics, mechanisms of transport, and role of silicon solubilizer microbiomes in sustainable agriculture. Pedosphere 2022, in press. [Google Scholar] [CrossRef]
- Brown, P.H.; Zhao, F.-J.; Dobermann, A. What is a plant nutrient? Changing definitions to advance science and innovation in plant nutrition. Plant Soil 2022, 476, 11–23. [Google Scholar] [CrossRef]
- Savvas, D.; Ntatsi, G. Biostimulant activity of silicon in horticulture. Sci. Hortic. 2015, 196, 66–81. [Google Scholar] [CrossRef]
- Constantinescu-Aruxandei, D.; Lupu, C.; Oancea, F. Siliceous Natural Nanomaterials as Biorationals—Plant Protectants and Plant Health Strengtheners. Agronomy 2020, 10, 1791. [Google Scholar] [CrossRef]
- Kandhol, N.; Singh, V.P.; Peralta-Videa, J.; Corpas, F.J.; Tripathi, D.K. Silica nanoparticles: The rising star in plant disease protection. Trends Plant Sci. 2022, 27, 7–9. [Google Scholar] [CrossRef]
- Schaller, J.; Puppe, D.; Kaczorek, D.; Ellerbrock, R.; Sommer, M. Silicon Cycling in Soils Revisited. Plants 2021, 10, 295. [Google Scholar] [CrossRef]
- Meunier, J.-D.; Cornu, S.; Keller, C.; Barboni, D. The role of silicon in the supply of terrestrial ecosystem services. Environ. Chem. Lett. 2022, 20, 2109–2121. [Google Scholar] [CrossRef]
- Chuetor, S.; Luque, R.; Barron, C.; Solhy, A.; Rouau, X.; Barakat, A. Innovative combined dry fractionation technologies for rice straw valorization to biofuels. Green Chem. 2015, 17, 926–936. [Google Scholar] [CrossRef]
- Zhu, H.-G.; Tang, H.-Q.; Cheng, Y.-Q.; Li, Z.-G.; Tong, L.-T. Electrostatic separation technology for obtaining plant protein concentrates: A review. Trends Food Sci. Technol. 2021, 113, 66–76. [Google Scholar] [CrossRef]
- Xing, Q.; de Wit, M.; Kyriakopoulou, K.; Boom, R.M.; Schutyser, M.A. Protein enrichment of defatted soybean flour by fine milling and electrostatic separation. Innov. Food Sci. Emerg. Technol. 2018, 50, 42–49. [Google Scholar] [CrossRef]
- Tabtabaei, S.; Konakbayeva, D.; Rajabzadeh, A.R.; Legge, R.L. Functional properties of navy bean (Phaseolus vulgaris) protein concentrates obtained by pneumatic tribo-electrostatic separation. Food Chem. 2019, 283, 101–110. [Google Scholar] [CrossRef]
- Cancelli, U.; Montevecchi, G.; Masino, F.; Mayer-Laigle, C.; Rouau, X.; Antonelli, A. Grape stalk: A first attempt to disentangle its fibres via electrostatic separation. Food Bioprod. Process. 2020, 124, 455–468. [Google Scholar] [CrossRef]
- Wang, J.; Smits, E.; Boom, R.M.; Schutyser, M.A. Arabinoxylans concentrates from wheat bran by electrostatic separation. J. Food Eng. 2015, 155, 29–36. [Google Scholar] [CrossRef]
- Kdidi, S.; Vaca-Medina, G.; Peydecastaing, J.; Oukarroum, A.; Fayoud, N.; Barakat, A. Electrostatic separation for sustainable production of rapeseed oil cake protein concentrate: Effect of mechanical disruption on protein and lignocellulosic fiber separation. Powder Technol. 2019, 344, 10–16. [Google Scholar] [CrossRef]
- Xing, Q.; Utami, D.P.; Demattey, M.B.; Kyriakopoulou, K.; de Wit, M.; Boom, R.M.; Schutyser, M.A. A two-step air classification and electrostatic separation process for protein enrichment of starch-containing legumes. Innov. Food Sci. Emerg. Technol. 2020, 66, 102480. [Google Scholar] [CrossRef]
- Frouel, S.; Pailler, S.; Spiraers, A. A Dry Oilseed Meal Protein Fraction. European patent 3530122A1, 28 August 2019. [Google Scholar]
- Hoang, A.T.; Nizetic, S.; Ong, H.C.; Chong, C.T.; Atabani, A.E.; Pham, V.V. Acid-based lignocellulosic biomass biorefinery for bioenergy production: Advantages, application constraints, and perspectives. J. Environ. Manag. 2021, 296, 113194. [Google Scholar] [CrossRef]
- Zhou, P.-P.; Meng, J.; Bao, J. Fermentative production of high titer citric acid from corn stover feedstock after dry dilute acid pretreatment and biodetoxification. Bioresour. Technol. 2017, 224, 563–572. [Google Scholar] [CrossRef]
- Baghel, R.S.; Trivedi, N.; Gupta, V.; Neori, A.; Reddy, C.R.K.; Lali, A.; Jha, B. Biorefining of marine macroalgal biomass for production of biofuel and commodity chemicals. Green Chem. 2015, 17, 2436–2443. [Google Scholar] [CrossRef]
- O’Neill, M.A.; Warrenfeltz, D.; Kates, K.; Pellerin, P.; Doco, T.; Darvill, A.G.; Albersheim, P. Rhamnogalacturonan-II, a Pectic Polysaccharide in the Walls of Growing Plant Cell, Forms a Dimer That Is Covalently Cross-linked by a Borate Ester: In vitro conditions for the formation and hydrolysis of the dimer. J. Biol. Chem. 1996, 271, 22923–22930. [Google Scholar] [CrossRef]
- O’Neill, M.A.; Eberhard, S.; Albersheim, P.; Darvill, A.G. Requirement of Borate Cross-Linking of Cell Wall Rhamnogalacturonan II for Arabidopsis Growth. Science 2001, 294, 846–849. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Mafra, I.; Soares, M.R.; Evtuguin, D.V.; Coimbra, M.A. Dimeric calcium complexes of arabinan-rich pectic polysaccharides from Olea europaea L. cell walls. Carbohydr. Polym. 2006, 65, 535–543. [Google Scholar] [CrossRef]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Awad, F.N.; Qi, X.; Sahu, J.N. Recent advances in biological pretreatment of microalgae and lignocellulosic biomass for biofuel production. Renew. Sustain. Energy Rev. 2019, 105, 105–128. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Z.; Shahadat Hossain, M.; Maurya, R.; Yang, Y.; Singh, V.; Kumar, D.; Salama, E.-S.; Sun, X.; Sindhu, R.; et al. Recent advances in lignocellulosic and algal biomass pretreatment and its biorefinery approaches for biochemicals and bioenergy conversion. Bioresour. Technol. 2023, 367, 128281. [Google Scholar] [CrossRef]
- Rojas, L.F.; Zapata, P.; Ruiz-Tirado, L. Agro-industrial waste enzymes: Perspectives in circular economy. Curr. Opin. Green Sustain. Chem. 2022, 34, 100585. [Google Scholar] [CrossRef]
- Dharmaraja, J.; Shobana, S.; Arvindnarayan, S.; Francis, R.R.; Jeyakumar, R.B.; Saratale, R.G.; Ashokkumar, V.; Bhatia, S.K.; Kumar, V.; Kumar, G. Lignocellulosic biomass conversion via greener pretreatment methods towards biorefinery applications. Bioresour. Technol. 2023, 369, 128328. [Google Scholar] [CrossRef] [PubMed]
- Vaaje-Kolstad, G.; Westereng, B.; Horn, S.J.; Liu, Z.; Zhai, H.; Sorlie, M.; Eijsink, V.G. An oxidative enzyme boosting the enzymatic conversion of recalcitrant polysaccharides. Science 2010, 330, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Eibinger, M.; Ganner, T.; Bubner, P.; Rosker, S.; Kracher, D.; Haltrich, D.; Ludwig, R.; Plank, H.; Nidetzky, B. Cellulose surface degradation by a lytic polysaccharide monooxygenase and its effect on cellulase hydrolytic efficiency. J. Biol. Chem. 2014, 289, 35929–35938. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.S. Lytic Polysaccharide Monooxygenases: The Microbial Power Tool for Lignocellulose Degradation. Trends Plant Sci. 2016, 21, 926–936. [Google Scholar] [CrossRef]
- Quinlan, R.J.; Sweeney, M.D.; Lo Leggio, L.; Otten, H.; Poulsen, J.C.; Johansen, K.S.; Krogh, K.B.; Jorgensen, C.I.; Tovborg, M.; Anthonsen, A.; et al. Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components. Proc. Natl. Acad. Sci. USA 2011, 108, 15079–15084. [Google Scholar] [CrossRef]
- Lo Leggio, L.; Simmons, T.J.; Poulsen, J.C.; Frandsen, K.E.; Hemsworth, G.R.; Stringer, M.A.; von Freiesleben, P.; Tovborg, M.; Johansen, K.S.; De Maria, L.; et al. Structure and boosting activity of a starch-degrading lytic polysaccharide monooxygenase. Nat. Commun. 2015, 6, 5961. [Google Scholar] [CrossRef]
- Vu, V.V.; Beeson, W.T.; Span, E.A.; Farquhar, E.R.; Marletta, M.A. A family of starch-active polysaccharide monooxygenases. Proc. Natl. Acad. Sci. USA 2014, 111, 13822–13827. [Google Scholar] [CrossRef]
- Agger, J.W.; Isaksen, T.; Varnai, A.; Vidal-Melgosa, S.; Willats, W.G.; Ludwig, R.; Horn, S.J.; Eijsink, V.G.; Westereng, B. Discovery of LPMO activity on hemicelluloses shows the importance of oxidative processes in plant cell wall degradation. Proc. Natl. Acad. Sci. USA 2014, 111, 6287–6292. [Google Scholar] [CrossRef]
- Borisova, A.S.; Isaksen, T.; Dimarogona, M.; Kognole, A.A.; Mathiesen, G.; Varnai, A.; Rohr, A.K.; Payne, C.M.; Sorlie, M.; Sandgren, M.; et al. Structural and Functional Characterization of a Lytic Polysaccharide Monooxygenase with Broad Substrate Specificity. J. Biol. Chem. 2015, 290, 22955–22969. [Google Scholar] [CrossRef]
- Bennati-Granier, C.; Garajova, S.; Champion, C.; Grisel, S.; Haon, M.; Zhou, S.; Fanuel, M.; Ropartz, D.; Rogniaux, H.; Gimbert, I.; et al. Substrate specificity and regioselectivity of fungal AA9 lytic polysaccharide monooxygenases secreted by Podospora anserina. Biotechnol. Biofuels 2015, 8, 90. [Google Scholar] [CrossRef]
- Müller, G.; Varnai, A.; Johansen, K.S.; Eijsink, V.G.; Horn, S.J. Harnessing the potential of LPMO-containing cellulase cocktails poses new demands on processing conditions. Biotechnol. Biofuels 2015, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.M.; Beeson, W.T.; Cate, J.H.; Marletta, M.A. Cellobiose dehydrogenase and a copper-dependent polysaccharide monooxygenase potentiate cellulose degradation by Neurospora crassa. ACS Chem. Biol. 2011, 6, 1399–1406. [Google Scholar] [CrossRef]
- Langston, J.A.; Shaghasi, T.; Abbate, E.; Xu, F.; Vlasenko, E.; Sweeney, M.D. Oxidoreductive cellulose depolymerization by the enzymes cellobiose dehydrogenase and glycoside hydrolase 61. Appl. Environ. Microbiol. 2011, 77, 7007–7015. [Google Scholar] [CrossRef]
- Cannella, D.; Mollers, K.B.; Frigaard, N.U.; Jensen, P.E.; Bjerrum, M.J.; Johansen, K.S.; Felby, C. Light-driven oxidation of polysaccharides by photosynthetic pigments and a metalloenzyme. Nat. Commun. 2016, 7, 11134. [Google Scholar] [CrossRef] [PubMed]
- Kracher, D.; Scheiblbrandner, S.; Felice, A.K.; Breslmayr, E.; Preims, M.; Ludwicka, K.; Haltrich, D.; Eijsink, V.G.; Ludwig, R. Extracellular electron transfer systems fuel cellulose oxidative degradation. Science 2016, 352, 1098–1101. [Google Scholar] [CrossRef]
- Rodríguez-Zúñiga, U.F.; Cannella, D.; Giordano, R.d.C.; Giordano, R.d.L.C.; Jørgensen, H.; Felby, C. Lignocellulose pretreatment technologies affect the level of enzymatic cellulose oxidation by LPMO. Green Chem. 2015, 17, 2896–2903. [Google Scholar] [CrossRef]
- Westereng, B.; Cannella, D.; Wittrup Agger, J.; Jorgensen, H.; Larsen Andersen, M.; Eijsink, V.G.; Felby, C. Enzymatic cellulose oxidation is linked to lignin by long-range electron transfer. Sci. Rep. 2015, 5, 18561. [Google Scholar] [CrossRef]
- Busk, P.K.; Lange, L. Classification of fungal and bacterial lytic polysaccharide monooxygenases. BMC Genom. 2015, 16, 368. [Google Scholar] [CrossRef]
- Lange, L.; Huang, Y.; Busk, P.K. Microbial decomposition of keratin in nature-a new hypothesis of industrial relevance. Appl. Microbiol. Biotechnol. 2016, 100, 2083–2096. [Google Scholar] [CrossRef]
- Muraleedharan, M.N.; Karnaouri, A.; Piatkova, M.; Ruiz-Caldas, M.-X.; Matsakas, L.; Liu, B.; Rova, U.; Christakopoulos, P.; Mathew, A.P. Isolation and modification of nano-scale cellulose from organosolv-treated birch through the synergistic activity of LPMO and endoglucanases. Int. J. Biol. Macromol. 2021, 183, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, J.; Ma, F.; Chen, Q.; Xiao, Q.; Zhang, X.; Xie, S.; Yu, H. Lytic polysaccharide monooxygenases promote oxidative cleavage of lignin and lignin–carbohydrate complexes during fungal degradation of lignocellulose. Environ. Microbiol. 2021, 23, 4547–4560. [Google Scholar] [CrossRef]
- Kont, R.; Pihlajaniemi, V.; Borisova, A.S.; Aro, N.; Marjamaa, K.; Loogen, J.; Büchs, J.; Eijsink, V.G.H.; Kruus, K.; Väljamäe, P. The liquid fraction from hydrothermal pretreatment of wheat straw provides lytic polysaccharide monooxygenases with both electrons and H2O2 co-substrate. Biotechnol. Biofuels 2019, 12, 235. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, L.; Zhang, K.; Walker, T.; Thy, P.; Jenkins, B.; Zheng, Y. Pretreatment of lignocellulosic biomass using bioleaching to reduce inorganic elements. Fuel 2019, 246, 386–393. [Google Scholar] [CrossRef]
- Zhang, N.; Walker, T.; Jenkins, B.; Anderson, S.; Zheng, Y. Bioleaching of Sorghum Straw in Bioreactors for Biomass Cleaning. Fermentation 2021, 7, 270. [Google Scholar] [CrossRef]
- Pernak, J.; Rzemieniecki, T.; Matern, K. Ionic liquids “in a nutshell” (history, properties and development). Chemik 2016, 70, 471–480. [Google Scholar]
- Mallakpour, S.; Dinari, M. Ionic Liquids as Green Solvents: Progress and Prospects. In Green Solvents II; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–32. [Google Scholar] [CrossRef]
- Hou, Q.; Ju, M.; Li, W.; Liu, L.; Chen, Y.; Yang, Q. Pretreatment of Lignocellulosic Biomass with Ionic Liquids and Ionic Liquid-Based Solvent Systems. Molecules 2017, 22, 490. [Google Scholar] [CrossRef]
- Liu, Y.-R.; Thomsen, K.; Nie, Y.; Zhang, S.-J.; Meyer, A.S. Predictive screening of ionic liquids for dissolving cellulose and experimental verification. Green Chem. 2016, 18, 6246–6254. [Google Scholar] [CrossRef]
- Pu, Y.; Jiang, N.; Ragauskas, A.J. Ionic Liquid as a Green Solvent for Lignin. J. Wood Chem. Technol. 2007, 27, 23–33. [Google Scholar] [CrossRef]
- Ji, W.; Ding, Z.; Liu, J.; Song, Q.; Xia, X.; Gao, H.; Wang, H.; Gu, W. Mechanism of Lignin Dissolution and Regeneration in Ionic Liquid. Energy Fuels 2012, 26, 6393–6403. [Google Scholar] [CrossRef]
- Tan, S.S.Y.; MacFarlane, D.R.; Upfal, J.; Edye, L.A.; Doherty, W.O.S.; Patti, A.F.; Pringle, J.M.; Scott, J.L. Extraction of lignin from lignocellulose at atmospheric pressure using alkylbenzenesulfonate ionic liquid. Green Chem. 2009, 11, 339–345. [Google Scholar] [CrossRef]
- Brandt, A.; Chen, L.; van Dongen, B.E.; Welton, T.; Hallett, J.P. Structural changes in lignins isolated using an acidic ionic liquid water mixture. Green Chem. 2015, 17, 5019–5034. [Google Scholar] [CrossRef]
- Yoo, C.G.; Pu, Y.; Ragauskas, A.J. Ionic liquids: Promising green solvents for lignocellulosic biomass utilization. Curr. Opin. Green Sustain. Chem. 2017, 5, 5–11. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Lee, D.-J. Pretreatment of biomass using ionic liquids: Research updates. Renew. Energy 2017, 111, 77–84. [Google Scholar] [CrossRef]
- Farrán, A.; Cai, C.; Sandoval, M.; Xu, Y.; Liu, J.; Hernáiz, M.J.; Linhardt, R.J. Green Solvents in Carbohydrate Chemistry: From Raw Materials to Fine Chemicals. Chem. Rev. 2015, 115, 6811–6853. [Google Scholar] [CrossRef]
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic Liquids and Cellulose: Dissolution, Chemical Modification and Preparation of New Cellulosic Materials. Int. J. Mol. Sci. 2014, 15, 11922. [Google Scholar] [CrossRef]
- Peleteiro, S.; Rivas, S.; Alonso, J.L.; Santos, V.; Parajo, J.C. Utilization of Ionic Liquids in Lignocellulose Biorefineries as Agents for Separation, Derivatization, Fractionation, or Pretreatment. J. Agric. Food Chem. 2015, 63, 8093–8102. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, Y.; Chen, Q.; Yu, Z.; Wang, C.; Jin, S.; Ding, Y.; Wu, G. Dissolution of cellulose with ionic liquids and its application: A mini-review. Green Chem. 2006, 8, 325–327. [Google Scholar] [CrossRef]
- Ang, T.N.; Ngoh, G.C.; Chua, A.S.M.; Lee, M.G. Elucidation of the effect of ionic liquid pretreatment on rice husk via structural analyses. Biotechnol. Biofuels 2012, 5, 67. [Google Scholar] [CrossRef]
- An, Y.-X.; Zong, M.-H.; Wu, H.; Li, N. Pretreatment of lignocellulosic biomass with renewable cholinium ionic liquids: Biomass fractionation, enzymatic digestion and ionic liquid reuse. Bioresour. Technol. 2015, 192, 165–171. [Google Scholar] [CrossRef]
- Miyafuji, H. Application of ionic liquids for effective use of woody biomass. J. Wood Sci. 2015, 61, 343–350. [Google Scholar] [CrossRef]
- Socha, A.M.; Parthasarathi, R.; Shi, J.; Pattathil, S.; Whyte, D.; Bergeron, M.; George, A.; Tran, K.; Stavila, V.; Venkatachalam, S.; et al. Efficient biomass pretreatment using ionic liquids derived from lignin and hemicellulose. Proc. Natl. Acad. Sci. USA 2014, 111, E3587–E3595. [Google Scholar] [CrossRef]
- Sadaf, A.; Morya, V.K.; Khare, S.K. Applicability of Sporotrichum thermophile xylanase in the in situ saccharification of wheat straw pre-treated with ionic liquids. Process Biochem. 2016, 51, 2090–2096. [Google Scholar] [CrossRef]
- Li, Q.; He, Y.-C.; Xian, M.; Jun, G.; Xu, X.; Yang, J.-M.; Li, L.-Z. Improving enzymatic hydrolysis of wheat straw using ionic liquid 1-ethyl-3-methyl imidazolium diethyl phosphate pretreatment. Bioresour. Technol. 2009, 100, 3570–3575. [Google Scholar] [CrossRef]
- Soudham, V.P.; Raut, D.G.; Anugwom, I.; Brandberg, T.; Larsson, C.; Mikkola, J.-P. Coupled enzymatic hydrolysis and ethanol fermentation: Ionic liquid pretreatment for enhanced yields. Biotechnol. Biofuels 2015, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Phanthong, P.; Karnjanakom, S.; Reubroycharoen, P.; Hao, X.; Abudula, A.; Guan, G. A facile one-step way for extraction of nanocellulose with high yield by ball milling with ionic liquid. Cellulose 2017, 24, 2083–2093. [Google Scholar] [CrossRef]
- Ullah, Z.; Bustam, M.A.; Man, Z. Biodiesel production from waste cooking oil by acidic ionic liquid as a catalyst. Renew. Energy 2015, 77, 521–526. [Google Scholar] [CrossRef]
- Ali Elsheikh, Y.; Hassan Akhtar, F. Biodiesel from Citrullus colocynthis Oil: Sulfonic-Ionic Liquid-Catalyzed Esterification of a Two-Step Process. Sci. World J. 2014, 2014, 540765. [Google Scholar] [CrossRef]
- Elgharbawy, A.A.; Alam, M.Z.; Kabbashi, N.A.; Moniruzzaman, M.; Jamal, P. Evaluation of several ionic liquids for in situ hydrolysis of empty fruit bunches by locally-produced cellulase. 3 Biotech 2016, 6, 128. [Google Scholar] [CrossRef]
- Passos, H.; Freire, M.G.; Coutinho, J.A.P. Ionic liquid solutions as extractive solvents for value-added compounds from biomass. Green Chem. 2014, 16, 4786–4815. [Google Scholar] [CrossRef]
- Da Costa Lopes, A.M.; Bogel-Łukasik, R. Acidic Ionic Liquids as Sustainable Approach of Cellulose and Lignocellulosic Biomass Conversion without Additional Catalysts. ChemSusChem 2015, 8, 947–965. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Liu, B.; Wang, Y.; Fang, Z.; Zhang, Z. Efficient conversion of cellulose into biofuel precursor 5-hydroxymethylfurfural in dimethyl sulfoxide–ionic liquid mixtures. Bioresour. Technol. 2014, 151, 361–366. [Google Scholar] [CrossRef]
- Carvalho, A.V.; da Costa Lopes, A.M.; Bogel-Lukasik, R. Relevance of the acidic 1-butyl-3-methylimidazolium hydrogen sulphate ionic liquid in the selective catalysis of the biomass hemicellulose fraction. RSC Adv. 2015, 5, 47153–47164. [Google Scholar] [CrossRef]
- Payal, R.S.; Bejagam, K.K.; Mondal, A.; Balasubramanian, S. Dissolution of Cellulose in Room Temperature Ionic Liquids: Anion Dependence. J. Phys. Chem. B 2015, 119, 1654–1659. [Google Scholar] [CrossRef]
- Sun, N.; Rodriguez, H.; Rahman, M.; Rogers, R.D. Where are ionic liquid strategies most suited in the pursuit of chemicals and energy from lignocellulosic biomass? Chem. Commun. 2011, 47, 1405–1421. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of Cellose with Ionic Liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Zakrzewska, M.E.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Solubility of Carbohydrates in Ionic Liquids. Energy Fuels 2010, 24, 737–745. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Wang, J.; Zhang, S. Effects of Cationic Structure on Cellulose Dissolution in Ionic Liquids: A Molecular Dynamics Study. ChemPhysChem 2012, 13, 3126–3133. [Google Scholar] [CrossRef]
- Hart, W.E.S.; Harper, J.B.; Aldous, L. The effect of changing the components of an ionic liquid upon the solubility of lignin. Green Chem. 2015, 17, 214–218. [Google Scholar] [CrossRef]
- Da Costa Lopes, A.M.; João, K.G.; Morais, A.R.C.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Ionic liquids as a tool for lignocellulosic biomass fractionation. Sustain. Chem. Process. 2013, 1, 3. [Google Scholar] [CrossRef]
- De Gregorio, G.F.; Weber, C.C.; Grasvik, J.; Welton, T.; Brandt, A.; Hallett, J.P. Mechanistic insights into lignin depolymerisation in acidic ionic liquids. Green Chem. 2016, 18, 5456–5465. [Google Scholar] [CrossRef]
- Brandt, A.; Hallett, J.P.; Leak, D.J.; Murphy, R.J.; Welton, T. The effect of the ionic liquid anion in the pretreatment of pine wood chips. Green Chem. 2010, 12, 672–679. [Google Scholar] [CrossRef]
- Wu, H.; Mora-Pale, M.; Miao, J.; Doherty, T.V.; Linhardt, R.J.; Dordick, J.S. Facile pretreatment of lignocellulosic biomass at high loadings in room temperature ionic liquids. Biotechnol. Bioeng. 2011, 108, 2865–2875. [Google Scholar] [CrossRef]
- Minnick, D.L.; Flores, R.A.; DeStefano, M.R.; Scurto, A.M. Cellulose Solubility in Ionic Liquid Mixtures: Temperature, Cosolvent, and Antisolvent Effects. J. Phys. Chem. B 2016, 120, 7906–7919. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Brandt, A.; Tran, K.; Zahari, S.M.S.N.S.; Klein-Marcuschamer, D.; Sun, N.; Sathitsuksanoh, N.; Shi, J.; Stavila, V.; Parthasarathi, R.; et al. Design of low-cost ionic liquids for lignocellulosic biomass pretreatment. Green Chem. 2015, 17, 1728–1734. [Google Scholar] [CrossRef]
- Brandt-Talbot, A.; Gschwend, F.J.V.; Fennell, P.S.; Lammens, T.M.; Tan, B.; Weale, J.; Hallett, J.P. An economically viable ionic liquid for the fractionation of lignocellulosic biomass. Green Chem. 2017, 19, 3078–3102. [Google Scholar] [CrossRef]
- Xia, S.; Baker, G.A.; Li, H.; Ravula, S.; Zhao, H. Aqueous Ionic Liquids and Deep Eutectic Solvents for Cellulosic Biomass Pretreatment and Saccharification. RSC Adv. 2014, 4, 10586–10596. [Google Scholar] [CrossRef]
- Minnick, D.L.; Scurto, A.M. Reversible and non-reactive cellulose separations from ionic liquid mixtures with compressed carbon dioxide. Chem. Commun. 2015, 51, 12649–12652. [Google Scholar] [CrossRef]
- Heinze, T.; Dicke, R.; Koschella, A.; Kull, A.H.; Klohr, E.-A.; Koch, W. Effective preparation of cellulose derivatives in a new simple cellulose solvent. Macromol. Chem. Phys. 2000, 201, 627–631. [Google Scholar] [CrossRef]
- Ema, T.; Komiyama, T.; Sunami, S.; Sakai, T. Synergistic effect of quaternary ammonium hydroxide and crown ether on the rapid and clear dissolution of cellulose at room temperature. RSC Adv. 2014, 4, 2523–2525. [Google Scholar] [CrossRef]
- Miao, J.; Sun, H.; Yu, Y.; Song, X.; Zhang, L. Quaternary ammonium acetate: An efficient ionic liquid for the dissolution and regeneration of cellulose. RSC Adv. 2014, 4, 36721–36724. [Google Scholar] [CrossRef]
- Abe, M.; Fukaya, Y.; Ohno, H. Fast and facile dissolution of cellulose with tetrabutylphosphonium hydroxide containing 40 wt% water. Chem. Commun. 2012, 48, 1808–1810. [Google Scholar] [CrossRef]
- Zhong, C.; Wang, C.; Huang, F.; Jia, H.; Wei, P. Wheat straw cellulose dissolution and isolation by tetra-n-butylammonium hydroxide. Carbohydr. Polym. 2013, 94, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-J.; Zhang, X.-Q.; Matharu, A.; Melo, E.; Li, R.-M.; Liu, C.-F.; Shi, Q.-S. Monitoring the Crystalline Structure of Sugar Cane Bagasse in Aqueous Ionic Liquids. ACS Sustain. Chem. Eng. 2017, 5, 7278–7283. [Google Scholar] [CrossRef]
- Viell, J.; Inouye, H.; Szekely, N.K.; Frielinghaus, H.; Marks, C.; Wang, Y.; Anders, N.; Spiess, A.C.; Makowski, L. Multi-scale processes of beech wood disintegration and pretreatment with 1-ethyl-3-methylimidazolium acetate/water mixtures. Biotechnol. Biofuels 2016, 9, 7. [Google Scholar] [CrossRef]
- Parthasarathi, R.; Sun, J.; Dutta, T.; Sun, N.; Pattathil, S.; Murthy Konda, N.V.S.N.; Peralta, A.G.; Simmons, B.A.; Singh, S. Activation of lignocellulosic biomass for higher sugar yields using aqueous ionic liquid at low severity process conditions. Biotechnol. Biofuels 2016, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Inoue, K.; Aomori, Y.; Ohnishi, A.; Ogino, C.; Shimizu, N.; Takahashi, K. Characterization of fractionated biomass component and recovered ionic liquid during repeated process of cholinium ionic liquid-assisted pretreatment and fractionation. Chem. Eng. J. 2015, 259, 323–329. [Google Scholar] [CrossRef]
- Liu, L.; Hu, Y.; Wen, P.; Li, N.; Zong, M.; Ou-Yang, B.; Wu, H. Evaluating the effects of biocompatible cholinium ionic liquids on microbial lipid production by Trichosporon fermentans. Biotechnol. Biofuels 2015, 8, 119. [Google Scholar] [CrossRef]
- Sarvaramini, A.; Gravel, O.; Larachi, F. Torrefaction of ionic-liquid impregnated lignocellulosic biomass and its comparison to dry torrefaction. Fuel 2013, 103, 814–826. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Abidi, N. Isolation of Chitin Nano-whiskers Directly from Crustacean Biomass Waste in a Single Step with Acidic Ionic Liquids. ACS Sustain. Chem. Eng. 2022, 10, 11846–11855. [Google Scholar] [CrossRef]
- Procentese, A.; Johnson, E.; Orr, V.; Garruto Campanile, A.; Wood, J.A.; Marzocchella, A.; Rehmann, L. Deep eutectic solvent pretreatment and subsequent saccharification of corncob. Bioresour. Technol. 2015, 192, 31–36. [Google Scholar] [CrossRef]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. Low-transition-temperature mixtures (LTTMs): A new generation of designer solvents. Angew. Chem. Int. Ed. Engl. 2013, 52, 3074–3085. [Google Scholar] [CrossRef]
- Tang, B.; Zhang, H.; Row, K.H. Application of deep eutectic solvents in the extraction and separation of target compounds from various samples. J. Sep. Sci. 2015, 38, 1053–1064. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as a new extraction media for phenolic metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.M.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents-Solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.; Witkamp, G.J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef]
- Lian, H.; Hong, S.; Carranza, A.; Mota-Morales, J.D.; Pojman, J.A. Processing of lignin in urea–zinc chloride deep-eutectic solvent and its use as a filler in a phenol-formaldehyde resin. RSC Adv. 2015, 5, 28778–28785. [Google Scholar] [CrossRef]
- Yiin, C.L.; Quitain, A.T.; Yusup, S.; Sasaki, M.; Uemura, Y.; Kida, T. Characterization of natural low transition temperature mixtures (LTTMs): Green solvents for biomass delignification. Bioresour. Technol. 2016, 199, 258–264. [Google Scholar] [CrossRef]
- Kumar, A.K.; Parikh, B.S.; Pravakar, M. Natural deep eutectic solvent mediated pretreatment of rice straw: Bioanalytical characterization of lignin extract and enzymatic hydrolysis of pretreated biomass residue. Environ. Sci. Pollut. Res. Int. 2016, 23, 9265–9275. [Google Scholar] [CrossRef]
- Zhang, C.W.; Xia, S.Q.; Ma, P.S. Facile pretreatment of lignocellulosic biomass using deep eutectic solvents. Bioresour. Technol. 2016, 219, 1–5. [Google Scholar] [CrossRef]
- Xu, G.-C.; Ding, J.-C.; Han, R.-Z.; Dong, J.-J.; Ni, Y. Enhancing cellulose accessibility of corn stover by deep eutectic solvent pretreatment for butanol fermentation. Bioresour. Technol. 2016, 203, 364–369. [Google Scholar] [CrossRef]
- Hiltunen, J.; Kuutti, L.; Rovio, S.; Puhakka, E.; Virtanen, T.; Ohra-Aho, T.; Vuoti, S. Using a low melting solvent mixture to extract value from wood biomass. Sci. Rep. 2016, 6, 32420. [Google Scholar] [CrossRef]
- Alvarez-Vasco, C.; Ma, R.; Quintero, M.; Guo, M.; Geleynse, S.; Ramasamy, K.K.; Wolcott, M.; Zhang, X. Unique low-molecular-weight lignin with high purity extracted from wood by deep eutectic solvents (DES): A source of lignin for valorization. Green Chem. 2016, 18, 5133–5141. [Google Scholar] [CrossRef]
- Jablonský, M.; Škulcová, A.; Kamenská, L.; Vrška, M.; Šíma, J. Deep Eutectic Solvents: Fractionation of Wheat Straw. BioResources 2015, 10, 8039–8047. [Google Scholar] [CrossRef]
- Fang, C.; Thomsen, M.H.; Frankær, C.G.; Brudecki, G.P.; Schmidt, J.E.; AlNashef, I.M. Reviving Pretreatment Effectiveness of Deep Eutectic Solvents on Lignocellulosic Date Palm Residues by Prior Recalcitrance Reduction. Ind. Eng. Chem. Res. 2017, 56, 3167–3174. [Google Scholar] [CrossRef]
- Marino, D.D.; Stöckmann, D.; Kriescher, S.; Stiefel, S.; Wessling, M. Electrochemical depolymerisation of lignin in a deep eutectic solvent. Green Chem. 2016, 18, 6021–6028. [Google Scholar] [CrossRef]
- Lynam, J.G.; Kumar, N.; Wong, M.J. Deep eutectic solvents’ ability to solubilize lignin, cellulose, and hemicellulose; thermal stability; and density. Bioresour. Technol. 2017, 238, 684–689. [Google Scholar] [CrossRef]
- Zhang, Q.; Benoit, M.; De Oliveira Vigier, K.; Barrault, J.; Jerome, F. Green and inexpensive choline-derived solvents for cellulose decrystallization. Chem. Eur. J. 2012, 18, 1043–1046. [Google Scholar] [CrossRef]
- Ramesh, S.; Shanti, R.; Morris, E. Characterization of conducting cellulose acetate based polymer electrolytes doped with “green” ionic mixture. Carbohydr. Polym. 2013, 91, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Sirviö, J.A.; Visanko, M.; Liimatainen, H. Deep eutectic solvent system based on choline chloride-urea as a pre-treatment for nanofibrillation of wood cellulose. Green Chem. 2015, 17, 3401–3406. [Google Scholar] [CrossRef]
- Wang, S.; Peng, X.; Zhong, L.; Sun, R. Choline chloride/urea as an effective plasticizer for production of cellulose films. Carbohydr. Polym. 2016, 117, 133–139. [Google Scholar] [CrossRef]
- Sharma, M.; Mukesh, C.; Mondal, D.; Prasad, K. Dissolution of α-chitin in deep eutectic solvents. RSC Adv. 2013, 3, 18149–18155. [Google Scholar] [CrossRef]
- Mukesh, C.; Mondal, D.; Sharma, M.; Prasad, K. Choline chloride–thiourea, a deep eutectic solvent for the production of chitin nanofibers. Carbohydr. Polym. 2014, 103, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Gunny, A.A.N.; Arbain, D.; Daud, M.Z.M.; Jamal, P. Synergistic action of deep eutectic solvents and cellulases for lignocellulosic biomass hydrolysis. Mater. Res. Innov. 2014, 18, S6-65. [Google Scholar] [CrossRef]
- Gunny, A.A.; Arbain, D.; Nashef, E.M.; Jamal, P. Applicability evaluation of Deep Eutectic Solvents-Cellulase system for lignocellulose hydrolysis. Bioresour. Technol. 2015, 181, 297–302. [Google Scholar] [CrossRef]
- Duan, L.; Dou, L.; Guo, L.; Liu, E. Comprehensive Evaluation of Deep Eutectic Solvents in Extraction of Bioactive Natural Products. ACS Sustain. Chem. Eng. 2016, 4, 2405–2411. [Google Scholar] [CrossRef]
- Dai, Y.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents providing enhanced stability of natural colorants from safflower (Carthamus tinctorius). Food Chem. 2014, 159, 116–121. [Google Scholar] [CrossRef]
- Chen, J.; Liu, M.; Wang, Q.; Du, H.; Zhang, L. Deep Eutectic Solvent-Based Microwave-Assisted Method for Extraction of Hydrophilic and Hydrophobic Components from Radix Salviae miltiorrhizae. Molecules 2016, 21, 1383. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.E.; Mangos, D.N.; Slattery, A.D.; Raston, C.L.; Boulos, R.A. Wool deconstruction using a benign eutectic melt. RSC Adv. 2016, 6, 20095–20101. [Google Scholar] [CrossRef]
- Kumar, A.K.; Parikh, B.S.; Shah, E.; Liu, L.Z.; Cotta, M.A. Cellulosic ethanol production from green solvent-pretreated rice straw. Biocatal. Agric. Biotechnol. 2016, 7, 14–23. [Google Scholar] [CrossRef]
- Wahlström, R.; Hiltunen, J.; Sirkka, M.P.d.S.N.; Vuoti, S.; Kruus, K. Comparison of three deep eutectic solvents and 1-ethyl-3-methylimidazolium acetate in the pretreatment of lignocellulose: Effect on enzyme stability, lignocellulose digestibility and one-pot hydrolysis. RSC Adv. 2016, 6, 68100–68110. [Google Scholar] [CrossRef]
- Lehmann, C.; Sibilla, F.; Maugeri, Z.; Streit, W.R.; Dominguez de Maria, P.; Martinez, R.; Schwaneberg, U. Reengineering CelA2 cellulase for hydrolysis in aqueous solutions of deep eutectic solvents and concentrated seawater. Green Chem. 2012, 14, 2719–2726. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Al-Saadi, M.A.; Hayyan, A.; AlNashef, I.M.; Mirghani, M.E. Assessment of cytotoxicity and toxicity for phosphonium-based deep eutectic solvents. Chemosphere 2013, 93, 455–459. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; AlNashef, I.M.; Mirghani, M.E.; Saheed, O.K. Are deep eutectic solvents benign or toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef]
- Juneidi, I.; Hayyan, M.; Mohd Ali, O. Toxicity profile of choline chloride-based deep eutectic solvents for fungi and Cyprinus carpio fish. Environ. Sci. Pollut. Res. Int. 2016, 23, 7648–7659. [Google Scholar] [CrossRef]
- Huang, Z.-L.; Wu, B.-P.; Wen, Q.; Yang, Z. Deep eutectic solvents can be viable enzyme activators and stabilizers. J. Chem. Technol. Biotechnol. 2014, 89, 1975–1981. [Google Scholar] [CrossRef]
- Sharma, V.; Tsai, M.-L.; Chen, C.-W.; Sun, P.-P.; Patel, A.K.; Singhania, R.R.; Nargotra, P.; Dong, C.-D. Deep eutectic solvents as promising pretreatment agents for sustainable lignocellulosic biorefineries: A review. Bioresour. Technol. 2022, 360, 127631. [Google Scholar] [CrossRef]
- Loow, Y.-L.; New, E.K.; Yang, G.H.; Ang, L.Y.; Foo, L.Y.W.; Wu, T.Y. Potential use of deep eutectic solvents to facilitate lignocellulosic biomass utilization and conversion. Cellulose 2017, 24, 3591–3618. [Google Scholar] [CrossRef]
- McReynolds, C.; Adrien, A.; Castejon, N.; Fernandes, S.C.M. Green in the deep blue: Deep eutectic solvents as versatile systems for the processing of marine biomass. Green Chem. Lett. Rev. 2022, 15, 383–404. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; Radojčić Redovniković, I.; Duarte, A.R.C.; Matias, A.A.; Paiva, A. Low-Phytotoxic Deep Eutectic Systems as Alternative Extraction Media for the Recovery of Chitin from Brown Crab Shells. ACS Omega 2021, 6, 28729–28741. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Li, J.; Yan, T.; Wang, X.; Huang, J.; Kuang, Z.; Ye, M.; Pan, M. Selectivity of deproteinization and demineralization using natural deep eutectic solvents for production of insect chitin (Hermetia illucens). Carbohydr. Polym. 2019, 225, 115255. [Google Scholar] [CrossRef]
- Mirmohamadsadeghi, S.; Karimi, K. Chapter 21—Recovery of silica from rice straw and husk. In Current Developments in Biotechnology and Bioengineering; Varjani, S., Pandey, A., Gnansounou, E., Khanal, S.K., Raveendran, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 411–433. [Google Scholar] [CrossRef]
- Yadav, M.; Dwibedi, V.; Sharma, S.; George, N. Biogenic silica nanoparticles from agro-waste: Properties, mechanism of extraction and applications in environmental sustainability. J. Environ. Chem. Eng. 2022, 10, 108550. [Google Scholar] [CrossRef]
- Liou, T.-H.; Wu, S.-J. Kinetics Study and Characteristics of Silica Nanoparticles Produced from Biomass-Based Material. Ind. Eng. Chem. Res. 2010, 49, 8379–8387. [Google Scholar] [CrossRef]
- Rehman, M.S.U.; Umer, M.A.; Rashid, N.; Kim, I.; Han, J.-I. Sono-assisted sulfuric acid process for economical recovery of fermentable sugars and mesoporous pure silica from rice straw. Ind. Crops Prod. 2013, 49, 705–711. [Google Scholar] [CrossRef]
- San, N.O.; Kurşungöz, C.; Tümtaş, Y.; Yaşa, Ö.; Ortaç, B.; Tekinay, T. Novel one-step synthesis of silica nanoparticles from sugarbeet bagasse by laser ablation and their effects on the growth of freshwater algae culture. Particuology 2014, 17, 29–35. [Google Scholar] [CrossRef]
- Chen, H.; Wang, W.; Martin, J.C.; Oliphant, A.J.; Doerr, P.A.; Xu, J.F.; DeBorn, K.M.; Chen, C.; Sun, L. Extraction of Lignocellulose and Synthesis of Porous Silica Nanoparticles from Rice Husks: A Comprehensive Utilization of Rice Husk Biomass. ACS Sustain. Chem. Eng. 2013, 1, 254–259. [Google Scholar] [CrossRef]
- Okur, M.; Eslek Koyuncu, D.D. Investigation of pretreatment parameters in the delignification of paddy husks with deep eutectic solvents. Biomass Bioenergy 2020, 142, 105811. [Google Scholar] [CrossRef]
- Adam, F.; Chew, T.-S.; Andas, J. A simple template-free sol–gel synthesis of spherical nanosilica from agricultural biomass. J. Sol-Gel Sci. Technol. 2011, 59, 580–583. [Google Scholar] [CrossRef]
- Yuan, Z.; Kapu, N.S.; Martinez, D.M. An eco-friendly scheme to eliminate silica problems during bamboo biomass fractionation. Nord. Pulp Pap. Res. J. 2017, 32, 4–13. [Google Scholar] [CrossRef]
- Khaleghian, H.; Molaverdi, M.; Karimi, K. Silica Removal from Rice Straw To Improve its Hydrolysis and Ethanol Production. Ind. Eng. Chem. Res. 2017, 56, 9793–9798. [Google Scholar] [CrossRef]
- Abdul, P.M.; Jahim, J.M.; Harun, S.; Markom, M.; Lutpi, N.A.; Hassan, O.; Balan, V.; Dale, B.E.; Mohd Nor, M.T. Effects of changes in chemical and structural characteristic of ammonia fibre expansion (AFEX) pretreated oil palm empty fruit bunch fibre on enzymatic saccharification and fermentability for biohydrogen. Bioresour. Technol. 2016, 211, 200–208. [Google Scholar] [CrossRef]
- Corrales-Ureña, Y.R.; Villalobos-Bermúdez, C.; Pereira, R.; Camacho, M.; Estrada, E.; Argüello-Miranda, O.; Vega-Baudrit, J.R. Biogenic silica-based microparticles obtained as a sub-product of the nanocellulose extraction process from pineapple peels. Sci. Rep. 2018, 8, 10417. [Google Scholar] [CrossRef] [PubMed]
- Bansal, V.; Ahmad, A.; Sastry, M. Fungus-Mediated Biotransformation of Amorphous Silica in Rice Husk to Nanocrystalline Silica. J. Am. Chem. Soc. 2006, 128, 14059–14066. [Google Scholar] [CrossRef]
- El-Gazzar, N.; Almanaa, T.N.; Reda, R.M.; El Gaafary, M.N.; Rashwan, A.A.; Mahsoub, F. Assessment the using of silica nanoparticles (SiO2NPs) biosynthesized from rice husks by Trichoderma harzianum MF780864 as water lead adsorbent for immune status of Nile tilapia (Oreochromis niloticus). Saudi J. Biol. Sci. 2021, 28, 5119–5130. [Google Scholar] [CrossRef]
- Zielonka, A.; Żymańczyk-Duda, E.; Brzezińska-Rodak, M.; Duda, M.; Grzesiak, J.; Klimek-Ochab, M. Nanosilica synthesis mediated by Aspergillus parasiticus strain. Fungal Biol. 2018, 122, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Pieła, A.; Żymańczyk-Duda, E.; Brzezińska-Rodak, M.; Duda, M.; Grzesiak, J.; Saeid, A.; Mironiuk, M.; Klimek-Ochab, M. Biogenic synthesis of silica nanoparticles from corn cobs husks. Dependence of the productivity on the method of raw material processing. Bioorganic Chem. 2020, 99, 103773. [Google Scholar] [CrossRef]
- Estevez, M.; Vargas, S.; Castaño, V.M.; Rodriguez, R. Silica nano-particles produced by worms through a bio-digestion process of rice husk. J. Non-Cryst. Solids 2009, 355, 844–850. [Google Scholar] [CrossRef]
- Yunus, R.; Salleh, S.F.; Abdullah, N.; Biak, D.R.A. Effect of ultrasonic pre-treatment on low temperature acid hydrolysis of oil palm empty fruit bunch. Bioresour. Technol. 2010, 101, 9792–9796. [Google Scholar] [CrossRef]
- Salleh, N.; Hamid, K.; Hussain, N. Removal of Silica Bodies on Oil Palm Empty Fruit Bunch Surfaces and Application for Biogas Production. Adv. Mater. Res. 2013, 709, 895–899. [Google Scholar] [CrossRef]
- Kwei-Nam, L.; Wan Daud, W.R.; Ghazali, A. Morphological and chemical nature of fiber strands of Oil Palm Empty-Fruit-Bunch (OPEFB). BioResources 2007, 2, 351–362. [Google Scholar]
- Negrão, D.R.; Driemeier, C. Fate of silica phytoliths in the industrial crushing of sugarcane stalks. Ind. Crops Prod. 2022, 185, 115132. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Tran, N.T.; Phan, T.P.; Nguyen, A.T.; Nguyen, M.X.T.; Nguyen, N.N.; Ko, Y.H.; Nguyen, D.H.; Van, T.T.T.; Hoang, D. The extraction of lignocelluloses and silica from rice husk using a single biorefinery process and their characteristics. J. Ind. Eng. Chem. 2022, 108, 150–158. [Google Scholar] [CrossRef]
- Donner, M.; Gohier, R.; de Vries, H. A new circular business model typology for creating value from agro-waste. Sci. Total Environ. 2020, 716, 137065. [Google Scholar] [CrossRef]
- Setiawan, W.K.; Chiang, K.-Y. Crop Residues as Potential Sustainable Precursors for Developing Silica Materials: A Review. Waste Biomass Valorization 2021, 12, 2207–2236. [Google Scholar] [CrossRef]
- Menon, V.; Rao, M. Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept. Prog. Energy Combust. Sci. 2012, 38, 522–550. [Google Scholar] [CrossRef]
- Phutela, U.G.; Sahni, N. Microscopic structural changes in paddy straw pretreated with Trichoderma reesei MTCC 164 and Coriolus versicolor MTCC 138. Indian J. Microbiol. 2013, 53, 227–231. [Google Scholar] [CrossRef]
- Torres, M.G.; Muñoz, S.V.; Martínez, A.R.; Hernández, V.S.; Saucedo, A.V.; Cervantes, E.R.; Talavera, R.R.; Rivera, M.; del Pilar Carreón Castro, M. Morphology-controlled silicon oxide particles produced by red wiggler worms. Powder Technol. 2017, 310, 205–212. [Google Scholar] [CrossRef]
- Kauldhar, B.S.; Yadav, S.K. Turning waste to wealth: A direct process for recovery of nano-silica and lignin from paddy straw agro-waste. J. Clean. Prod. 2018, 194, 158–166. [Google Scholar] [CrossRef]
- Barana, D.; Salanti, A.; Orlandi, M.; Ali, D.S.; Zoia, L. Biorefinery process for the simultaneous recovery of lignin, hemicelluloses, cellulose nanocrystals and silica from rice husk and Arundo donax. Ind. Crops Prod. 2016, 86, 31–39. [Google Scholar] [CrossRef]
- Minu, K.; Jiby, K.K.; Kishore, V.V.N. Isolation and purification of lignin and silica from the black liquor generated during the production of bioethanol from rice straw. Biomass Bioenergy 2012, 39, 210–217. [Google Scholar] [CrossRef]
- Hamzah, F.; Idris, A.; Shuan, T.K. Preliminary study on enzymatic hydrolysis of treated oil palm (Elaeis) empty fruit bunches fibre (EFB) by using combination of cellulase and β 1-4 glucosidase. Biomass Bioenergy 2011, 35, 1055–1059. [Google Scholar] [CrossRef]
- Yuan, Z.; Wen, Y.; Kapu, N.S.; Beatson, R. Evaluation of an organosolv-based biorefinery process to fractionate wheat straw into ethanol and co-products. Ind. Crops Prod. 2018, 121, 294–302. [Google Scholar] [CrossRef]
- Thamsee, T.; Choojit, S.; Cheirsilp, B.; Yamseangsung, R.; Ruengpeerakul, T.; Sangwichien, C. Combination of Superheated Steam Explosion and Alkaline Autoclaving Pretreatment for Improvement of Enzymatic Digestibility of the Oil Palm Tree Residues as Alternative Sugar Sources. Waste Biomass Valorization 2019, 10, 3009–3023. [Google Scholar] [CrossRef]
- Mahmud, N.A.N.; Baharuddin, A.S.; Bahrin, E.K.; Sulaiman, A.; Naim, M.N.; Zakaria, R.; Hassan, M.A.; Nishida, H.; Shirai, Y. Enzymatic Saccharification of Oil Palm Mesocarp Fiber (OPMF) Treated with Superheated Steam. BioResources 2013, 8, 1320–1331. [Google Scholar] [CrossRef]
- Pinheiro, F.G.C.; Leitão, R.C.; Frollini, E. Removing silica from oil palm mesocarp fibers. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Jahan, M.S.; Rahman, M.M.; Ni, Y. Alternative initiatives for non-wood chemical pulping and integration with the biorefinery concept: A. review. Biofuels Bioprod. Biorefining 2021, 15, 100–118. [Google Scholar] [CrossRef]
- Thongma, B.; Chiarakorn, S. Recovery of silica and carbon black from rice husk ash disposed from a biomass power plant by precipitation method. IOP Conf. Ser. Earth Environ. Sci. 2019, 373, 012026. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, S.; Arumugam, S.M.; Miglani, C.; Elumalai, S. Biphasic Separation Approach in the DES Biomass Fractionation Facilitates Lignin Recovery for Subsequent Valorization to Phenolics. ACS Sustain. Chem. Eng. 2020, 8, 19140–19154. [Google Scholar] [CrossRef]
- Yue, X.; Suopajärvi, T.; Sun, S.; Mankinen, O.; Mikkelson, A.; Huttunen, H.; Komulainen, S.; Romakkaniemi, I.; Ahola, J.; Telkki, V.-V.; et al. High-purity lignin fractions and nanospheres rich in phenolic hydroxyl and carboxyl groups isolated with alkaline deep eutectic solvent from wheat straw. Bioresour. Technol. 2022, 360, 127570. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.H.; Zhang, Y.; Davis, R.; Heath, G.; Ravi, V. Biorefinery upgrading of herbaceous biomass to renewable hydrocarbon fuels, Part 2: Air pollutant emissions and permitting implications. J. Clean. Prod. 2022, 362, 132409. [Google Scholar] [CrossRef]
- Weikard, H.-P. Phosphorus recycling and food security in the long run: A conceptual modelling approach SpringerLink. Food Secur. 2016, 8, 405–414. [Google Scholar] [CrossRef]
- Pimentel, O.; Bailey, O.; Kim, P.; Mullaney, E.; Calabrese, J.; Walman, L.; Nelson, F.; Yao, X. Will Limits of the Earth’s Resources Control Human Numbers? | SpringerLink. Environ. Dev. Sustain. 1999, 1, 19–39. [Google Scholar] [CrossRef]
- Mayer, B.K.; Baker, L.A.; Boyer, T.H.; Drechsel, P.; Gifford, M.; Hanjra, M.A.; Parameswaran, P.; Stoltzfus, J.; Westerhoff, P.; Rittmann, B.E. Total Value of Phosphorus Recovery. Environ. Sci. Technol. 2016, 50, 6606–6620. [Google Scholar] [CrossRef] [PubMed]
- Peccia, J.; Westerhoff, P. We Should Expect More out of Our Sewage Sludge. Environ. Sci. Technol. 2015, 49, 8271–8276. [Google Scholar] [CrossRef] [PubMed]
- Donatello, S.; Cheeseman, C.R. Recycling and recovery routes for incinerated sewage sludge ash (ISSA): A review. Waste Manag. 2013, 33, 2328–2340. [Google Scholar] [CrossRef] [PubMed]
- Ottosen, L.M.; Jensen, P.E.; Kirkelund, G.M. Phosphorous recovery from sewage sludge ash suspended in water in a two-compartment electrodialytic cell. Waste Manag. 2016, 51, 142–148. [Google Scholar] [CrossRef]
- Chojnacka, K.; Gorazda, K.; Witek-Krowiak, A.; Moustakas, K. Recovery of fertilizer nutrients from materials—Contradictions, mistakes and future trends. Renew. Sustain. Energy Rev. 2019, 110, 485–498. [Google Scholar] [CrossRef]
- Mancini, E.; Raggi, A. A review of circularity and sustainability in anaerobic digestion processes. J. Environ. Manag. 2021, 291, 112695. [Google Scholar] [CrossRef] [PubMed]
- Martens, W.; Böhm, R. Overview of the ability of different treatment methods for liquid and solid manure to inactivate pathogens. Bioresour. Technol. 2009, 100, 5374–5378. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.Y.; Shen, Q.; Zhao, F. Antibiotics and antibiotic resistance from animal manures to soil: A review. Eur. J. Soil Sci. 2018, 69, 181–195. [Google Scholar] [CrossRef]
- Moreno-Caselles, J.; Moral, R.; Perez-Murcia, M.; Perez-Espinosa, A.; Rufete, B. Nutrient value of animal manures in front of environmental hazards. Commun. Soil Sci. Plant Anal. 2002, 33, 3023–3032. [Google Scholar] [CrossRef]
- Weiland, K.; Wlcek, B.; Krexner, T.; Kral, I.; Kontturi, E.; Mautner, A.; Bauer, A.; Bismarck, A. Excellence in Excrements: Upcycling of Herbivore Manure into Nanocellulose and Biogas. ACS Sustain. Chem. Eng. 2021, 9, 15506–15513. [Google Scholar] [CrossRef]
- Demichelis, F.; Pleissner, D.; Fiore, S.; Mariano, S.; Gutiérrez, I.M.N.; Schneider, R.; Venus, J. Investigation of food waste valorization through sequential lactic acid fermentative production and anaerobic digestion of fermentation residues. Bioresour. Technol. 2017, 241, 508–516. [Google Scholar] [CrossRef]
- Gottardo, M.; Bolzonella, D.; Tuci, G.A.; Valentino, F.; Majone, M.; Pavan, P.; Battista, F. Producing volatile fatty acids and polyhydroxyalkanoates from foods by-products and waste: A review. Bioresour. Technol. 2022, 361, 127716. [Google Scholar] [CrossRef]
- Lang, Q.; Liu, Z.; Li, Y.; Xu, J.; Li, J.; Liu, B.; Sun, Q. Combustion characteristics, kinetic and thermodynamic analyses of hydrochars derived from hydrothermal carbonization of cattle manure. J. Environ. Chem. Eng. 2022, 10, 106938. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, D.; Yang, J.; Yan, Z.; Zhang, Z.; Zhong, B.; Wang, X. Treatment of distiller grain with wet-process phosphoric acid leads to biochar for the sustained release of nutrients and adsorption of Cr(VI). J. Hazard. Mater. 2023, 441, 129949. [Google Scholar] [CrossRef]
- Wang, B.; Lian, G.; Lee, X.; Gao, B.; Li, L.; Liu, T.; Zhang, X.; Zheng, Y. Phosphogypsum as a novel modifier for distillers grains biochar removal of phosphate from water. Chemosphere 2020, 238, 124684. [Google Scholar] [CrossRef]
- Feng, Y.; Bu, T.; Zhang, Q.; Han, M.; Tang, Z.; Yuan, G.; Chen, D.; Hu, Y. Pyrolysis characteristics of anaerobic digestate from kitchen waste and availability of Phosphorus in pyrochar. J. Anal. Appl. Pyrolysis 2022, 168, 105729. [Google Scholar] [CrossRef]
- Tayibi, S.; Monlau, F.; Marias, F.; Thevenin, N.; Jimenez, R.; Oukarroum, A.; Alboulkas, A.; Zeroual, Y.; Barakat, A. Industrial symbiosis of anaerobic digestion and pyrolysis: Performances and agricultural interest of coupling biochar and liquid digestate. Sci. Total Environ. 2021, 793, 148461. [Google Scholar] [CrossRef]
- Monlau, F.; Francavilla, M.; Sambusiti, C.; Antoniou, N.; Solhy, A.; Libutti, A.; Zabaniotou, A.; Barakat, A.; Monteleone, M. Toward a functional integration of anaerobic digestion and pyrolysis for a sustainable resource management. Comparison between solid-digestate and its derived pyrochar as soil amendment. Appl. Energy 2016, 169, 652–662. [Google Scholar] [CrossRef]
- Bento, L.R.; Castro, A.J.R.; Moreira, A.B.; Ferreira, O.P.; Bisinoti, M.C.; Melo, C.A. Release of nutrients and organic carbon in different soil types from hydrochar obtained using sugarcane bagasse and vinasse. Geoderma 2019, 334, 24–32. [Google Scholar] [CrossRef]
- Zhao, X.; Becker, G.C.; Faweya, N.; Rodriguez Correa, C.; Yang, S.; Xie, X.; Kruse, A. Fertilizer and activated carbon production by hydrothermal carbonization of digestate. Biomass Convers. Biorefinery 2018, 8, 423–436. [Google Scholar] [CrossRef]
- Wood, B.M.; Jader, L.R.; Schendel, F.J.; Hahn, N.J.; Valentas, K.J.; McNamara, P.J.; Novak, P.M.; Heilmann, S.M. Industrial symbiosis: Corn ethanol fermentation, hydrothermal carbonization, and anaerobic digestion. Biotechnol. Bioeng. 2013, 110, 2624–2632. [Google Scholar] [CrossRef]
- Liu, G.; Bao, J. Maximizing phosphorus and potassium recycling by supplementation of lignin combustion ash from dry biorefining of lignocellulose. Biochem. Eng. J. 2019, 144, 104–109. [Google Scholar] [CrossRef]
- Alotaibi, K.D.; Schoenau, J.J.; Fonstad, T. Possible utilization of ash from meat and bone meal and dried distillers grains gasification as a phosphorus fertilizer: Crop growth response and changes in soil chemical properties. J. Soils Sediments 2013, 13, 1024–1031. [Google Scholar] [CrossRef]
- Eriksson, G.; Grimm, A.; Skoglund, N.; Boström, D.; Öhman, M. Combustion and fuel characterisation of wheat distillers dried grain with solubles (DDGS) and possible combustion applications. Fuel 2012, 102, 208–220. [Google Scholar] [CrossRef]
- Carpanez, T.G.; Moreira, V.R.; Magalhães, N.C.; Assis, I.R.; Lange, L.C.; Amaral, M.C.S. Integrated membrane-based processes to obtain organo-mineral fertilizer, water, and energy from sugarcane vinasse. Sep. Purif. Technol. 2022, 302, 122180. [Google Scholar] [CrossRef]
- Gienau, T.; Brüß, U.; Kraume, M.; Rosenberger, S. Nutrient recovery from anaerobic sludge by membrane filtration: Pilot tests at a 2.5 MWe biogas plant. Int. J. Recycl. Org. Waste Agric. 2018, 7, 325–334. [Google Scholar] [CrossRef]
- Kratky, L.; Zamazal, P. Economic feasibility and sensitivity analysis of fish waste processing biorefinery. J. Clean. Prod. 2020, 243, 118677. [Google Scholar] [CrossRef]
- Zhang, Q.; Hogen, T.; Zhou, K.; Berendts, S.; Hu, K.; Zhang, Y.; Geißen, S.-U. Dynamic and equilibrium precipitation of struvite from the concentrated cellulosic ethanol stillage. Water Sci. Technol. 2021, 84, 3859–3870. [Google Scholar] [CrossRef]
- Silva, A.F.R.; Lebron, Y.A.R.; Brasil, Y.L.; Lange, L.C.; Amaral, M.C.S. Effect of electrolyte solution recycling on the potassium recovery from vinasse by integrated electrodialysis and K-struvite precipitation processes. Chem. Eng. J. 2022, 450, 137975. [Google Scholar] [CrossRef]
- Teymouri, A.; Stuart, B.J.; Kumar, S. Hydroxyapatite and dittmarite precipitation from algae hydrolysate. Algal Res. 2018, 29, 202–211. [Google Scholar] [CrossRef]
- Pastor, L.; Mangin, D.; Ferrer, J.; Seco, A. Struvite formation from the supernatants of an anaerobic digestion pilot plant. Bioresour. Technol. 2010, 101, 118–125. [Google Scholar] [CrossRef]
- Juneja, A.; Cusick, R.; Singh, V. Recovering phosphorus as a coproduct from corn dry grind plants: A techno-economic evaluation. Cereal Chem. 2020, 97, 449–458. [Google Scholar] [CrossRef]
- Pepè Sciarria, T.; Vacca, G.; Tambone, F.; Trombino, L.; Adani, F. Nutrient recovery and energy production from digestate using microbial electrochemical technologies (METs). J. Clean. Prod. 2019, 208, 1022–1029. [Google Scholar] [CrossRef]
- Hou, H.; Li, Z.; Liu, B.; Liang, S.; Xiao, K.; Zhu, Q.; Hu, S.; Yang, J.; Hu, J. Biogas and phosphorus recovery from waste activated sludge with protocatechuic acid enhanced Fenton pretreatment, anaerobic digestion and microbial electrolysis cell. Sci. Total Environ. 2020, 704, 135274. [Google Scholar] [CrossRef]
- Szymańska, M.; Szara, E.; Sosulski, T.; Wąs, A.; Van Pruissen, G.W.; Cornelissen, R.L.; Borowik, M.; Konkol, M. A bio-refinery concept for N and P recovery—A chance for biogas plant development. Energies 2019, 12, 155. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Z.; Shen, B.; Liu, L. Insights into biochar and hydrochar production and applications: A review. Energy 2019, 171, 581–598. [Google Scholar] [CrossRef]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A Review of Biochar and Its Use and Function in Soil. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2010; Volume 105, pp. 47–82. [Google Scholar]
- Huang, Y.; Tao, B.; Lal, R.; Lorenz, K.; Jacinthe, P.A.; Shrestha, R.K.; Bai, X.; Singh, M.P.; Lindsey, L.E.; Ren, W. A global synthesis of biochar’s sustainability in climate-smart agriculture—Evidence from field and laboratory experiments. Renew. Sustain. Energy Rev. 2023, 172, 113042. [Google Scholar] [CrossRef]
- Rombel, A.; Krasucka, P.; Oleszczuk, P. Sustainable biochar-based soil fertilizers and amendments as a new trend in biochar research. Sci. Total Environ. 2022, 816, 151588. [Google Scholar] [CrossRef]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, W.; Lu, L.; Yan, L.; Yu, D. Utilization of biochar for the removal of nitrogen and phosphorus. J. Clean. Prod. 2020, 257, 120573. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Awasthi, S.K.; Wang, Q.; Wang, Z.; Lahori, A.H.; Ren, X.; Chen, H.; Wang, M.; Zhao, J.; Zhang, Z. Influence of biochar on volatile fatty acids accumulation and microbial community succession during biosolids composting. Bioresour. Technol. 2018, 251, 158–164. [Google Scholar] [CrossRef]
- Luo, D.; Wang, L.; Nan, H.; Cao, Y.; Wang, H.; Kumar, T.V.; Wang, C. Phosphorus adsorption by functionalized biochar: A review. Environ. Chem. Lett. 2022, 1–28. [Google Scholar] [CrossRef]
- Jellali, S.; El-Bassi, L.; Charabi, Y.; Usman, M.; Khiari, B.; Al-Wardy, M.; Jeguirim, M. Recent advancements on biochars enrichment with ammonium and nitrates from wastewaters: A critical review on benefits for environment and agriculture. J. Environ. Manag. 2022, 305, 114368. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Ye, Z.-L.; Chen, S. Engineered biochars for recovering phosphate and ammonium from wastewater: A review. Sci. Total Environ. 2021, 779, 146240. [Google Scholar] [CrossRef]
- Huygens, D.; Saveyn, H.; Tonini, D.; Eder, P.; Delgado Sancho, L. Technical Proposals for Selected New Fertilising Materials Under the Fertilising Products Regulation (Regulation (EU) 2019/1009); Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Huygens, D.; Saveyn, H.G.M. Agronomic efficiency of selected phosphorus fertilisers derived from secondary raw materials for European agriculture. A meta-analysis. Agron. Sustain. Dev. 2018, 38, 52. [Google Scholar] [CrossRef]
- Chen, Q.; Qin, J.; Sun, P.; Cheng, Z.; Shen, G. Cow dung-derived engineered biochar for reclaiming phosphate from aqueous solution and its validation as slow-release fertilizer in soil-crop system. J. Clean. Prod. 2018, 172, 2009–2018. [Google Scholar] [CrossRef]
- Shang, L.; Xu, H.; Huang, S.; Zhang, Y. Adsorption of Ammonium in Aqueous Solutions by the Modified Biochar and its Application as an Effective N-Fertilizer. Water Air Soil Pollut. 2018, 229, 320. [Google Scholar] [CrossRef]
- Kratz, S.; Vogel, C.; Adam, C. Agronomic performance of P recycling fertilizers and methods to predict it: A review. Nutr. Cycl. Agroecosyst. 2019, 115, 1–39. [Google Scholar] [CrossRef]
- Morash, D.; Lejeune, M.; Zicari, S. Organic Potassium Compositions Derived from Plant Ash. U.S. Patent Application 2022106237 A1, 7 April 2022. [Google Scholar]
- Herbert, G.; René, D.; Doris, M. A Process for Obtaining a Soluble Phosphate Fraction from Phosphate Containing Ash. European Patent 3495323 B1, 9 September 2020. [Google Scholar]
- Urano, T.; Sato, Y. Phosphate and Potash (PK)-Containing Compound Fertilizer. U.S. Patent 7452398 B2, 18 November 2008. [Google Scholar]
- Fang, L.; Wang, Q.; Li, J.-S.; Poon, C.S.; Cheeseman, C.; Donatello, S.; Tsang, D.C. Feasibility of wet-extraction of phosphorus from incinerated sewage sludge ash (ISSA) for phosphate fertilizer production: A critical review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 939–971. [Google Scholar] [CrossRef]
- Egle, L.; Rechberger, H.; Krampe, J.; Zessner, M. Phosphorus recovery from municipal wastewater: An integrated comparative technological, environmental and economic assessment of P recovery technologies. Sci. Total Environ. 2016, 571, 522–542. [Google Scholar] [CrossRef]
- Kehrein, P.; Van Loosdrecht, M.; Osseweijer, P.; Garfí, M.; Dewulf, J.; Posada, J. A critical review of resource recovery from municipal wastewater treatment plants–market supply potentials, technologies and bottlenecks. Environ. Sci. Water Res. Technol. 2020, 6, 877–910. [Google Scholar] [CrossRef]
- Di Capua, F.; de Sario, S.; Ferraro, A.; Petrella, A.; Race, M.; Pirozzi, F.; Fratino, U.; Spasiano, D. Phosphorous removal and recovery from urban wastewater: Current practices and new directions. Sci. Total Environ. 2022, 823, 153750. [Google Scholar] [CrossRef]
- Gherghel, A.; Teodosiu, C.; De Gisi, S. A review on wastewater sludge valorisation and its challenges in the context of circular economy. J. Clean. Prod. 2019, 228, 244–263. [Google Scholar] [CrossRef]
- Mladenović, M.; Paprika, M.; Marinković, A. Denitrification techniques for biomass combustion. Renew. Sustain. Energy Rev. 2018, 82, 3350–3364. [Google Scholar] [CrossRef]
- Torres, W.; Pansare, S.S.; Goodwin, J.G., Jr. Hot gas removal of tars, ammonia, and hydrogen sulfide from biomass gasification gas. Catal. Rev. 2007, 49, 407–456. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, Z.; Ge, P.; Wang, M.; Zhang, C.; Wang, H.; Zhao, L.; Wu, J.; Li, Y. The preparation of slow-release fertilizers with biomass ash and water/waste acid solutions from desulfurization and denitrification of flue gas. Environ. Sci. Pollut. Res. 2022, 29, 57566–57578. [Google Scholar] [CrossRef] [PubMed]
- Van Voorthuizen, E.M.; Zwijnenburg, A.; Wessling, M. Nutrient removal by NF and RO membranes in a decentralized sanitation system. Water Res. 2005, 39, 3657–3667. [Google Scholar] [CrossRef]
- Bilstad, T. Nitrogen separation from domestic wastewater by reverse osmosis. J. Membr. Sci. 1995, 102, 93–102. [Google Scholar] [CrossRef]
- Jenkins, B.M.; Mannapperuma, J.D.; Bakker, R.R. Biomass leachate treatment by reverse osmosis. Fuel Process. Technol. 2003, 81, 223–246. [Google Scholar] [CrossRef]
- Rajabzadeh, A.R.; Ruzich, N.; Zendehboudi, S.; Rahbari, M. Biomass Leachate Treatment and Nutrient Recovery Using Reverse Osmosis: Experimental Study and Hybrid Artificial Neural Network Modeling. Energy Fuels 2012, 26, 7155–7163. [Google Scholar] [CrossRef]
- Kumar, M.; Badruzzaman, M.; Adham, S.; Oppenheimer, J. Beneficial phosphate recovery from reverse osmosis (RO) concentrate of an integrated membrane system using polymeric ligand exchanger (PLE). Water Res. 2007, 41, 2211–2219. [Google Scholar] [CrossRef]
- Jupp, A.R.; Beijer, S.; Narain, G.C.; Schipper, W.; Slootweg, J.C. Phosphorus recovery and recycling–closing the loop. Chem. Soc. Rev. 2021, 50, 87–101. [Google Scholar] [CrossRef]
- Ryu, H.D.; Lim, C.S.; Kim, Y.K.; Lee, S.I. Recovery of Struvite Obtained from Semiconductor Wastewater and Reuse as a Slow-Release Fertilizer. Environ. Eng. Sci. 2012, 29, 540–548. [Google Scholar] [CrossRef]
- Wang, S.; Hawkins, G.L.; Kiepper, B.H.; Das, K.C. Struvite Precipitation as a Means of Recovering Nutrients and Mitigating Ammonia Toxicity in a Two-Stage Anaerobic Digester Treating Protein-Rich Feedstocks. Molecules 2016, 21, 1011. [Google Scholar] [CrossRef]
- Morales, N.; Boehler, M.; Buettner, S.; Liebi, C.; Siegrist, H. Recovery of N and P from Urine by Struvite Precipitation Followed by Combined Stripping with Digester Sludge Liquid at Full Scale. Water 2013, 5, 1262–1278. [Google Scholar] [CrossRef]
- Ganrot, Z.; Dave, G.; Nilsson, E. Recovery of N and P from human urine by freezing, struvite precipitation and adsorption to zeolite and active carbon. Bioresour. Technol. 2007, 98, 3112–3121. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Wang, C.; van Loosdrecht, M.C.; Hu, Y. Looking beyond struvite for P-recovery. Environ. Sci. Technol. 2013, 47, 4965–4966. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Pal, P. Assessing the feasibility of N and P recovery by struvite precipitation from nutrient-rich wastewater: A review. Environ. Sci. Pollut. Res. Int. 2015, 22, 17453–17464. [Google Scholar] [CrossRef]
- Kabdaşlı, I.; Siciliano, A.; Limonti, C.; Tünay, O. Is K-Struvite Precipitation a Plausible Nutrient Recovery Method from Potassium-Containing Wastes?—A Review. Sustainability 2022, 14, 11680. [Google Scholar] [CrossRef]
- Massey, M.S.; Ippolito, J.A.; Davis, J.G.; Sheffield, R.E. Macroscopic and microscopic variation in recovered magnesium phosphate materials: Implications for phosphorus removal processes and product re-use. Bioresour. Technol. 2010, 101, 877–885. [Google Scholar] [CrossRef]
- Dai, H.; Lu, X.; Peng, Y.; Yang, Z.; Zhsssu, H. Effects of supersaturation control strategies on hydroxyapatite (HAP) crystallization for phosphorus recovery from wastewater. Environ. Sci. Pollut. Res. 2017, 24, 5791–5799. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Zhang, X.; Feng, H.; Li, C.; Xu, T. Phosphate Recovery from Excess Sludge by Conventional Electrodialysis (CED) and Electrodialysis with Bipolar Membranes (EDBM). Ind. Eng. Chem. Res. 2013, 52, 15896–15904. [Google Scholar] [CrossRef]
- Zhang, Y.; Desmidt, E.; Looveren, A.V.; Pinoy, L.; Meesschaert, B.; Bruggen, B.V.d. Phosphate Separation and Recovery from Wastewater by Novel Electrodialysis. Environ. Sci. Technol. 2013, 47, 5888–5895. [Google Scholar] [CrossRef]
- Wu, C.; Yan, H.; Li, Z.; Lu, X. Ammonia recovery from high concentration wastewater of soda ash industry with membrane distillation process. Desalin Water Treat 2015, 57, 6792–6800. [Google Scholar] [CrossRef]
- Zarebska, A.; Nieto, D.R.; Christensen, K.V.; Norddahl, B. Ammonia recovery from agricultural wastes by membrane distillation: Fouling characterization and mechanism. Water Res. 2014, 56, 1–10. [Google Scholar] [CrossRef]
- Xie, M.; Shon, H.K.; Gray, S.R.; Elimelech, M. Membrane-based processes for wastewater nutrient recovery: Technology, challenges, and future direction. Water Res. 2015, 89, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Wang, Y.; Du, Y.; Feng, H.; Xu, T. Simultaneous recovery of ammonium and phosphorus via the integration of electrodialysis with struvite reactor—ScienceDirect. J. Memb. Sci. 2015, 490, 65–71. [Google Scholar] [CrossRef]
- Zhang, C.; Guisasola, A.; Baeza, J.A. A review on the integration of mainstream P-recovery strategies with enhanced biological phosphorus removal. Water Res. 2022, 212, 118102. [Google Scholar] [CrossRef]
- Kelly, P.T.; He, Z. Nutrients removal and recovery in bioelectrochemical systems: A review. Bioresour. Technol. 2014, 153, 351–360. [Google Scholar] [CrossRef]
- Cord-Ruwisch, R.; Law, Y.; Cheng, K.Y. Ammonium as a sustainable proton shuttle in bioelectrochemical systems. Bioresour. Technol. 2011, 102, 9691–9696. [Google Scholar] [CrossRef]
- Kim, J.R.; Cheng, S.; Oh, S.E.; Logan, B.E. Power generation using different cation, anion, and ultrafiltration membranes in microbial fuel cells. Environ. Sci. Technol. 2007, 41, 1004–1009. [Google Scholar] [CrossRef]
- Kim, J.R.; Zuo, Y.; Regan, J.M.; Logan, B.E. Analysis of ammonia loss mechanisms in microbial fuel cells treating animal wastewater. Biotechnol. Bioeng. 2008, 99, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Kuntke, P.; Geleji, M.; Bruning, H.; Zeeman, G.; Hamelers, H.V.; Buisman, C.J. Effects of ammonium concentration and charge exchange on ammonium recovery from high strength wastewater using a microbial fuel cell. Bioresour. Technol. 2011, 102, 4376–4382. [Google Scholar] [CrossRef] [PubMed]
- Zang, G.L.; Sheng, G.P.; Li, W.W.; Tong, Z.H.; Zeng, R.J.; Shi, C.; Yu, H.Q. Nutrient removal and energy production in a urine treatment process using magnesium ammonium phosphate precipitation and a microbial fuel cell technique. Phys. Chem. Chem. Phys. 2012, 14, 1978–1984. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Abu-Reesh, I.; He, Z. Development of Bioelectrochemical Systems to Promote Sustainable Agriculture. Agriculture 2015, 5, 367–388. [Google Scholar] [CrossRef]
- Puyol, D.; Batstone, D.J.; Hülsen, T.; Astals, S.; Peces, M.; Krömer, J.O. Resource Recovery from Wastewater by Biological Technologies: Opportunities, Challenges, and Prospects. Front. Microbiol. 2016, 7, 2106. [Google Scholar] [CrossRef] [PubMed]
- Nakhate, P.H.; Joshi, N.T.; Marathe, K.V. A critical review of bioelectrochemical membrane reactor (BECMR) as cutting-edge sustainable wastewater treatment. Rev. Chem. Eng. 2017, 33, 143–161. [Google Scholar] [CrossRef]
- Iskander, S.M.; Brazil, B.; Novak, J.T.; He, Z. Resource recovery from landfill leachate using bioelectrochemical systems: Opportunities, challenges, and perspectives. Bioresour. Technol. 2016, 201, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Ledezma, P.; Jermakka, J.; Keller, J.; Freguia, S. Recovering Nitrogen as a Solid without Chemical Dosing: Bio-Electroconcentration for Recovery of Nutrients from Urine. Environ. Sci. Technol. 2017, 4, 119–124. [Google Scholar] [CrossRef]
- Kuntke, P.; Sleutels, T.H.J.A.; Saakes, M.; Buisman, C.J.N. Hydrogen production and ammonium recovery from urine by a Microbial Electrolysis Cell. Int. J. Hydrog. Energy 2014, 39, 4771–4778. [Google Scholar] [CrossRef]
- Kuntke, P.; Śmiech, K.M.; Bruning, H.; Zeeman, G.; Saakes, M.; Sleutels, T.H.J.A.; Hamelers, H.V.M.; Buisman, C.J.N. Ammonium recovery and energy production from urine by a microbial fuel cell. Water Res. 2012, 46, 2627–2636. [Google Scholar] [CrossRef]
- Ichihashi, O.; Hirooka, K. Removal and recovery of phosphorus as struvite from swine wastewater using microbial fuel cell. Bioresour. Technol. 2012, 114, 303–307. [Google Scholar] [CrossRef]
- Chen, X.; Sun, D.; Zhang, X.; Liang, P.; Huang, X. Novel Self-driven Microbial Nutrient Recovery Cell with Simultaneous Wastewater Purification. Sci. Rep. 2015, 5, 15744. [Google Scholar] [CrossRef]
- Zhang, F.; Li, J.; He, Z. A new method for nutrients removal and recovery from wastewater using a bioelectrochemical system. Bioresour. Technol. 2014, 166, 630–634. [Google Scholar] [CrossRef]
- Desloover, J.; De Vrieze, J.; Van de Vijver, M.; Mortelmans, J.; Rozendal, R.; Rabaey, K. Electrochemical nutrient recovery enables ammonia toxicity control and biogas desulfurization in anaerobic digestion. Environ. Sci. Technol. 2014, 49, 948–955. [Google Scholar] [CrossRef]
- Zhang, Y.; Angelidaki, I. Submersible microbial desalination cell for simultaneous ammonia recovery and electricity production from anaerobic reactors containing high levels of ammonia. Bioresour. Technol. 2015, 177, 233–239. [Google Scholar] [CrossRef]
- Zhang, Y.; Angelidaki, I. Counteracting ammonia inhibition during anaerobic digestion by recovery using submersible microbial desalination cell. Biotechnol. Bioeng. 2015, 112, 1478–1482. [Google Scholar] [CrossRef]
- Wu, X.; Modin, O. Ammonium recovery from reject water combined with hydrogen production in a bioelectrochemical reactor. Bioresour. Technol. 2013, 146, 530–536. [Google Scholar] [CrossRef]
- Yuan, P.; Kim, Y. Increasing phosphorus recovery from dewatering centrate in microbial electrolysis cells. Biotechnol. Biofuels 2017, 10, 70. [Google Scholar] [CrossRef]
- Kim, J.R.; Song, Y.E.; Munussami, G.; Kim, C.; Jeon, B.-H. Recent applications of bioelectrochemical system for useful resource recovery: Retrieval of nutrient and metal from wastewater. Geosyst. Eng. 2015, 18, 173–180. [Google Scholar] [CrossRef]
- Luo, S.; Hongyue, S.; Qingyun, P.; Ran, J.; He, Z. A Review of Modeling Bioelectrochemical Systems: Engineering and Statistical Aspects. Energies 2016, 9, 111. [Google Scholar] [CrossRef]
- Qin, M.; He, Z. Resource Recovery by Osmotic Bioelectrochemical Systems towards Sustainable Wastewater Treatment. Environ. Sci. Water Res. Technol. 2017, accepted. [Google Scholar] [CrossRef]
- Shaffer, D.L.; Werber, J.R.; Jaramillo, H.; Lin, S.; Elimelech, M. Forward osmosis: Where are we now? Desalination 2015, 356, 271–284. [Google Scholar] [CrossRef]
- Lutchmiah, K.; Verliefde, A.R.; Roest, K.; Rietveld, L.C.; Cornelissen, E.R. Forward osmosis for application in wastewater treatment: A review. Water Res. 2014, 58, 179–197. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, J.; Tang, J.; Wang, X.; Wu, Z. A pilot-scale forward osmosis membrane system for concentrating low-strength municipal wastewater: Performance and implications. Sci. Rep. 2016, 6, 21653. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.J.; Hai, F.I.; Price, W.E.; Drewes, J.E.; Nghiem, L.D. Forward osmosis as a platform for resource recovery from municipal wastewater—A critical assessment of the literature. J. Membr. Sci. 2017, 529, 195–206. [Google Scholar] [CrossRef]
- Lu, Y.; Qin, M.; Yuan, H.; Abu-Reesh, I.M.; He, Z. When Bioelectrochemical Systems Meet Forward Osmosis: Accomplishing Wastewater Treatment and Reuse through Synergy. Water 2014, 7, 38–50. [Google Scholar] [CrossRef]
- Qin, M.; He, Z. Self-Supplied Ammonium Bicarbonate Draw Solute for Achieving Wastewater Treatment and Recovery in a Microbial Electrolysis Cell-Forward Osmosis-Coupled System. Environ. Sci. Technol. Lett 2014, 1, 437–441. [Google Scholar] [CrossRef]
- Zou, S.; Qin, M.; Moreau, Y.; He, Z. Nutrient-energy-water recovery from synthetic sidestream centrate using a microbial electrolysis cell—forward osmosis hybrid system. J. Clean. Prod. 2017, 154, 16–25. [Google Scholar] [CrossRef]
- Hou, D.; Lu, L.; Ren, Z.J. Microbial fuel cells and osmotic membrane bioreactors have mutual benefits for wastewater treatment and energy production. Water Res. 2016, 98, 183–189. [Google Scholar] [CrossRef]
- Qin, M.; Hynes, E.A.; Abu-Reesh, I.M.; He, Z. Ammonium removal from synthetic wastewater promoted by current generation and water flux in an osmotic microbial fuel cell. J. Clean. Prod. 2017, 149, 856–862. [Google Scholar] [CrossRef]
- Chozhavendhan, S.; Karthigadevi, G.; Bharathiraja, B.; Praveen Kumar, R.; Abo, L.D.; Venkatesa Prabhu, S.; Balachandar, R.; Jayakumar, M. Current and prognostic overview on the strategic exploitation of anaerobic digestion and digestate: A review. Environ. Res. 2023, 216, 114526. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, M.; Wu, C.; Wang, Q.; Gao, M.; Huang, Q.; Liu, Y. A comprehensive review on food waste anaerobic digestion: Research updates and tendencies. Bioresour. Technol. 2018, 247, 1069–1076. [Google Scholar] [CrossRef]
- Wainaina, S.; Awasthi, M.K.; Sarsaiya, S.; Chen, H.; Singh, E.; Kumar, A.; Ravindran, B.; Awasthi, S.K.; Liu, T.; Duan, Y.; et al. Resource recovery and circular economy from organic solid waste using aerobic and anaerobic digestion technologies. Bioresour. Technol. 2020, 301, 122778. [Google Scholar] [CrossRef]
- Ampese, L.C.; Sganzerla, W.G.; Di Domenico Ziero, H.; Mudhoo, A.; Martins, G.; Forster-Carneiro, T. Research progress, trends, and updates on anaerobic digestion technology: A bibliometric analysis. J. Clean. Prod. 2022, 331, 130004. [Google Scholar] [CrossRef]
- Wang, T.-T.; Sun, Z.-Y.; Huang, Y.-L.; Tan, L.; Tang, Y.-Q.; Kida, K. Biogas Production from Distilled Grain Waste by Thermophilic Dry Anaerobic Digestion: Pretreatment of Feedstock and Dynamics of Microbial Community. Appl. Biochem. Biotechnol. 2018, 184, 685–702. [Google Scholar] [CrossRef] [PubMed]
- Sganzerla, W.G.; Buller, L.S.; Mussatto, S.I.; Forster-Carneiro, T. Techno-economic assessment of bioenergy and fertilizer production by anaerobic digestion of brewer’s spent grains in a biorefinery concept. J. Clean. Prod. 2021, 297, 126600. [Google Scholar] [CrossRef]
- Gunes, B.; Stokes, J.; Davis, P.; Connolly, C.; Lawler, J. Pre-treatments to enhance biogas yield and quality from anaerobic digestion of whiskey distillery and brewery wastes: A review. Renew. Sustain. Energy Rev. 2019, 113, 109281. [Google Scholar] [CrossRef]
- Moraes, B.S.; Zaiat, M.; Bonomi, A. Anaerobic digestion of vinasse from sugarcane ethanol production in Brazil: Challenges and perspectives. Renew. Sustain. Energy Rev. 2015, 44, 888–903. [Google Scholar] [CrossRef]
- Silva, A.; Brasil, Y.; Koch, K.; Amaral, M. Resource recovery from sugarcane vinasse by anaerobic digestion–A review. J. Environ. Manag. 2021, 295, 113137. [Google Scholar] [CrossRef]
- Junior, A.D.N.F.; Etchebehere, C.; Perecin, D.; Teixeira, S.; Woods, J. Advancing anaerobic digestion of sugarcane vinasse: Current development, struggles and future trends on production and end-uses of biogas in Brazil. Renew. Sustain. Energy Rev. 2022, 157, 112045. [Google Scholar] [CrossRef]
- Usman Khan, M.; Kiaer Ahring, B. Anaerobic digestion of biorefinery lignin: Effect of different wet explosion pretreatment conditions. Bioresour. Technol. 2020, 298, 122537. [Google Scholar] [CrossRef]
- Wagner, A.O.; Lackner, N.; Mutschlechner, M.; Prem, E.M.; Markt, R.; Illmer, P. Biological pretreatment strategies for second-generation lignocellulosic resources to enhance biogas production. Energies 2018, 11, 1797. [Google Scholar] [CrossRef]
- Malhotra, M.; Aboudi, K.; Pisharody, L.; Singh, A.; Banu, J.R.; Bhatia, S.K.; Varjani, S.; Kumar, S.; González-Fernández, C.; Kumar, S.; et al. Biorefinery of anaerobic digestate in a circular bioeconomy: Opportunities, challenges and perspectives. Renew. Sustain. Energy Rev. 2022, 166, 112642. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.-S. Integrated role of algae in the closed-loop circular economy of anaerobic digestion. Bioresour. Technol. 2022, 360, 127618. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.C.; Cheng, Y.W.; Ishak, S.; Lam, M.K.; Lim, J.W.; Tan, I.S.; Show, P.L.; Lee, K.T. Anaerobic digestate as a low-cost nutrient source for sustainable microalgae cultivation: A way forward through waste valorization approach. Sci. Total Environ. 2022, 803, 150070. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.-B.; Yang, L.-B.; Zhang, W.-W.; Zhao, X.-C. Lipids production and nutrients recycling by microalgae mixotrophic culture in anaerobic digestate of sludge using wasted organics as carbon source. Bioresour. Technol. 2020, 297, 122379. [Google Scholar] [CrossRef]
- Velea, S.; Oancea, F.; Fischer, F. Heterotrophic and mixotrophic microalgae cultivation. In Microalgae-Based Biofuels and Bioproducts; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 45–65. [Google Scholar]
- Xia, A.; Murphy, J.D. Microalgal cultivation in treating liquid digestate from biogas systems. Trends Biotechnol. 2016, 34, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, S.; Qu, M.; Dai, D.; Liu, D.; Wang, W.; Tang, C.; Zhu, L. Microalgal cultivation for the upgraded biogas by removing CO2, coupled with the treatment of slurry from anaerobic digestion: A review. Bioresour. Technol. 2022, 364, 128118. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, D.; Lee, D.-J.; Chang, J.-S. Integration of anaerobic digestion and microalgal cultivation for digestate bioremediation and biogas upgrading. Bioresour. Technol. 2019, 290, 121804. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; Lin, R.; Rajendran, K.; O’Shea, R.; Xia, A.; Murphy, J.D. How to optimise photosynthetic biogas upgrading: A perspective on system design and microalgae selection. Biotechnol. Adv. 2019, 37, 107444. [Google Scholar] [CrossRef]
- Lins, L.P.; Martinez, D.G.; Furtado, A.C.; Padilha, J.C. Biomethane generation and CO2 recovery through biogas production using brewers’ spent Grains. Biocatal. Agric. Biotechnol. 2022, 102579, in press. [Google Scholar] [CrossRef]
- Sun, Z.-Y.; Yamaji, S.; Cheng, Q.-S.; Yang, L.; Tang, Y.-Q.; Kida, K. Simultaneous decrease in ammonia and hydrogen sulfide inhibition during the thermophilic anaerobic digestion of protein-rich stillage by biogas recirculation and air supply at 60 °C. Process Biochem. 2014, 49, 2214–2219. [Google Scholar] [CrossRef]
- Barrera, E.L.; Spanjers, H.; Dewulf, J.; Romero, O.; Rosa, E. The sulfur chain in biogas production from sulfate-rich liquid substrates: A review on dynamic modeling with vinasse as model substrate. J. Chem. Technol. Biotechnol. 2013, 88, 1405–1420. [Google Scholar] [CrossRef]
- Kiani Deh Kiani, M.; Parsaee, M.; Safieddin Ardebili, S.M.; Reyes, I.P.; Fuess, L.T.; Karimi, K. Different bioreactor configurations for biogas production from sugarcane vinasse: A comprehensive review. Biomass Bioenergy 2022, 161, 106446. [Google Scholar] [CrossRef]
- Jung, H.; Kim, D.; Choi, H.; Lee, C. A review of technologies for in-situ sulfide control in anaerobic digestion. Renew. Sustain. Energy Rev. 2022, 157, 112068. [Google Scholar] [CrossRef]
- Yellezuome, D.; Zhu, X.; Wang, Z.; Liu, R. Mitigation of ammonia inhibition in anaerobic digestion of nitrogen-rich substrates for biogas production by ammonia stripping: A review. Renew. Sustain. Energy Rev. 2022, 157, 112043. [Google Scholar] [CrossRef]
- Jo, Y.; Cayetano, R.D.A.; Kim, G.-B.; Park, J.; Kim, S.-H. The effects of ammonia acclimation on biogas recovery and the microbial population in continuous anaerobic digestion of swine manure. Environ. Res. 2022, 212, 113483. [Google Scholar] [CrossRef] [PubMed]
- Fakkaew, K.; Polprasert, C. Air stripping pre-treatment process to enhance biogas production in anaerobic digestion of chicken manure wastewater. Bioresour. Technol. Rep. 2021, 14, 100647. [Google Scholar] [CrossRef]
- Yin, D.-M.; Qiao, W.; Negri, C.; Adani, F.; Fan, R.; Dong, R.-J. Enhancing hyper-thermophilic hydrolysis pre-treatment of chicken manure for biogas production by in-situ gas phase ammonia stripping. Bioresour. Technol. 2019, 287, 121470. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Usman, M.; Alsareii, S.A.; Harraz, F.A.; Al-Assiri, M.; Jalalah, M.; Li, X.; Salama, E.-S. Synergistic ammonia and fatty acids inhibition of microbial communities during slaughterhouse waste digestion for biogas production. Bioresour. Technol. 2021, 337, 125383. [Google Scholar] [CrossRef]
- Wang, H.-Z.; Li, J.; Yi, Y.; Nobu, M.K.; Narihiro, T.; Tang, Y.-Q. Response to inhibitory conditions of acetate-degrading methanogenic microbial community. J. Biosci. Bioeng. 2020, 129, 476–485. [Google Scholar] [CrossRef]
- Kadam, R.; Panwar, N.L. Recent advancement in biogas enrichment and its applications. Renew. Sustain. Energy Rev. 2017, 73, 892–903. [Google Scholar] [CrossRef]
- Shi, X.; Zuo, J.; Zhang, M.; Wang, Y.; Yu, H.; Li, B. Enhanced biogas production and in situ ammonia recovery from food waste using a gas-membrane absorption anaerobic reactor. Bioresour. Technol. 2019, 292, 121864. [Google Scholar] [CrossRef]
- Brienza, C.; Sigurnjak, I.; Meier, T.; Michels, E.; Adani, F.; Schoumans, O.; Vaneeckhaute, C.; Meers, E. Techno-economic assessment at full scale of a biogas refinery plant receiving nitrogen rich feedstock and producing renewable energy and biobased fertilisers. J. Clean. Prod. 2021, 308, 127408. [Google Scholar] [CrossRef] [PubMed]
- Costamagna, P.; Giordano, A.; Lazzarini, Y.; Delucchi, M.; Busca, G. Process of ammonia removal from anaerobic digestion and associated ammonium sulphate production: Pilot plant demonstration. J. Environ. Manag. 2020, 259, 109841. [Google Scholar] [CrossRef]
- Becker, C.M.; Marder, M.; Junges, E.; Konrad, O. Technologies for biogas desulfurization—An overview of recent studies. Renew. Sustain. Energy Rev. 2022, 159, 112205. [Google Scholar] [CrossRef]
- Lawrence, S.G. Hydrogen Sulfide Control in Biodigestion Processes. U.S. Patent 2015122120, 7 May 2015. [Google Scholar]
- Münster-Swendsen, J.E.; Jakobsson, N.B.; Egeblad, K.; Christensen, K. A Process for Cleaning Biogas While Producing a Sulfur-Containing Fertilizer. WO 2019158474, 22 August 2019. [Google Scholar]
- Jackson, D.; Gomach, J.B.; Hardy, M. Hydrogen Sulfide Removal Process. U.S. Patent 10974190B2, 13 April 2021. [Google Scholar]
- Mulu, E.; M’Arimi, M.M.; Ramkat, R.C. A review of recent developments in application of low cost natural materials in purification and upgrade of biogas. Renew. Sustain. Energy Rev. 2021, 145, 111081. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Ficara, E.; Aboulkas, A.; Barakat, A.; Carrère, H. New opportunities for agricultural digestate valorization: Current situation and perspectives. Energy Environ. Sci. 2015, 8, 2600–2621. [Google Scholar] [CrossRef]
- Golovko, O.; Ahrens, L.; Schelin, J.; Sörengård, M.; Bergstrand, K.-J.; Asp, H.; Hultberg, M.; Wiberg, K. Organic micropollutants, heavy metals and pathogens in anaerobic digestate based on food waste. J. Environ. Manag. 2022, 313, 114997. [Google Scholar] [CrossRef]
- Lamolinara, B.; Pérez-Martínez, A.; Guardado-Yordi, E.; Fiallos, C.G.; Diéguez-Santana, K.; Ruiz-Mercado, G.J. Anaerobic digestate management, environmental impacts, and techno-economic challenges. Waste Manag. 2022, 140, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Yin, R.; Gao, X.; Chen, J.; Wang, R.; Xu, Z.; Luo, W.; Li, G.; Li, Y. Development of Solid-State Anaerobic Digestion and Aerobic Composting Hybrid Processes for Organic Solid Waste Treatment and Resource Recovery: A Review. Curr. Pollut. Rep. 2022, 8, 221–233. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, Q.; Li, Y. A review on sulfur transformation during anaerobic digestion of organic solid waste: Mechanisms, influencing factors and resource recovery. Sci. Total Environ. 2022, 865, 161193. [Google Scholar] [CrossRef] [PubMed]
- Flores-Alsina, X.; Solon, K.; Mbamba, C.K.; Tait, S.; Gernaey, K.V.; Jeppsson, U.; Batstone, D.J. Modelling phosphorus (P), sulfur (S) and iron (Fe) interactions for dynamic simulations of anaerobic digestion processes. Water Res. 2016, 95, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, C.; Patel, D.; Jung, H.; Liu, P.; Wan, B.; Pavlostathis, S.G.; Tang, Y. Coevolution of iron, phosphorus, and sulfur speciation during anaerobic digestion with hydrothermal pretreatment of sewage sludge. Environ. Sci. Technol. 2020, 54, 8362–8372. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Deng, F.; Zhang, L. Phosphorus recovery from sludge by rusty scrap iron enhanced anaerobic digestion and vivianite crystallization. J. Water Process Eng. 2022, 47, 102697. [Google Scholar] [CrossRef]
- Su, L.; Zhen, G.; Zhang, L.; Zhao, Y.; Niu, D.; Chai, X. The use of the core–shell structure of zero-valent iron nanoparticles (NZVI) for long-term removal of sulphide in sludge during anaerobic digestion. Environ. Sci. Process. Impacts 2015, 17, 2013–2021. [Google Scholar] [CrossRef]
- Caicedo-Ramirez, A.; Laroco, N.; Bilgin, A.A.; Shiokari, S.; Grubb, D.G.; Hernandez, M. Engineered addition of slag fines for the sequestration of phosphate and sulfide during mesophilic anaerobic digestion. Water Environ. Res. 2020, 92, 455–464. [Google Scholar] [CrossRef] [PubMed]
- König, R.; Cuomo, M.; Pianta, E.; Buetti, A.; Mauri, F.; Tanadini, M.; Principi, P. Addition of conductive materials to support syntrophic microorganisms in anaerobic digestion. Fermentation 2022, 8, 354. [Google Scholar] [CrossRef]
- Jung, H.; Baek, G.; Lee, C. Magnetite-assisted in situ microbial oxidation of H2S to S0 during anaerobic digestion: A new potential for sulfide control. Chem. Eng. J. 2020, 397, 124982. [Google Scholar] [CrossRef]
- Jeníček, P.; Horejš, J.; Pokorná-Krayzelová, L.; Bindzar, J.; Bartáček, J. Simple biogas desulfurization by microaeration–Full scale experience. Anaerobe 2017, 46, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Robbens, J.; Heyndrickx, M.; Debode, J.; Raes, K. Bioprocessing of marine crustacean side-streams into bioactives: A review. J. Chem. Technol. Biotechnol. 2021, 96, 1465–1474. [Google Scholar] [CrossRef]
- Rodrigues, I.M.; Carvalho, M.G.V.; Rocha, J.M. Increase of protein extraction yield from rapeseed meal through a pretreatment with phytase. J. Sci. Food Agric. 2017, 97, 2641–2646. [Google Scholar] [CrossRef]
- Carraresi, L.; Bröring, S. How does business model redesign foster resilience in emerging circular value chains? J. Clean. Prod. 2021, 289, 125823. [Google Scholar] [CrossRef]
- Di Lena, G.; Sanchez del Pulgar, J.; Lucarini, M.; Durazzo, A.; Ondrejíčková, P.; Oancea, F.; Frincu, R.-M.; Aguzzi, A.; Ferrari Nicoli, S.; Casini, I.; et al. Valorization Potentials of Rapeseed Meal in a Biorefinery Perspective: Focus on Nutritional and Bioactive Components. Molecules 2021, 26, 6787. [Google Scholar] [CrossRef] [PubMed]
- Mele, A.; Castiglione, F.; Ferro, M.; Colombo Dugoni, G.; Di Pietro, M.E.; Mannu, A.; Panzeri, W. Process for Biomass Treatment. US 2022213276 A1, 24 February 2022. [Google Scholar]
- Santos, A.F.; Almeida, P.V.; Alvarenga, P.; Gando-Ferreira, L.M.; Quina, M.J. From wastewater to fertilizer products: Alternative paths to mitigate phosphorus demand in European countries. Chemosphere 2021, 284, 131258. [Google Scholar] [CrossRef] [PubMed]
- Ricci, M.; Tilbury, L.; Daridon, B.; Sukalac, K. General principles to justify plant biostimulant claims. Front. Plant Sci. 2019, 10, 494. [Google Scholar] [CrossRef]
- Kirkby, E. Chapter 1—Introduction, Definition and Classification of Nutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 3–5. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Bélanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef] [PubMed]
- De Tombeur, F.; Raven, J.A.; Toussaint, A.; Lambers, H.; Cooke, J.; Hartley, S.E.; Johnson, S.N.; Coq, S.; Katz, O.; Schaller, J.; et al. Why do plants silicify? Trends Ecol. Evol. 2022. [Google Scholar] [CrossRef]
- Exley, C. A possible mechanism of biological silicification in plants. Front. Plant Sci. 2015, 6, 853. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Bhat, J.A.; Siddiqui, M.H.; Rinklebe, J.; Ahmad, P. Integration of silicon and secondary metabolites in plants: A significant association in stress tolerance. J. Exp. Bot. 2020, 71, 6758–6774. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Rizwan, M.; Brestic, M.; Xie, W. Silicon: An essential element for plant nutrition and phytohormones signaling mechanism under stressful conditions. Plant Growth Regul. 2022. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Vishwakarma, K.; Singh, V.P.; Prakash, V.; Sharma, S.; Muneer, S.; Nikolic, M.; Deshmukh, R.; Vaculík, M.; Corpas, F.J. Silicon crosstalk with reactive oxygen species, phytohormones and other signaling molecules. J. Hazard. Mater. 2021, 408, 124820. [Google Scholar] [CrossRef]
- De Tombeur, F.; Turner, B.L.; Laliberté, E.; Lambers, H.; Mahy, G.; Faucon, M.-P.; Zemunik, G.; Cornelis, J.-T. Plants sustain the terrestrial silicon cycle during ecosystem retrogression. Science 2020, 369, 1245–1248. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, J.-T.; Delvaux, B. Soil processes drive the biological silicon feedback loop. Funct. Ecol. 2016, 30, 1298–1310. [Google Scholar] [CrossRef]
- Haynes, R.J. A contemporary overview of silicon availability in agricultural soils. J. Plant Nutr. Soil Sci. 2014, 177, 831–844. [Google Scholar] [CrossRef]
- Carey, J.C.; Fulweiler, R.W. Human appropriation of biogenic silicon—The increasing role of agriculture. Funct. Ecol. 2016, 30, 1331–1339. [Google Scholar] [CrossRef]
- Zellner, W.; Tubaña, B.; Rodrigues, F.A.; Datnoff, L.E. Silicon’s Role in Plant Stress Reduction and Why This Element Is Not Used Routinely for Managing Plant Health. Plant Dis. 2021, 105, 2033–2049. [Google Scholar] [CrossRef] [PubMed]
- Seyfferth, A.L.; Kocar, B.D.; Lee, J.A.; Fendorf, S. Seasonal dynamics of dissolved silicon in a rice cropping system after straw incorporation. Geochim. Cosmochim. Acta 2013, 123, 120–133. [Google Scholar] [CrossRef]
- Sible, C.N.; Seebauer, J.R.; Below, F.E. Plant biostimulants: A categorical review, their implications for row crop production, and relation to soil health indicators. Agronomy 2021, 11, 1297. [Google Scholar] [CrossRef]
- European Commission, Regulation 2019/1009 of the European Parliament and of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003. J. Eur. Union 2019, 62, 1–114.
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Chojnacka, K.; Mikula, K.; Skrzypczak, D.; Izydorczyk, G.; Gorazda, K.; Kulczycka, J.; Kominko, H.; Moustakas, K.; Witek-Krowiak, A. Practical aspects of biowastes conversion to fertilizers. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Guntzer, F.; Keller, C.; Meunier, J.-D. Benefits of plant silicon for crops: A review. Agron. Sustain. Dev. 2012, 32, 201–213. [Google Scholar] [CrossRef]
- Mathur, P.; Roy, S. Nanosilica facilitates silica uptake, growth and stress tolerance in plants. Plant Physiol. Biochem. 2020, 157, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Yazdimamaghani, M.; Moos, P.J.; Dobrovolskaia, M.A.; Ghandehari, H. Genotoxicity of amorphous silica nanoparticles: Status and prospects. Nanomed. Nanotechnol. Biol. Med. 2019, 16, 106–125. [Google Scholar] [CrossRef]
- Guo, C.; Liu, Y.; Li, Y. Adverse effects of amorphous silica nanoparticles: Focus on human cardiovascular health. J. Hazard. Mater. 2021, 406, 124626. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Jha, S. Amorphous nanosilica induced toxicity, inflammation and innate immune responses: A critical review. Toxicology 2020, 441, 152519. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, J.; Zhang, Y.; Zhang, G.; Kang, Y.; Chen, A.; Feng, X.; Shao, L. The toxicity of silica nanoparticles to the immune system. Nanomedicine 2018, 13, 1939–1962. [Google Scholar] [CrossRef]
- Oancea, F. Development of Safe Nanoagrochemicals—The Nanoporous Route. Chem. Proc. 2022, 7, 68. [Google Scholar]
- Bruins, M.E.; Sanders, J.P.M. Small-scale processing of biomass for biorefinery. Biofuels Bioprod. Biorefining 2012, 6, 135–145. [Google Scholar] [CrossRef]
- Ait Sair, A.; Kansou, K.; Michaud, F.; Cathala, B. Multicriteria Definition of Small-Scale Biorefineries Based on a Statistical Classification. Sustainability 2021, 13, 7310. [Google Scholar] [CrossRef]
- Hu, X.; Tian, Z.; Li, X.; Wang, S.; Pei, H.; Sun, H.; Zhang, Z. Green, Simple, and Effective Process for the Comprehensive Utilization of Shrimp Shell Waste. ACS Omega 2020, 5, 19227–19235. [Google Scholar] [CrossRef]
- Pérez, W.A.; Marín, J.A.; López, J.N.; Burgos, M.A.; Rios, L.A. Development of a Pilot-ecofriendly Process for Chitosan Production from Waste Shrimp Shells. Environ. Process. 2022, 9, 55. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Pou, J.; Comesaña, R.; Lusquiños, F.; de Carlos, A.; León, B. Biological hydroxyapatite obtained from fish bones. Mater. Sci. Eng. C 2012, 32, 478–486. [Google Scholar] [CrossRef]
- Carella, F.; Seck, M.; Esposti, L.D.; Diadiou, H.; Maienza, A.; Baronti, S.; Vignaroli, P.; Vaccari, F.P.; Iafisco, M.; Adamiano, A. Thermal conversion of fish bones into fertilizers and biostimulants for plant growth—A low tech valorization process for the development of circular economy in least developed countries. J. Environ. Chem. Eng. 2021, 9, 104815. [Google Scholar] [CrossRef]
- Cooper, J.; Lombardi, R.; Boardman, D.; Carliell-Marquet, C. The future distribution and production of global phosphate rock reserves. Resour. Conserv. Recycl. 2011, 57, 78–86. [Google Scholar] [CrossRef]
- Le Moal, M.; Gascuel-Odoux, C.; Ménesguen, A.; Souchon, Y.; Étrillard, C.; Levain, A.; Moatar, F.; Pannard, A.; Souchu, P.; Lefebvre, A.; et al. Eutrophication: A new wine in an old bottle? Sci. Total Environ. 2019, 651, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mallin, M.A.; Cahoon, L.B. The Hidden Impacts of Phosphorus Pollution to Streams and Rivers. BioScience 2020, 70, 315–329. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament, the European Council, the European Economic and Social Committee and the Committee of the Regions, Critical Raw Materials Resilience: Charting a Path towards Greater Security and Sustainability; COM(2020) 474 final; EC: Brussels, Belgium, 2020. [Google Scholar]
- Meers, E.; Velthof, G.; Michels, E.; Rietra, R. Biorefinery of Inorganics: Recovering Mineral Nutrients from Biomass and Organic Waste; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Ruffatto, K.; Emaminejad, S.A.; Juneja, A.; Kurambhatti, C.; Margenot, A.; Singh, V.; Cusick, R.D. Mapping the National Phosphorus Recovery Potential from Centralized Wastewater and Corn Ethanol Infrastructure. Environ. Sci. Technol. 2022, 56, 8691–8701. [Google Scholar] [CrossRef]
- Campos, J.L.; Crutchik, D.; Franchi, Ó.; Pavissich, J.P.; Belmonte, M.; Pedrouso, A.; Mosquera-Corral, A.; Val del Río, Á. Nitrogen and Phosphorus Recovery From Anaerobically Pretreated Agro-Food Wastes: A Review. Front. Sustain. Food Syst. 2019, 2, 91. [Google Scholar] [CrossRef]
- Jarvie, H.P.; Flaten, D.; Sharpley, A.N.; Kleinman, P.J.A.; Healy, M.G.; King, S.M. Future Phosphorus: Advancing New 2D Phosphorus Allotropes and Growing a Sustainable Bioeconomy. J. Environ. Qual. 2019, 48, 1145–1155. [Google Scholar] [CrossRef]
- Yesigat, A.; Worku, A.; Mekonnen, A.; Bae, W.; Feyisa, G.L.; Gatew, S.; Han, J.-L.; Liu, W.; Wang, A.; Guadie, A. Phosphorus recovery as K-struvite from a waste stream: A review of influencing factors, advantages, disadvantages and challenges. Environ. Res. 2022, 214, 114086. [Google Scholar] [CrossRef]
- Li, B.; Udugama, I.A.; Mansouri, S.S.; Yu, W.; Baroutian, S.; Gernaey, K.V.; Young, B.R. An exploration of barriers for commercializing phosphorus recovery technologies. J. Clean. Prod. 2019, 229, 1342–1354. [Google Scholar] [CrossRef]
- Rivero, R.M.; Mittler, R.; Blumwald, E.; Zandalinas, S.I. Developing climate-resilient crops: Improving plant tolerance to stress combination. Plant J. 2022, 109, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Berni, R.; Hausman, J.-F.; Guerriero, G. A Review on the Beneficial Role of Silicon against Salinity in Non-Accumulator Crops: Tomato as a Model. Biomolecules 2020, 10, 1284. [Google Scholar] [CrossRef]
- Yee, R.A.; Leifels, M.; Scott, C.; Ashbolt, N.J.; Liu, Y. Evaluating microbial and chemical hazards in commercial struvite recovered from wastewater. Environ. Sci. Technol. 2019, 53, 5378–5386. [Google Scholar] [CrossRef]
- Chen, Q.-L.; An, X.-L.; Zhu, Y.-G.; Su, J.-Q.; Gillings, M.R.; Ye, Z.-L.; Cui, L. Application of struvite alters the antibiotic resistome in soil, rhizosphere, and phyllosphere. Environ. Sci. Technol. 2017, 51, 8149–8157. [Google Scholar] [CrossRef]
- Kang, W.; Zhang, Y.-J.; Shi, X.; He, J.-Z.; Hu, H.-W. Short-term copper exposure as a selection pressure for antibiotic resistance and metal resistance in an agricultural soil. Environ. Sci. Pollut. Res. 2018, 25, 29314–29324. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Song, H.; Lu, J.; Yuan, Z.; Guo, J. Copper nanoparticles and copper ions promote horizontal transfer of plasmid-mediated multi-antibiotic resistance genes across bacterial genera. Environ. Int. 2019, 129, 478–487. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Wang, Y.; Wang, F.; Xia, S.; Zhao, J. Struvite-supported biochar composite effectively lowers Cu bio-availability and the abundance of antibiotic-resistance genes in soil. Sci. Total Environ. 2020, 724, 138294. [Google Scholar] [CrossRef]
- Jing, H.-P.; Wang, X.; Xia, P.; Zhao, J. Sustainable utilization of a recovered struvite/diatomite compound for lead immobilization in contaminated soil: Potential, mechanism, efficiency, and risk assessment. Environ. Sci. Pollut. Res. 2019, 26, 4890–4900. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Li, Y.; Liu, Y.; Sun, Y.; Hansen, H.C.B.; Xia, S.; Zhao, J. Effects of struvite-loaded zeolite amendment on the fate of copper, tetracycline and antibiotic resistance genes in microplastic-contaminated soil. Chem. Eng. J. 2022, 430, 130478. [Google Scholar] [CrossRef]
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 2011, 348, 439. [Google Scholar] [CrossRef]
- Yuan, C.; Gao, B.; Peng, Y.; Gao, X.; Fan, B.; Chen, Q. A meta-analysis of heavy metal bioavailability response to biochar aging: Importance of soil and biochar properties. Sci. Total Environ. 2021, 756, 144058. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Fonte, S.J.; Parikh, S.J.; Six, J.; Scow, K.M. Biochar additions can enhance soil structure and the physical stabilization of C in aggregates. Geoderma 2017, 303, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, A.E.; Horn, R. Biochar-Induced Changes in Soil Resilience: Effects of Soil Texture and Biochar Dosage. Pedosphere 2017, 27, 236–247. [Google Scholar] [CrossRef]
- An, N.; Zhang, L.; Liu, Y.; Shen, S.; Li, N.; Wu, Z.; Yang, J.; Han, W.; Han, X. Biochar application with reduced chemical fertilizers improves soil pore structure and rice productivity. Chemosphere 2022, 298, 134304. [Google Scholar] [CrossRef]
- Liu, Z.; Ogunmokun, F.A.; Wallach, R. Does biochar affect soil wettability and flow pattern? Geoderma 2022, 417, 115826. [Google Scholar] [CrossRef]
- Jindo, K.; Audette, Y.; Higashikawa, F.S.; Silva, C.A.; Akashi, K.; Mastrolonardo, G.; Sánchez-Monedero, M.A.; Mondini, C. Role of biochar in promoting circular economy in the agriculture sector. Part 1: A review of the biochar roles in soil N, P and K cycles. Chem. Biol. Technol. Agric. 2020, 7, 15. [Google Scholar] [CrossRef]
- Bolan, N.; Sarmah, A.K.; Bordoloi, S.; Bolan, S.; Padhye, L.P.; Van Zwieten, L.; Sooriyakumar, P.; Khan, B.A.; Ahmad, M.; Solaiman, Z.M.; et al. Soil acidification and the liming potential of biochar. Environ. Pollut. 2023, 317, 120632. [Google Scholar] [CrossRef]
- Hussain, R.; Ravi, K.; Garg, A. Influence of biochar on the soil water retention characteristics (SWRC): Potential application in geotechnical engineering structures. Soil Tillage Res. 2020, 204, 104713. [Google Scholar] [CrossRef]
- Edeh, I.G.; Mašek, O.; Buss, W. A meta-analysis on biochar’s effects on soil water properties—New insights and future research challenges. Sci. Total Environ. 2020, 714, 136857. [Google Scholar] [CrossRef]
- Adhikari, S.; Timms, W.; Mahmud, M.A.P. Optimising water holding capacity and hydrophobicity of biochar for soil amendment—A review. Sci. Total Environ. 2022, 851, 158043. [Google Scholar] [CrossRef] [PubMed]
- Alkharabsheh, H.M.; Seleiman, M.F.; Battaglia, M.L.; Shami, A.; Jalal, R.S.; Alhammad, B.A.; Almutairi, K.F.; Al-Saif, A.M. Biochar and Its Broad Impacts in Soil Quality and Fertility, Nutrient Leaching and Crop Productivity: A Review. Agronomy 2021, 11, 993. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Bai, S.H.; Omidvar, N.; Gallart, M.; Kämper, W.; Tahmasbian, I.; Farrar, M.B.; Singh, K.; Zhou, G.; Muqadass, B.; Xu, C.-Y.; et al. Combined effects of biochar and fertilizer applications on yield: A review and meta-analysis. Sci. Total Environ. 2022, 808, 152073. [Google Scholar] [CrossRef] [PubMed]
- Imran, S.; Sarker, P.; Hoque, M.N.; Paul, N.C.; Mahamud, M.A.; Chakrobortty, J.; Tahjib-Ul-Arif, M.; Latef, A.A.H.A.; Hasanuzzaman, M.; Rhaman, M.S. Biochar actions for the mitigation of plant abiotic stress. Crop Pasture Sci. 2023, 74, 6–20. [Google Scholar] [CrossRef]
- Keabetswe, L.; Shao, G.C.; Cui, J.; Lu, J.; Stimela, T. A combination of biochar and regulated deficit irrigation improves tomato fruit quality: A comprehensive quality analysis. Folia Hortic. 2019, 31, 181–193. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Saud, S.; Hassan, S.; Tanveer, M.; Ihsan, M.Z.; Shah, A.N.; Ullah, A.; Nasrullah; Khan, F.; et al. A combined application of biochar and phosphorus alleviates heat-induced adversities on physiological, agronomical and quality attributes of rice. Plant Physiol. Biochem. 2016, 103, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Parvin, K.; Bardhan, K.; Nahar, K.; Anee, T.I.; Masud, A.A.C.; Fotopoulos, V. Biostimulants for the Regulation of Reactive Oxygen Species Metabolism in Plants under Abiotic Stress. Cells 2021, 10, 2537. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xiong, X.; He, M.; Xu, Z.; Hou, D.; Zhang, W.; Ok, Y.S.; Rinklebe, J.; Wang, L.; Tsang, D.C.W. Roles of biochar-derived dissolved organic matter in soil amendment and environmental remediation: A critical review. Chem. Eng. J. 2021, 424, 130387. [Google Scholar] [CrossRef]
- Dos Santos, J.V.; Fregolente, L.G.; Moreira, A.B.; Ferreira, O.P.; Mounier, S.; Viguier, B.; Hajjoul, H.; Bisinoti, M.C. Humic-like acids from hydrochars: Study of the metal complexation properties compared with humic acids from anthropogenic soils using PARAFAC and time-resolved fluorescence. Sci. Total Environ. 2020, 722, 137815. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Shan, S.; Yang, C.; Zhang, C.; Zhou, X.; Ma, Q.; Yrjälä, K.; Zheng, H.; Cao, Y. The comparison of dissolved organic matter in hydrochars and biochars from pig manure. Sci. Total Environ. 2020, 720, 137423. [Google Scholar] [CrossRef] [PubMed]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Marín-Alzate, N.; Tobón, J.I.; Bertolotti, B.; Quintana Cáceda, M.E.; Flores, E. Evaluation of the Properties of Diatomaceous Earth in Relation to Their Performance in the Removal of Heavy Metals from Contaminated Effluents. Water Air Soil Pollut. 2021, 232, 122. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Rizwan, M.; Ali, S.; Hassan, M.J.; Brestic, M.; Zhang, X.; Huang, L. Effects of silicon on heavy metal uptake at the soil-plant interphase: A review. Ecotoxicol. Environ. Saf. 2021, 222, 112510. [Google Scholar] [CrossRef] [PubMed]
- Zargar, S.M.; Mahajan, R.; Bhat, J.A.; Nazir, M.; Deshmukh, R. Role of silicon in plant stress tolerance: Opportunities to achieve a sustainable cropping system. 3 Biotech 2019, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Minkina, T.; Feizi, M.; Kumari, A.; Khan, M.; Mandzhieva, S.; Sushkova, S.; El-Ramady, H.; Verma, K.K.; Singh, A. Effects of silicon and silicon-based nanoparticles on rhizosphere microbiome, plant stress and growth. Biology 2021, 10, 791. [Google Scholar] [CrossRef]
- Vasanthi, N.; Saleena, L.M.; Raj, S.A. Silica Solubilization Potential of Certain Bacterial Species in the Presence of Different Silicate Minerals. Silicon 2018, 10, 267–275. [Google Scholar] [CrossRef]
- Swoboda, P.; Döring, T.F.; Hamer, M. Remineralizing soils? The agricultural usage of silicate rock powders: A review. Sci. Total Environ. 2022, 807, 150976. [Google Scholar] [CrossRef]
- De Tombeur, F.; Roux, P.; Cornelis, J.-T. Silicon dynamics through the lens of soil-plant-animal interactions: Perspectives for agricultural practices. Plant Soil 2021, 467, 1–28. [Google Scholar] [CrossRef]
- Novy, V.; Nielsen, F.; Seiboth, B.; Nidetzky, B. The influence of feedstock characteristics on enzyme production in Trichoderma reesei: A review on productivity, gene regulation and secretion profiles. Biotechnol. Biofuels 2019, 12, 238. [Google Scholar] [CrossRef]
- Bischof, R.H.; Ramoni, J.; Seiboth, B. Cellulases and beyond: The first 70 years of the enzyme producer Trichoderma reesei. Microb. Cell Factories 2016, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Cao, M.; Sang, C.; Li, T.; Zhang, Y.; Chang, Y.; Li, L. Trichoderma Bio-Fertilizer Decreased C Mineralization in Aggregates on the Southern North China Plain. Agriculture 2022, 12, 1001. [Google Scholar] [CrossRef]
- Sriariyanun, M.; Tantayotai, P.; Yasurin, P.; Pornwongthong, P.; Cheenkachorn, K. Production, purification and characterization of an ionic liquid tolerant cellulase from Bacillus sp. isolated from rice paddy field soil. Electron. J. Biotechnol. 2016, 19, 23–28. [Google Scholar] [CrossRef]
- Ganesan, M.; Mathivani Vinayakamoorthy, R.; Thankappan, S.; Muniraj, I.; Uthandi, S. Thermotolerant glycosyl hydrolases-producing Bacillus aerius CMCPS1 and its saccharification efficiency on HCR-laccase (LccH)-pretreated corncob biomass. Biotechnol. Biofuels 2020, 13, 124. [Google Scholar] [CrossRef]
- Xie, S.-S.; Wu, H.-J.; Zang, H.-Y.; Wu, L.-M.; Zhu, Q.-Q.; Gao, X.-W. Plant Growth Promotion by Spermidine-Producing Bacillus subtilis OKB105. Mol. Plant-Microbe Interact. 2014, 27, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.-M.; Zheng, F.-L.; Wu, Q.-S. Elucidating the dialogue between arbuscular mycorrhizal fungi and polyamines in plants. World J. Microbiol. Biotechnol. 2022, 38, 159. [Google Scholar] [CrossRef]
- Veresoglou, S.D.; Verbruggen, E.; Makarova, O.; Mansour, I.; Sen, R.; Rillig, M.C. Arbuscular Mycorrhizal Fungi Alter the Community Structure of Ammonia Oxidizers at High Fertility via Competition for Soil NH4+. Microb. Ecol. 2019, 78, 147–158. [Google Scholar] [CrossRef]
- Dudáš, M.; Pjevac, P.; Kotianová, M.; Gančarčíková, K.; Rozmoš, M.; Hršelová, H.; Bukovská, P.; Jansa, J. Arbuscular Mycorrhiza and Nitrification: Disentangling Processes and Players by Using Synthetic Nitrification Inhibitors. Appl. Environ. Microbiol. 2022, 88, e0136922. [Google Scholar] [CrossRef]
- Agnihotri, R.; Sharma, M.P.; Prakash, A.; Ramesh, A.; Bhattacharjya, S.; Patra, A.K.; Manna, M.C.; Kurganova, I.; Kuzyakov, Y. Glycoproteins of arbuscular mycorrhiza for soil carbon sequestration: Review of mechanisms and controls. Sci. Total Environ. 2022, 806, 150571. [Google Scholar] [CrossRef]
- Gavito, M.E.; Jakobsen, I.; Mikkelsen, T.N.; Mora, F. Direct evidence for modulation of photosynthesis by an arbuscular mycorrhiza-induced carbon sink strength. New Phytol. 2019, 223, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Mosa, A.; Zhan, L.; Gao, B. Biochar modulates mineral nitrogen dynamics in soil and terrestrial ecosystems: A critical review. Chemosphere 2021, 278, 130378. [Google Scholar] [CrossRef]
- Borchard, N.; Schirrmann, M.; Cayuela, M.L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizábal, T.; Sigua, G.; Spokas, K.; Ippolito, J.A.; et al. Biochar, soil and land-use interactions that reduce nitrate leaching and N2O emissions: A meta-analysis. Sci. Total Environ. 2019, 651, 2354–2364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jing, Y.; Chen, C.; Xiang, Y.; Rezaei Rashti, M.; Li, Y.; Deng, Q.; Zhang, R. Effects of biochar application on soil nitrogen transformation, microbial functional genes, enzyme activity, and plant nitrogen uptake: A meta-analysis of field studies. GCB Bioenergy 2021, 13, 1859–1873. [Google Scholar] [CrossRef]
- European Commission. Sustainable Carbon Cycles for a 2050 Climate-Neutral EU Technical Assessment Brussels; SWD(2021) 450, The European Green Deal; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the European Council, the European Economic and Social Committee and the Committee of the Regions, EU Biodiversity Strategy for 2030 Bringing Nature back into Our Lives; COM (2020) 380 Final; EC: Brussels, Belgium, 2020. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. A Farm to Fork Strategy for a Fair, Healthy and Environmen-Tally-Friendly Food System; COM/2020/381 Final; EC: Brussels, Belgium, 2020. [Google Scholar]
- Montanarella, L.; Panagos, P. The relevance of sustainable soil management within the European Green Deal. Land Use Policy 2021, 100, 104950. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament, the European Council, the European Economic and Social Committee and the Committee of the Regions, Sustainable Carbon Cycles; COM(2021) 800; EC: Brussels, Belgium, 2021. [Google Scholar]
- Almaraz, M.; Wong, M.Y.; Geoghegan, E.K.; Houlton, B.Z. A review of carbon farming impacts on nitrogen cycling, retention, and loss. Ann. N. Y. Acad. Sci. 2021, 1505, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.T.; Basit, A.; Mohamed, H.I.; Ullah, I.; Sajid, M.; Sohrab, A. Use of Mulches in Various Tillage Conditions Reduces the Greenhouse Gas Emission—An Overview. Gesunde Pflanz. 2022. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.L.; Pu, C.; Zhang, X.Q.; Xue, J.F.; Zhang, R.; Wang, Y.Q.; Lal, R.; Zhang, H.L.; Chen, F. Methane and nitrous oxide emissions under no-till farming in China: A meta-analysis. Glob. Change Biol. 2016, 22, 1372–1384. [Google Scholar] [CrossRef]
- Zhang, C.; Song, X.; Zhang, Y.; Wang, D.; Rees, R.M.; Ju, X. Using nitrification inhibitors and deep placement to tackle the trade-offs between NH3 and N2O emissions in global croplands. Glob. Change Biol. 2022, 28, 4409–4422. [Google Scholar] [CrossRef]
- Bhakta, I.; Phadikar, S.; Majumder, K. State-of-the-art technologies in precision agriculture: A systematic review. J. Sci. Food Agric. 2019, 99, 4878–4888. [Google Scholar] [CrossRef]
- Lowenberg-DeBoer, J.; Erickson, B. Setting the Record Straight on Precision Agriculture Adoption. Agron. J. 2019, 111, 1552–1569. [Google Scholar] [CrossRef]
- Basso, B.; Antle, J. Digital agriculture to design sustainable agricultural systems. Nat. Sustain. 2020, 3, 254–256. [Google Scholar] [CrossRef]
- Shafi, U.; Mumtaz, R.; García-Nieto, J.; Hassan, S.A.; Zaidi, S.A.R.; Iqbal, N. Precision Agriculture Techniques and Practices: From Considerations to Applications. Sensors 2019, 19, 3796. [Google Scholar] [CrossRef] [PubMed]
- Torky, M.; Hassanein, A.E. Integrating blockchain and the internet of things in precision agriculture: Analysis, opportunities, and challenges. Comput. Electron. Agric. 2020, 178, 105476. [Google Scholar] [CrossRef]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
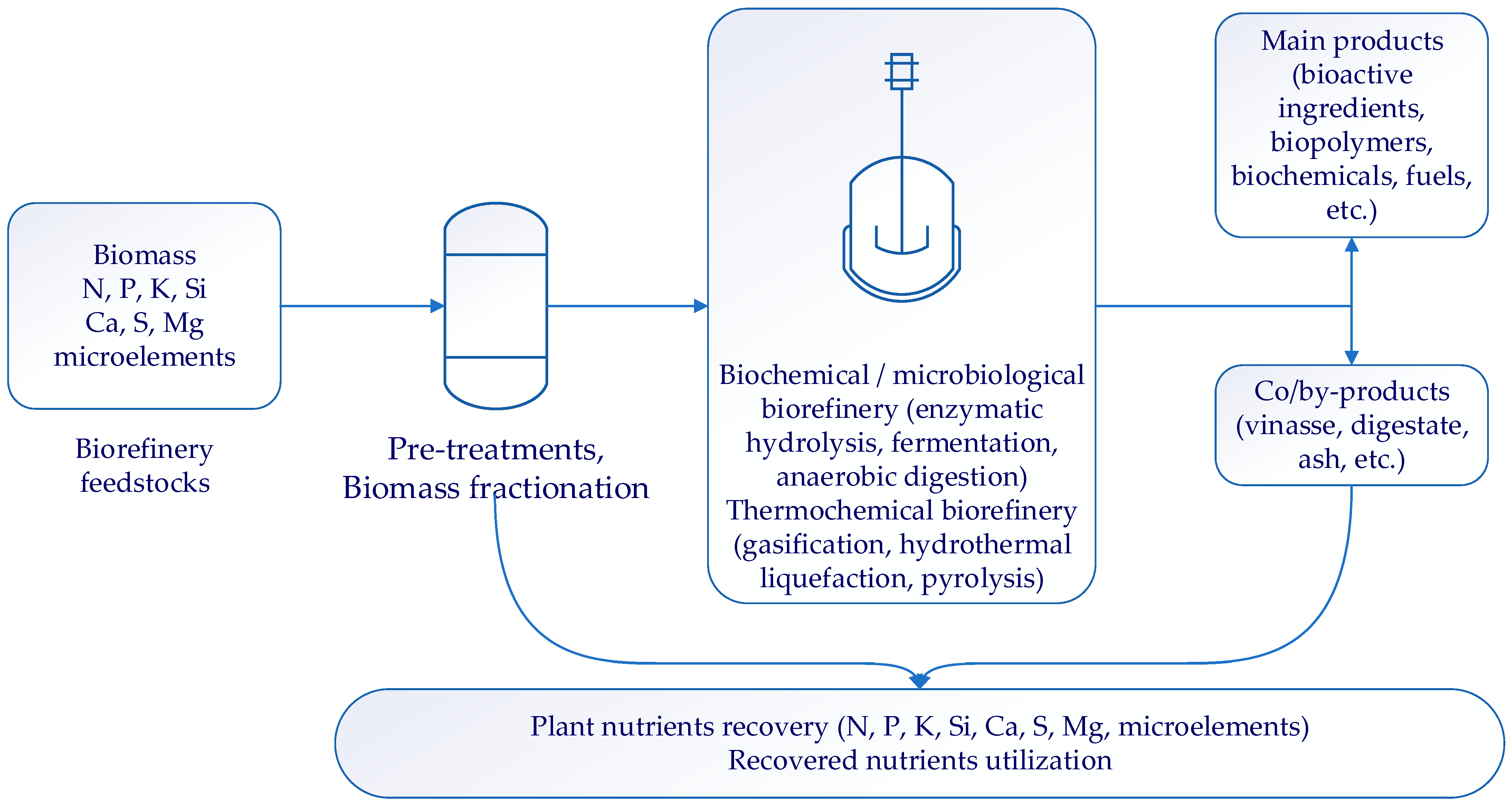
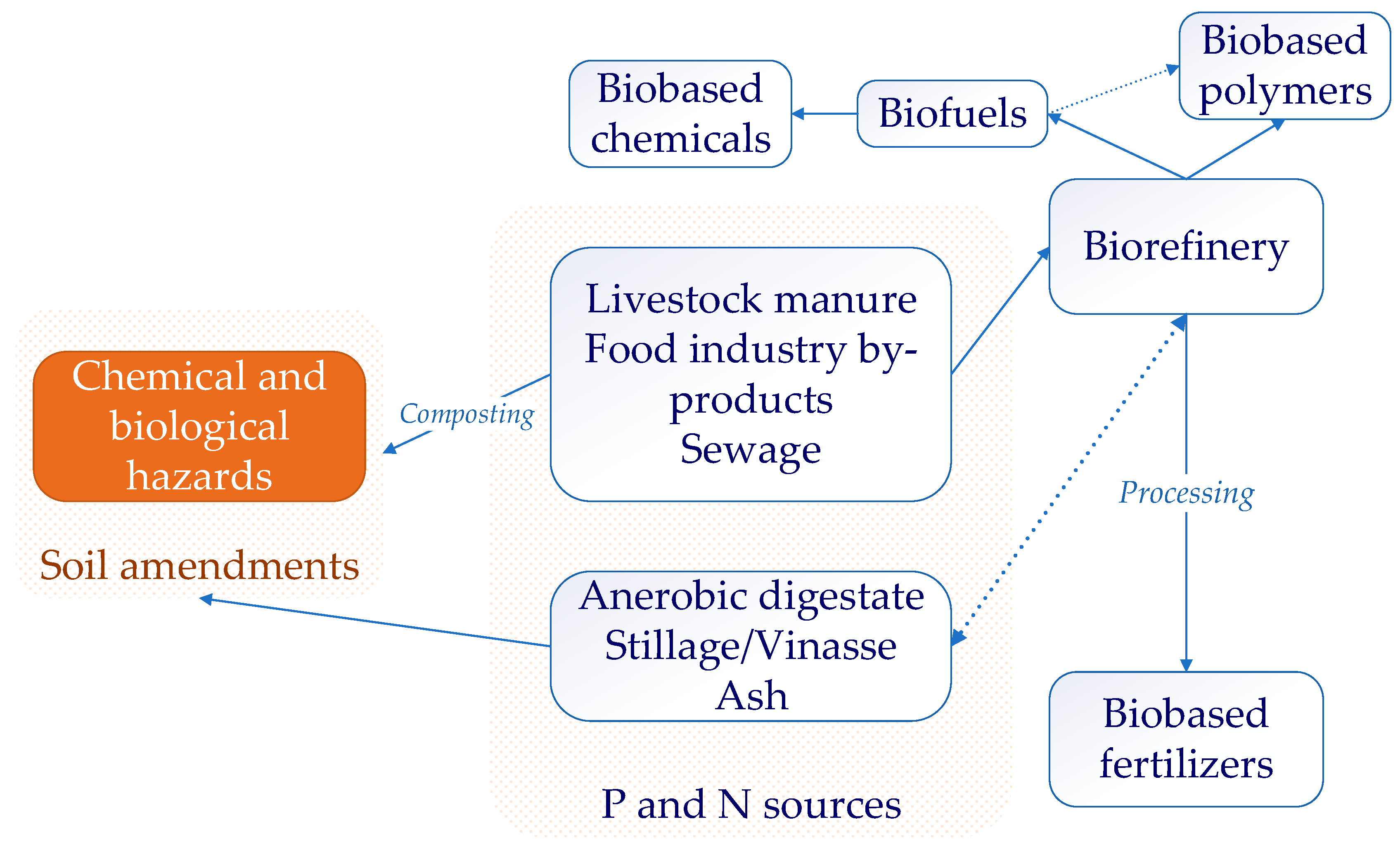
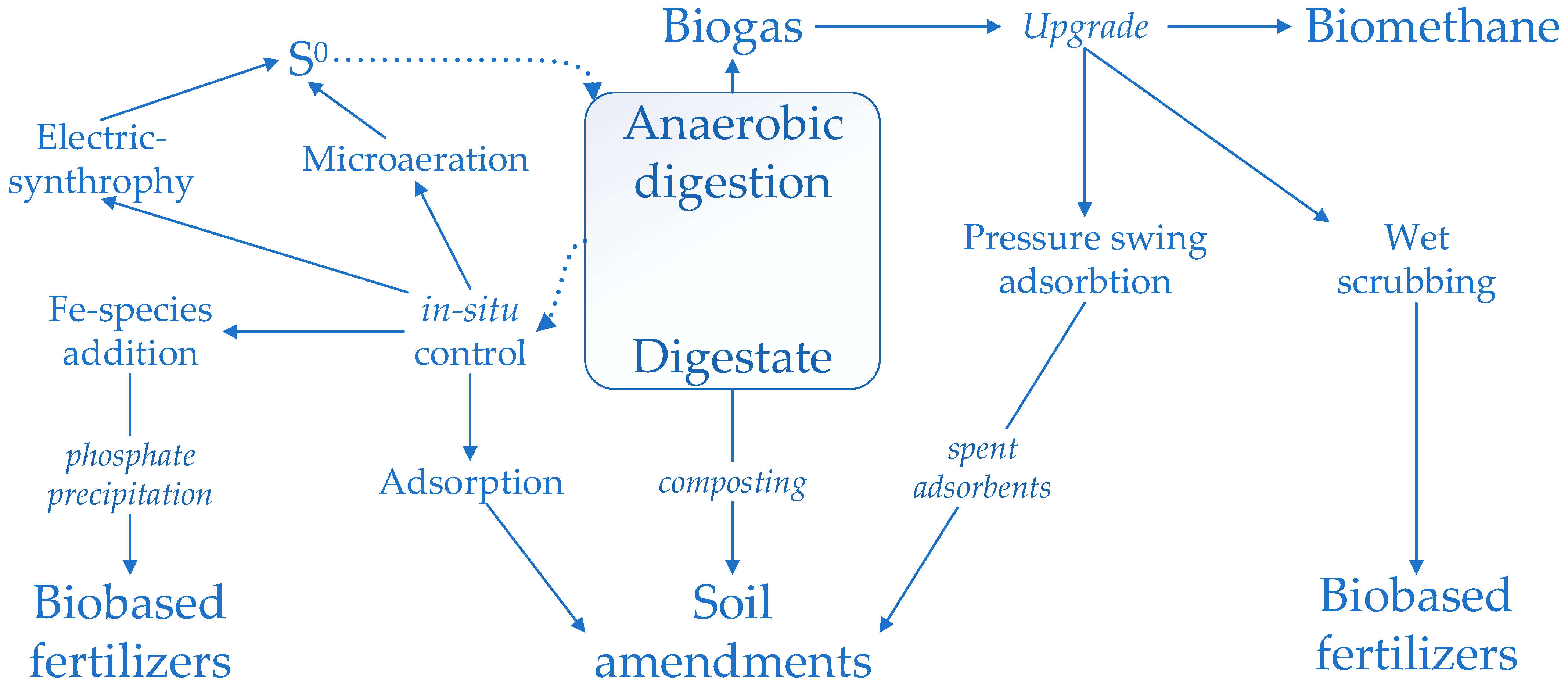
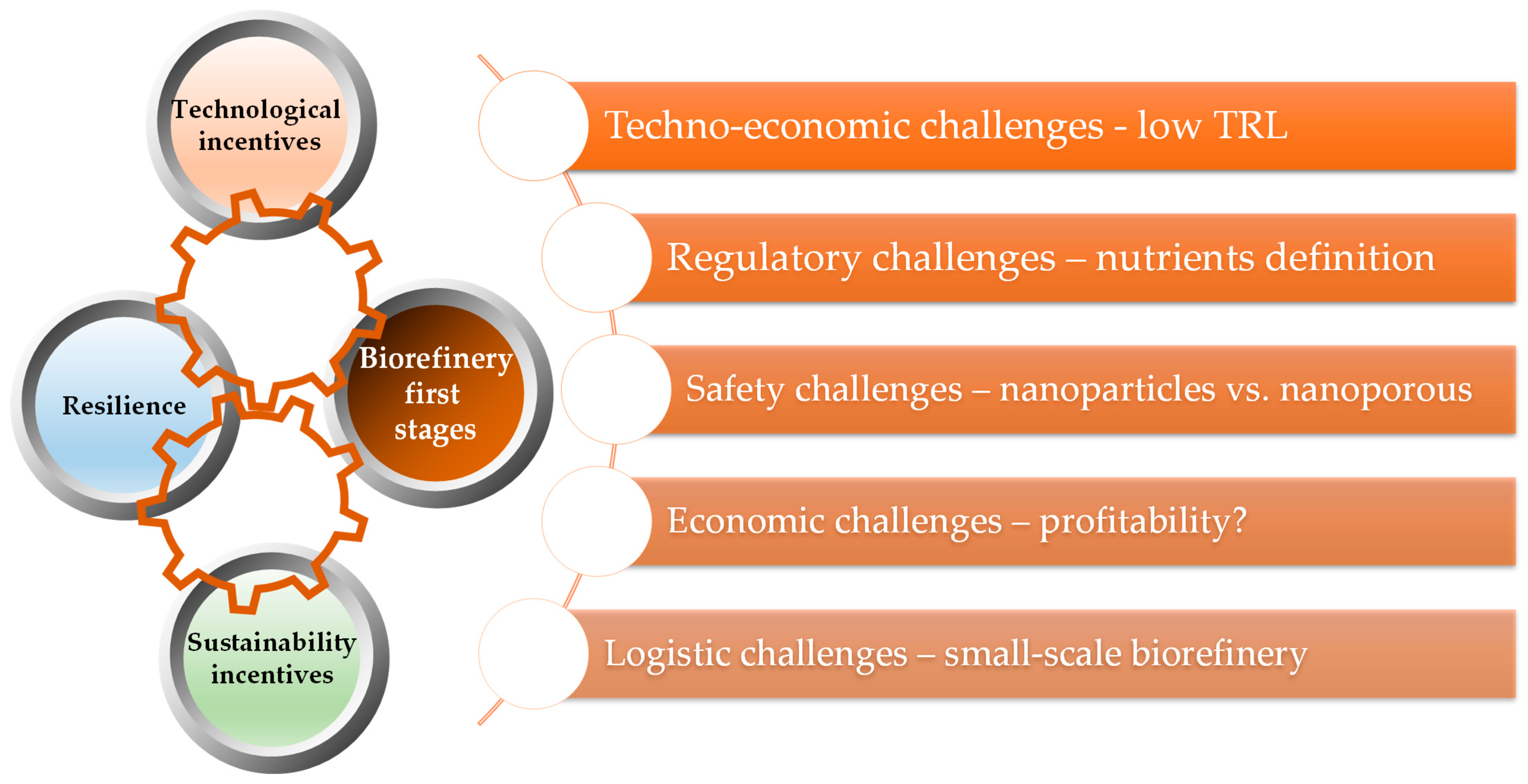
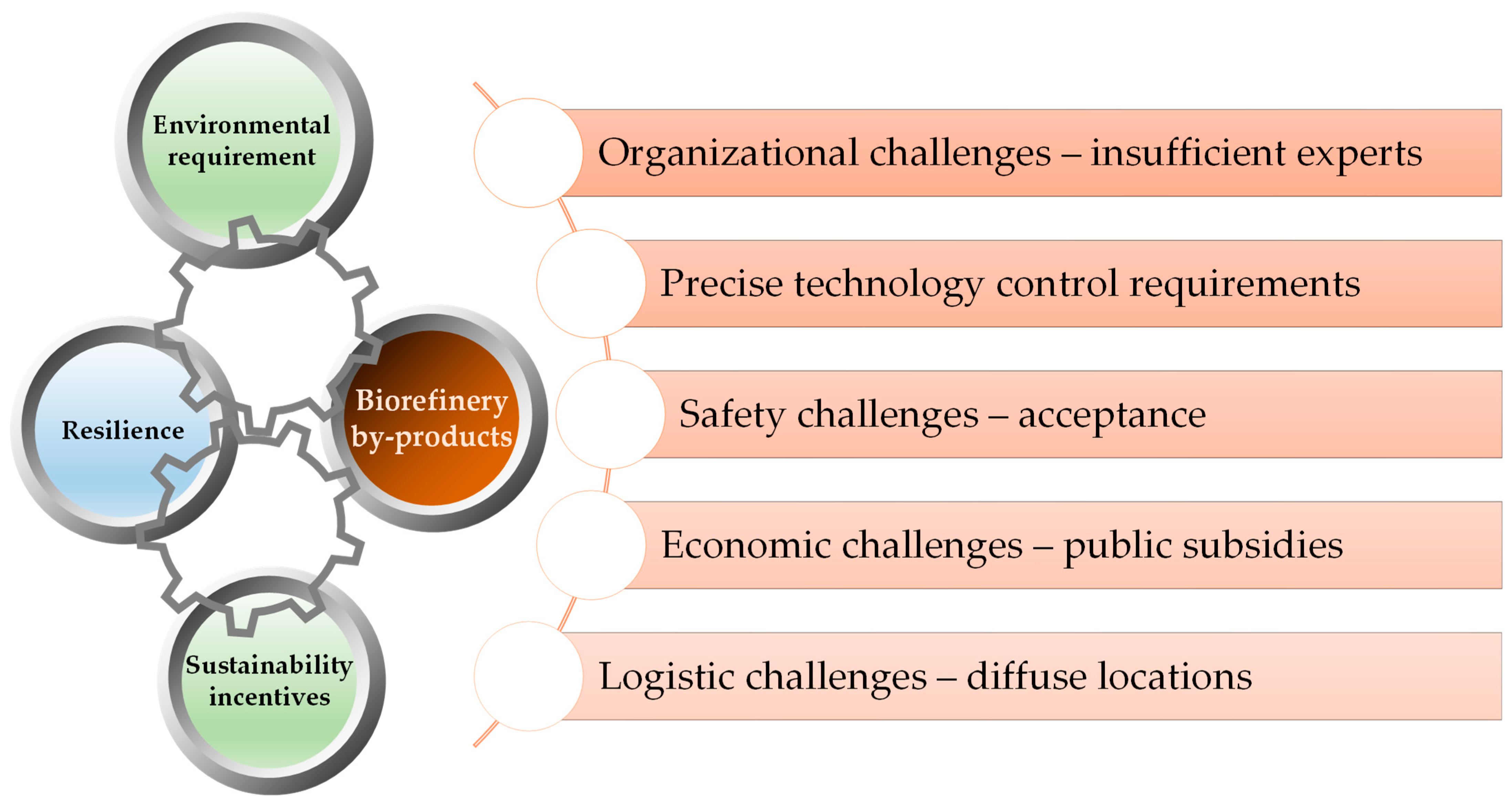

| Mineral | (Possible) Biorefinery Step | Biomass | (Na)DES/Yield | Ref |
|---|---|---|---|---|
| General | Pre-treatment | Various types | (Dry) acid-based pre-treatment | Reviewed in [76] |
| General, mainly Cl, K, P, Mg | Pre-treatment | Sorghum, switchgrass, corn stover, wheat straw | Bioleaching with Aspergillus niger | [107] |
| Hydroxyapatite | Pre-treatment | Fish (Aristichthys nobilis) scales | ChCl:glycerol/>46% | [206] |
| Hydroxyapatite | Pre-treatment | Freshwater carp (Carassius sp.) scales | ChCl:1,4 butanediol/>40% | [206] |
| General, mostly CaCO3 | Pre-treatment | Brown crab (Cancer pagurus) shell | ChCl:carboxylic acids (lactic, malic, malonic)/up to 100% | [207] |
| General, mostly CaCO3 | Pre-treatment | Insect (Hermetia illucens) | ChCl, betaine:lactic, oxalic, butyric acid, urea, glycerol/up to 98% | [208] |
| Silica Form | (Possible) Biorefinery Step | Biomass | Method | Ref |
|---|---|---|---|---|
| Silica nanoparticles (NPs) | Final step | Rice husks | Acid pre-treatment/ Calcination | [211] |
| Mesoporous silica | Final step | Rice straw | Ultrasound-H2SO4 pretreatment + calcination | [212] |
| Silica NPs | Pre-treatment/ final step | Sugarbeet bagasse | Laser ablation | [213] |
| Porous silica NPs | Pre-treatment/ Final step | Rice husks | Lignocellulose extraction with ionic liquids + calcination | [214] |
| N/A | Pre-treatment | Paddy husks | Delignification with deep eutectic solvent (DES) | [215] |
| Spherical nanosilica | Pre-treatment | Rice husks | Nitric acid + NaOH + sol–gel synthesis | [216] |
| Insoluble colloid H2SiO3 | Pre-treatment | Bamboo | NaOH cold extraction+ CO2 precipitation | [217] |
| N/A | Pre-treatment | Rice straw | Organosolv, Na2CO3 | [218] |
| N/A | Pre-treatment | Oil palm empty fruit bunch (OPEFB) | Ammonia fiber expansion (AFEX) | [219] |
| Silica rosette-like microparticles | Final step | Pineapple peels | By-product of nanocellulose extraction | [220] |
| Nanocrystalline silica | Pre-treatment | Rice husks | Fungal-based bioleaching (Fusarium oxysporum) | [221] |
| Silica NPs | Pre-treatment | Rice husks | Fungal bioprocessing (Trichoderma harzianum) | [222] |
| Nanosilica | Pre-treatment | Rice husks | Fungal-based bioleaching and biotranformation (Aspergillus parasiticus) | [223] |
| Nanosilica | Pre-treatment | Corn cobs husks | Fungal-based biotransformation (Fusarium culmorum) | [224] |
| Nanosilica | Pre-treatment | Rice husks | Californian red worms | [225] |
| N/A | Pre-treatment | OPEFB, Sugarcane stalks | Mechanical (ultrasonic, hammering, crushing + size fractionation) | [226,227,228,229] |
| Technological Solutions | Biorefinery Side-Streams | Resulted Fertilizing Product | Ref. |
|---|---|---|---|
| Pyrolysis | Distiller grains | Biochar with multinutrients, produced from distiller grains treated with wet-process phosphoric acid, neutralized with KOH, and pyrolyzed at 400 °C | [264] |
| Distiller grains | Biochar composite, produced from distiller grains mixed with phosphogypsum that adsorb phosphate from water | [265] | |
| Anaerobic digestate from kitchen waste | Pyrochar (at 500 °C) with bioavailable phosphorus | [266] | |
| Anaerobic digestate of wastewater sludge and quinoa residues | Biochar that, at 25 t/ha, with liquid digestate (170 kg N/ha) increase by 25% growth of tomato plant | [267] | |
| Anaerobic digestate from commercial biogas producing plant | Pyrochar—soil amendment | [268] | |
| Hydrothermal carbonisation | Sugarcane bagasse and vinasse | Hydrochar releasing nutrients and carbon, according to the sol and the applied dose | [269] |
| Anaerobic digestate | Aqueous phosphate fertilizers, released from hydrochar by leaching with sulfuric acid | [270] | |
| Thin stillage | Hydrochar that collect the mineral nutrients from thin stillage | [271] | |
| Combustion | Lignin from cellulosic ethanol | Ash rich in potassium and phosphorus | [272] |
| Dried distiller grains | Ash rich in phosphorus that is available for Argentine canola (Brassica napus L.L. 5030) plants | [273] | |
| Distiller grains | Ash with high content of sulfur, nitrogen, phosphorus, and potassium | [274] | |
| Reverse osmosis | Sugarcane vinasse | Organo-mineral fertilizer | [275] |
| Anaerobic digestate from a commercial 2.5 MWe biogas plant | Liquid N/K-fertiliser, solid N/P-fertiliser, produced in a three-stage reverse osmosis unit, included in a pilot plant | [276] | |
| Anaerobic digestate from fish waste and cow dung | Concentrated liquid mineral fertilizer, solid fertilizer | [277] | |
| Phosphorus precipitation | Concentrated cellulosic ethanol stillage | Struvite—MgNH4PO4·6H2O | [278] |
| Vinasse, concentrate of the cathode area of the electrodialysis membrane | K-struvite—KMgPO4· 6H2O) | [279] | |
| Hydrolysate of microalgae, Scenedesmus sp. | Hydroxyapatite—Ca10(PO4)6(OH)2 Dittmarite—MgNH4PO4·H2O | [280] | |
| Liquid anerobic digestate | Struvite—MgNH4PO4·6H2O | [281] | |
| Thin stillage from corn ethanol | Ca-phytate, precipitated at pH 9.0 and 1.5:1 Ca-P ratio | [282] | |
| Microbial fuel cell (MFC) and a Microbial electrolysis cell (MEC) | Anerobic digestate | Struvite—MgNH4PO4·6H2O | [283] |
| Microbial electrolysis cell (MEC) | Anerobic digestate | Struvite—MgNH4PO4·6H2O | [284] |
| Treatment | Substrate | Operating Conditions | References |
|---|---|---|---|
| Dissolution in C1–4 monocarboxylic acid (formic, acetic, lactic, propionic, or butyric) solutions | Ash from fruits skin (hull), e.g., sunflower hulls | Solutions up to 35% organic acid, temperatures from 20 to 50 °C | US2022106237 A1 [302] |
| Dissolution of the phosphate-containing ash by adding nitric acid, separation of soluble phosphate fraction and soluble calcium fraction, precipitation of calcium nitrate, phosphate solution concentration | Sewage sludge ash (SSA) and/or meat-and-bone meal, plus phosphate rock | The rate between nitric acid and phosphate from the substrate is 1—to 1.8; phosphate-containing ash is dissolved at 50–60 °C for 70–80 min | EP3495323 B1 [303] |
| Mixing ash with a calcium or magnesium compound, followed by dissolution in phosphoric or sulfuric acid | Incinerated ash residue of chicken droppings | 5–200 parts of calcium or magnesium compound mixed with 100 ash parts | US7452398 B2 [304] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantinescu-Aruxandei, D.; Oancea, F. Closing the Nutrient Loop—The New Approaches to Recovering Biomass Minerals during the Biorefinery Processes. Int. J. Environ. Res. Public Health 2023, 20, 2096. https://doi.org/10.3390/ijerph20032096
Constantinescu-Aruxandei D, Oancea F. Closing the Nutrient Loop—The New Approaches to Recovering Biomass Minerals during the Biorefinery Processes. International Journal of Environmental Research and Public Health. 2023; 20(3):2096. https://doi.org/10.3390/ijerph20032096
Chicago/Turabian StyleConstantinescu-Aruxandei, Diana, and Florin Oancea. 2023. "Closing the Nutrient Loop—The New Approaches to Recovering Biomass Minerals during the Biorefinery Processes" International Journal of Environmental Research and Public Health 20, no. 3: 2096. https://doi.org/10.3390/ijerph20032096
APA StyleConstantinescu-Aruxandei, D., & Oancea, F. (2023). Closing the Nutrient Loop—The New Approaches to Recovering Biomass Minerals during the Biorefinery Processes. International Journal of Environmental Research and Public Health, 20(3), 2096. https://doi.org/10.3390/ijerph20032096







