Evaluation of the Impact of Activated Biochar-Manure Compost Pellet Fertilizer on Volatile Organic Compound Emissions and Heavy Metal Saturation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the ABMCP
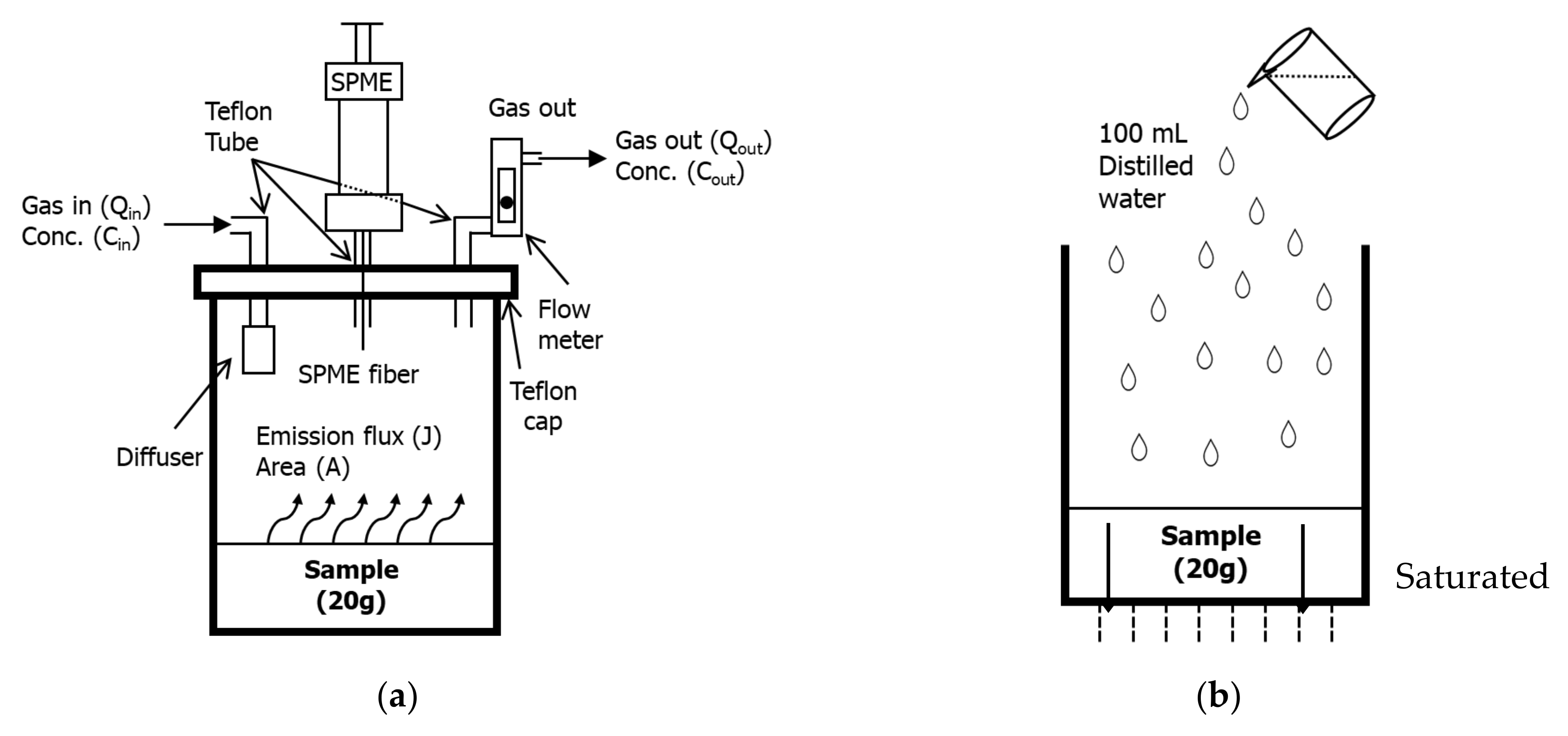
2.2. Experimental Setup, Odor, and VOC Analysis
2.3. Biochar Characteristics and Elemental Composition
2.4. Analysis of Heavy Metals in the Leachates
2.5. Statistical Analysis of Data
3. Results and Discussion
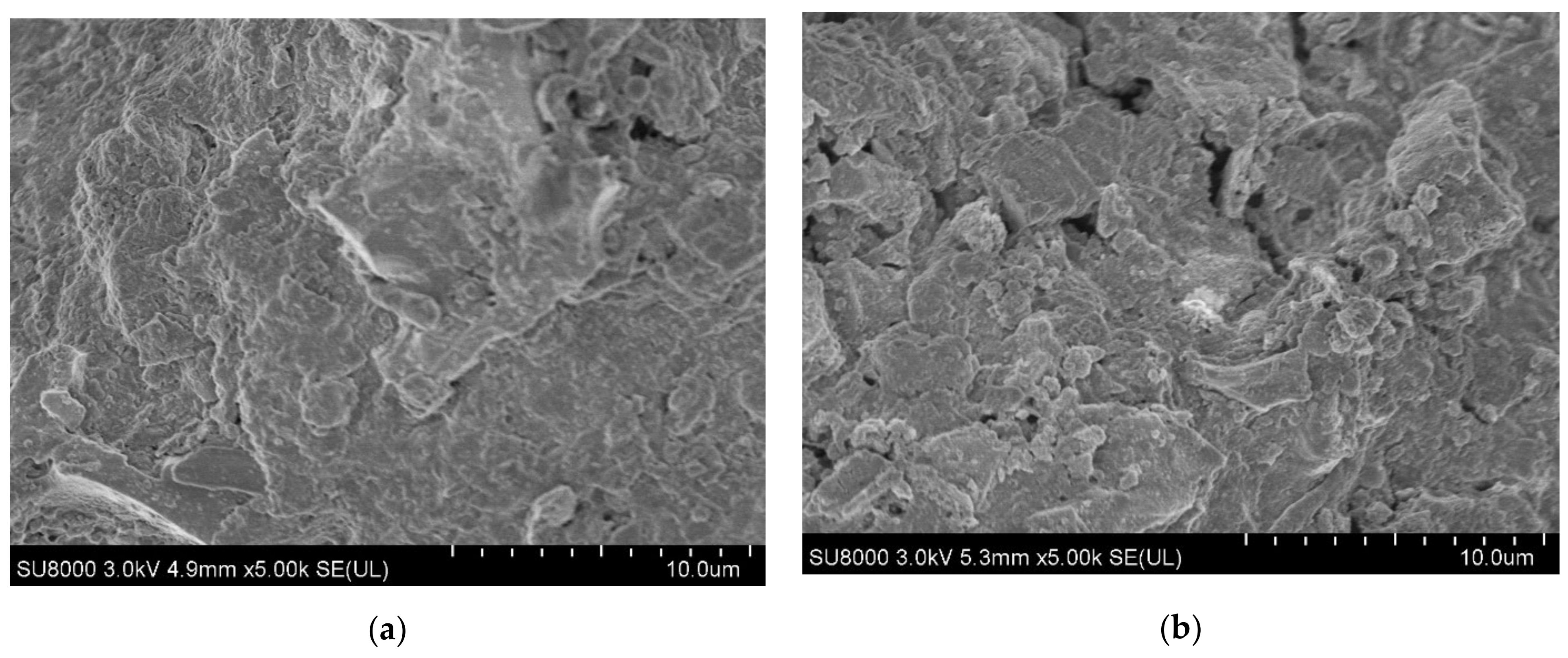
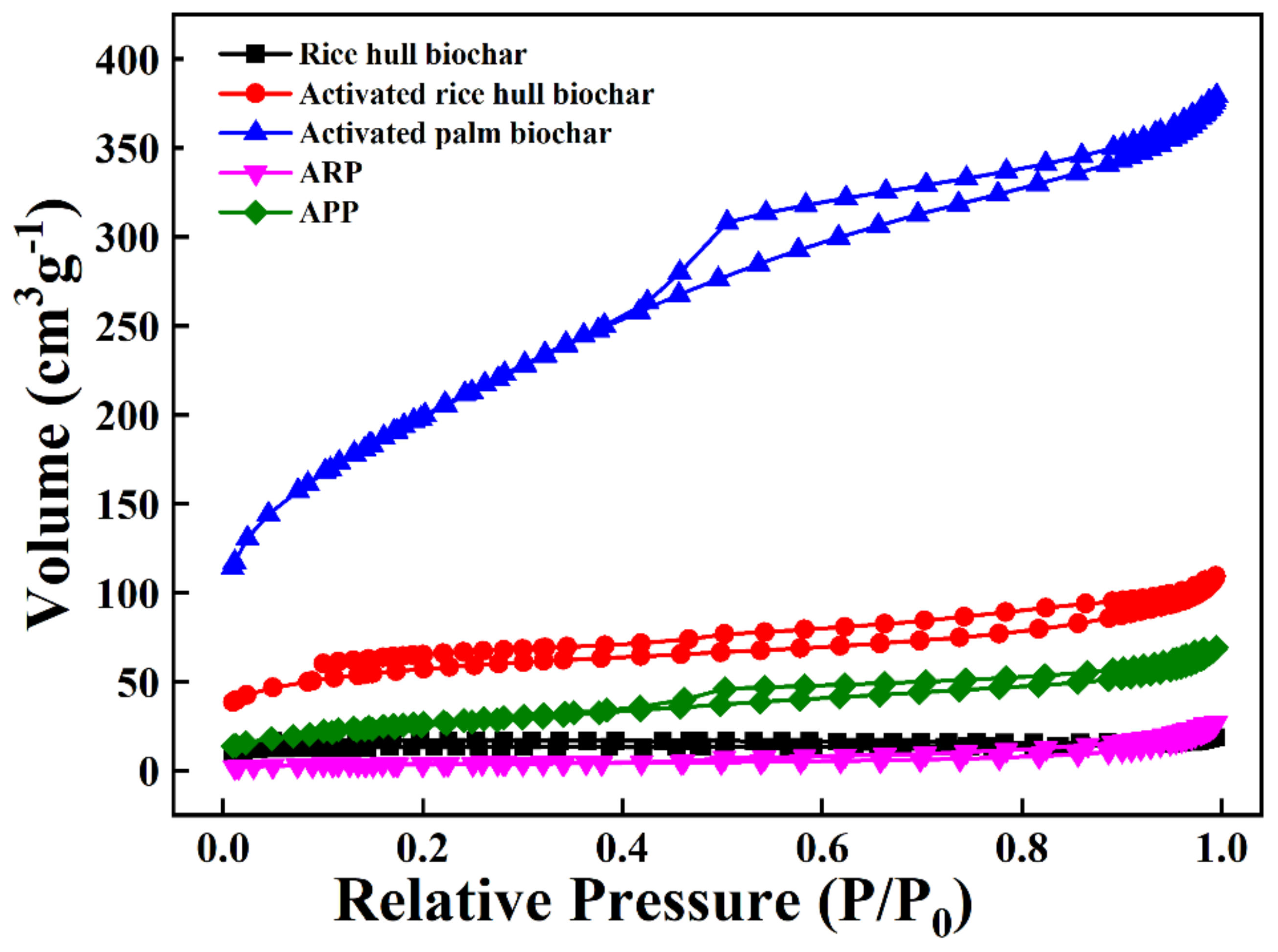
3.1. Characteristics of the ABMCP
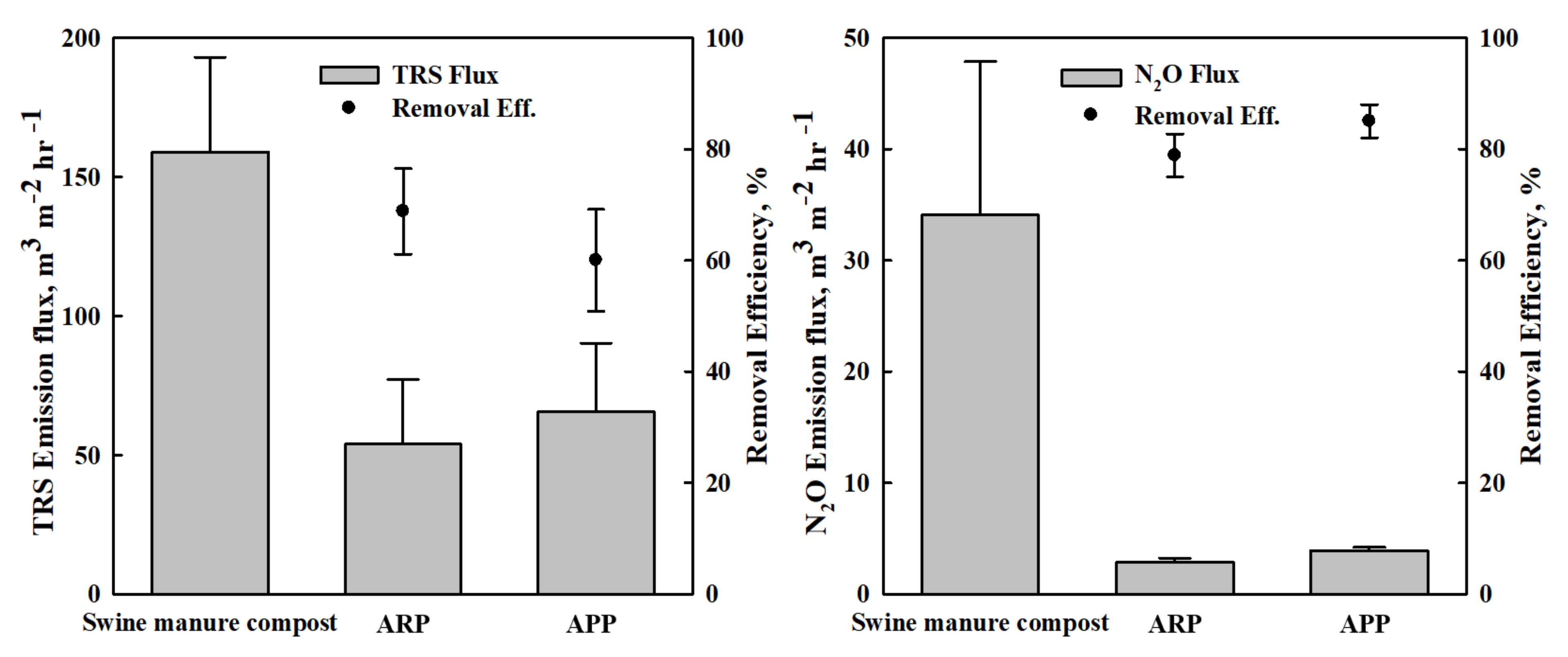
3.2. Impacts of the ABMCP on Odor Emission of TRS and N2O
3.3. Impacts of the ABMCP on VFAs Concentration and VOC Emission
3.4. Reduction in Heavy Metals from the Leachates through ABMCP Saturation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, T.W.; Halder, J.N.; Kim, S.; Yoon, Y.; Lee, M. Nutrient Composition and Heavy Metal Contents of Matured Livestock Liquid Fertilizer in Korea. J. Korea Org. Resour. Recycl. Assoc. 2017, 25, 31–39. [Google Scholar] [CrossRef]
- Lee, J.H.; Febrisiantosa, A. Improvement of nitrogen balance (land budget) in South Korea in terms of livestock manure: A review. IOP Conf. Ser. Earth Environ. Sci. 2020, 462, 012011. [Google Scholar] [CrossRef]
- Yuan, T.; Huang, W.; Lei, Z.; Zhao, Z.; Zhang, Z. Effects of different alkalis on hydrolysis of swine manure during dry anaerobic digestion and resultant nutrients availability. Int. Biodeterior. Biodegrad. 2017, 123, 138–145. [Google Scholar] [CrossRef]
- Zang, B.; Li, S.; Michel, F.C.; Li, G.; Zhang, D.; Li, Y. Control of dimethyl sulfide and dimethyl disulfide odors during pig manure composting using nitrogen amendment. Bioresour. Technol. 2017, 224, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.J.; Chen, M.L.; Ye, A.D.; Chou, M.S.; Shen, S.H.; Mao, I.F. The relationship of odor concentration and the critical components emitted from food waste composting plants. Atmos. Environ. 2008, 42, 8246–8251. [Google Scholar] [CrossRef]
- Maulii-Duran, C.; Artola, A.; Font, X.; Sánchez, A. A systematic study of the gaseous emissions from biosolids composting: Raw sludge versus anaerobically digested sludge. Bioresour. Technol. 2013, 147, 43–51. [Google Scholar] [CrossRef]
- Hansen, M.; Kasper, P.; Adamsen, A.; Feilberg, A. Key Odorants from Pig Production Based on Improved Measurements of Odor Threshold Values Combining Olfactometry and Proton-Transfer-Reaction Mass Spectrometry (PTR-MS). Sensors 2018, 18, 788. [Google Scholar] [CrossRef]
- Lee, S.W.; Daud, W.M.A.W.; Lee, M.G. Adsorption characteristics of methyl mercaptan, dimethyl disulfide, and trimethylamine on coconut-based activated carbons modified with acid and base. J. Ind. Eng. Chem. 2010, 16, 973–977. [Google Scholar] [CrossRef]
- Zhao, S.; Yi, H.; Tang, X.; Gao, F.; Zhang, B.; Wang, Z.; Zuo, Y. Methyl mercaptan removal from gas streams using metal-modified activated carbon. J. Clean. Prod. 2015, 87, 856–861. [Google Scholar] [CrossRef]
- Blazy, V.; de Guardia, A.; Benoist, J.C.; Daumoin, M.; Lemasle, M.; Wolbert, D.; Barrington, S. Odorous gaseous emissions as influence by process condition for the forced aeration composting of pig slaughterhouse sludge. Waste Manag. 2014, 34, 1125–1138. [Google Scholar] [CrossRef]
- Lo, Y.C.M.; Koziel, J.A.; Cai, L.; Hoff, S.J.; Jenks, W.S.; Xin, H. Simultaneous chemical sensory characterization of volatile organic compounds and sim-volatile organic compounds emitted from swine manure using solid phase microextraction and multidimensional gas chromatography-mass spectrometry-olfactometry. J. Environ. Qual. 2008, 37, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, S.S.; Bennett, J.L.; Raymer, J.H. Quantification of odors and odorants from swine operations in North Carolina. Agric. For. Meteorol. 2001, 108, 213–240. [Google Scholar] [CrossRef]
- Parker, D.B.; Gilley, J.; Woodbury, B.; Kim, K.H.; Galvin, G.; Bartelt-Hunt, S.L.; Li, X.; Snow, D.D. Odorous VOC emission following land application of swine manure slurry. Atmos. Environ. 2013, 66, 91–100. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, W.; Wang, X.; Lei, C.; Tang, R.; Zhang, F.; Yang, Q.; Zhu, F. Tracing heavy metals in ‘swine manure-maggot-chicken’ production chain. Sci. Rep. 2017, 7, 8417. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, J.; Xu, M.; Lv, J.; Sun, N. Accumulation, availability, and uptake of heavy metals in a red soil after 22-year fertilization and cropping. Environ. Sci. Pollut. Res. 2015, 22, 15154–15163. [Google Scholar] [CrossRef]
- Guan, D.X.; Sun, F.S.; Yu, G.H.; Polizzotto, M.L.; Liu, Y.G. Total and available metal concentrations in soils from six long-term fertilization sites across China. Environ. Sci. Pollut. Res. 2018, 25, 31666–31678. [Google Scholar] [CrossRef]
- Guo, T.; Lou, C.; Zhai, W.; Tang, X.; Hashmi, M.Z.; Murtaza, R.; Li, Y.; Liu, X.; Xu, J. Increased occurrence of heavy metals, antibiotics and resistance genes in surface soil after long-term application of manure. Sci. Total Environ. 2018, 635, 995–1003. [Google Scholar] [CrossRef]
- Qaswar, M.; Yiren, L.; Jing, H.; Kaillou, L.; Mudasir, M.; Zhenzhen, L.; Hongqian, H.; Xianjin, L.; Jianhua, J.; Ahmed, W.; et al. Soil nutrients and heavy metal availability under long-term combined application of swine manure and synthetic fertilizers in acidic paddy soil. J. Soils Sediments 2020, 20, 2093–2106. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, N.; Tong, L.; Lv, Y. Structural and adsorption characteristics of potassium carbonate activated biochar. RCS Adv. 2018, 8, 21012–21019. [Google Scholar] [CrossRef]
- Inyang, M.; Dickenson, E. The potential role of biochar in the removal of organic and microbial contaminants from potable and reuse water: A review. Chemosphere 2015, 134, 232–240. [Google Scholar] [CrossRef]
- Le-Minh, N.; Sivret, E.C.; Shammay, A.; Stuetz, R.M. Factors affecting the adsorption of gaseous environmental odors by activated carbon: A critical review. Crit. Rev. Environ. Sci. Technol. 2018, 48, 341–375. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Lin, C.; Hoang, H.G.; Sanderson, P.; Dang, B.T.; Bui, X.T.; Nguyen, N.H.; Vo, D.N.; Tran, H.T. Evaluate the role of biochar during the organic waste composting process: A critical review. Chemosphere 2022, 229, 134488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, B.; Zheng, Y.; Hu, X.; Creamer, A.E.; Annable, M.D.; Li, Y. Biochar for volatile organic compound (VOC) removal: Sorption performance and governing mechanisms. Bioresour. Technol. 2017, 245, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Dari, B.; Nair, V.D.; Harris, W.G.; Nair, P.K.R.; Sollenberger, L.; Mylavarapu, R. Relative influence of soil-vs. biochar properties on soil phosphorus retention. Geoderma 2016, 280, 82–87. [Google Scholar] [CrossRef]
- Pourhashem, G.; Rasool, Q.Z.; Zhang, R.; Medlock, K.B.; Cohan, D.S.; Masiello, C.A. Valuing the air quality effects of biochar reductions on soil NO emissions. Environ. Sci. Technol. 2017, 51, 9856–9863. [Google Scholar] [CrossRef] [PubMed]
- Sizmur, T.; Fresno, T.; Akgül, G.; Frost, H.; Moreno-Jiménez, E. Biochar modification to enhance sorption of inorganics from water. Bioresour. Technol. 2017, 246, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Wang, J.; Pan, Y.; Shen, B.; Wu, C. Review of biochar for the management of contaminated soil: Preparation, application and prospect. Sci. Total Environ. 2019, 659, 473–490. [Google Scholar] [CrossRef]
- Kim, H.; Yun, S.; An, N.; Shin, J. Effect of KOH concentrations and pyrolysis temperatures for enhancing NH4-N adsorption capacity of rice hull activated biochar. Korean J. Environ. Agric. 2020, 39, 171–177. [Google Scholar] [CrossRef]
- Sessa, F.; Merlin, G.; Ganu, P. Pine bark valorization by activated carbons production due to be used as VOCs adsorbents. Fuel 2002, 318, 123346. [Google Scholar] [CrossRef]
- Shin, J.; Choi, E.; Jang, E.; Hong, S.; Lee, S.; Ravindran, B. Adsorption characteristics of ammonium nitrogen and plant responses to biochar pellet. Sustainability 2018, 10, 1331. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Nochetto, C.; McConnell, L.L. Gas-phase analysis of trimethylamine, propionic and butyric acids, and sulfur compounds using solid-phase microextraction. Anal. Chem. 2002, 74, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Klasason, K.T. Biochar characterization and a method for estimating biochar quality from proximate analysis results. Biomass Bioenergy 2017, 96, 50–58. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, W.; Liang, G.; Song, D.; Zhang, X. Characteristics of maize biochar with different pyrolysis temperatures and its effects on organic carbon, nitrogen and enzymatic activities after addition to fluvo-aquic soil. Sci. Total Environ. 2015, 538, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, D.; Zhu, L. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef]
- Yang, H.; Huan, B.; Chen, Y.; Gao, Y.; Li, J.; Chen, H. Biomass-based Pyrolytic Poly generation system for bamboo industry waste: Evolution of the char structure and the pyrolysis mechanism. Energy Fuels 2016, 30, 6430–6439. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Bagreev, A.; Bandosz, T.J. Study of hydrogen sulfide adsorption on activated carbons using inverse gas chromatography at infinite dilution. J. Chem. Phys. B 2000, 104, 8841–8847. [Google Scholar] [CrossRef]
- Bashkova, S.; Armstrong, T.R.; Schwartz, V. Selective catalytic oxidation of hydrogen sulfide on activated carbons impregnated with sodium hydroxide. Energy Fuels 2009, 23, 1674–1682. [Google Scholar] [CrossRef]
- Chun, Y.; Sheng, G.; Chiou, C.T.; Xing, B. Compositions and sorptive properties of crop residue-derived chars. Environ. Sci. Technol. 2004, 38, 4649–4655. [Google Scholar] [CrossRef] [PubMed]
- Inyang, M.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.R.; Pullammanappallil, P.; Cao, X. Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresour. Technol. 2012, 110, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, J.; Yong, X.; Luo, L.; Wong, J.W.; Zhang, Y.; Wu, H.; Zhou, J. Effect of biochar combined with a biotrickling filter on deodorization, nitrogen retention, and microbial community succession during chicken manure composting. Bioresour. Technol. 2022, 343, 126137. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Koziel, J.A.; Lee, M.; O’Brien, S.C.; Li, P.; Brown, R.C. Mitigation of acute hydrogen sulfide and ammonia emissions from swine manure during three-hour agitation using pelletized biochar. Atmosphere 2021, 12, 825. [Google Scholar] [CrossRef]
- Hwang, O.; Lee, S.R.; Cho, S.; Ro, K.S.; Spiehs, M.; Woodbury, B.; Silva, P.J.; Han, D.W.; Choi, H.; Kim, K.Y.; et al. Efficacy of different biochars in removing odorous volatile organic compounds (VOCs) emitted from swine manure. ACS Sustain. Chem. Eng. 2018, 6, 14239–14247. [Google Scholar] [CrossRef]
- Shang, G.; Li, Q.; Liu, L.; Chen, P.; Huang, X. Adsorption of hydrogen sulfide by biochars derived from pyrolysis of different agricultural/forestry wastes. J. Air Waste Manag. Assoc. 2016, 66, 8–16. [Google Scholar] [CrossRef]
- Giagnoni, L.; Maienza, A.; Baronti, S.; Vaccari, F.P.; Genesio, L.; Taiti, C.; Martellini, T.; Scodellini, R.; Cincinelli, A.; Costa, C.; et al. Long-term soil biological fertility, volatile organic compounds and chemical properties in a vineyard soil after biochar amendment. Geoderma 2019, 344, 127–136. [Google Scholar] [CrossRef]
- Duan, Y.; Awasthi, S.K.; Liu, T.; Zhang, Z.; Awasthi, M.K. Response of bamboo biochar amendment on volatile fatty acids accumulation reduction and humification during chicken manure composting. Bioresour. Technol. 2019, 291, 121845. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Awasthi, S.K.; Wang, Q.; Wang, Z.; Lahori, A.H.; Ren, X.; Chen, H.; Wang, M.; Zhao, J.; Zhang, Z. Influence of biochar on volatile fatty acids accumulation and microbial community succession during biosolids composting. Bioresour. Technol. 2018, 251, 158–164. [Google Scholar] [CrossRef]
- Korea Ministry of Environment. Soil Environment Conservation Act; Act No. 15102; Korea Ministry of Environment: Sejong City, Korea, 2017.
- Korea Ministry of Environment. Wastes Control Act; Act No. 15103; Korea Ministry of Environment: Sejong City, Korea, 2018.

| Materials | Rice Hull Biochar (%) | Activated Rice Hull Biochar (%) | Activated Palm Biochar (%) | Swine Manure Compost (%) | ARP 1 (%) | APP 2 (%) |
|---|---|---|---|---|---|---|
| C | 44.3 | 61.5 | 74.3 | 18.8 | 32.5 | 37.6 |
| H | 1.1 | 1.2 | 1.0 | 6.4 | 3.4 | 3.9 |
| O | 8.9 | 10.7 | 7.6 | 19.1 | 22.0 | 24.6 |
| N | 0.6 | 0.3 | 1.0 | 0.5 | 9.4 | 8.1 |
| S | N.D. 3 | N.D. | 1.3 | 0.6 | 0.7 | 0.7 |
| Others | 45.1 | 26.3 | 14.8 | 54.6 | 32.0 | 25.1 |
| Materials | Rice Hull Biochar (%) | Activated Rice Hull Biochar (%) | Activated Palm Biochar (%) | ARP 1 (%) | APP 2 (%) |
|---|---|---|---|---|---|
| Calcium (Ca) | 10.1 | 6.2 | 28.7 | 49.6 | 39.3 |
| Potassium (K) | 18.8 | 77.8 | 2.6 | 29.4 | 27.0 |
| Iron (Fe) | 1.5 | 1.9 | 35.9 | 7.0 | 16.9 |
| Chlorine (Cl) | N.D. | N.D. | N.D. | 8.2 | 9.9 |
| Silicon (Si) | 64.9 | 12.3 | 15.7 | 2.3 | 1.4 |
| Sulfur (S) | 0.7 | N.D. | 7.9 | N.D. | 1.2 |
| Phosphorus (P) | 0.6 | N.D. | N.D. | 1.1 | 1.2 |
| Manganese (Mn) | 3.0 | 1.2 | 1.7 | N.D. | N.D. |
| Titanium (Ti) | N.D. 3 | N.D. | 4.6 | N.D. | N.D. |
| Others | 0.4 | 0.6 | 2.9 | 2.4 | 3.1 |
| Characteristics | Rice Hull Biochar | Activated Rice Hull Biochar | Activated Palm Biochar | ARP 1 | APP 2 |
|---|---|---|---|---|---|
| BET Surface Area (m2 g−1) | 54.8 ± 1.07 | 206.0 ± 2.35 | 720.7 ± 2.32 | 12.8 ± 0.01 | 92.3 ± 0.36 |
| Total pore volume (at BJH desorption) (cm2 g−1) | 0.0307 ± 0.005 | 0.163 ± 0.011 | 0.578 ± 0.002 | 0.0454 ± 0.004 | 0.109 ± 0.02 |
| Average pore size (at BJH desorption) (nm) | 2.24 ± 0.03 | 3.17 ± 0.04 | 3.21 ± 0.01 | 10.6 ± 0.25 | 5.01 ± 0.15 |
| Heavy Metals | Swine Manure Compost (mg L−1) | ARP 1 (mg L−1) | APP 2 (mg L−1) | Method Detection Limit (mg L−1) | Soil Environmental Conservation Act (mg kg−1) | Waste Management Act (mg L−1) |
|---|---|---|---|---|---|---|
| As | N.D. 3 | N.D. | N.D. | 0.08 | 25 | 1.5 |
| Cd | N.D. | N.D. | N.D. | 0.001 | 4 | 0.3 |
| Cu | 0.36 ± 0.15 | 0.30 ± 0.18 | 0.01 ± 0.01 | 0.002 | 150 | 3 |
| Ni | 0.009 ± 0.01 | 0.074 ± 0.03 | 0.004 ± 0.03 | 0.002 | 100 | - |
| Pb | N.D. | N.D. | N.D. | 0.01 | 200 | 3 |
| Zn | 1.37 ± 0.69 | 0.48 ± 0.29 | 0.59 ± 0.37 | 0.001 | 300 | - |
| Fe | 5.07 ± 2.60 | 3.17 ± 2.04 | 15.7 ± 10.6 | 0.001 | - | - |
| Ca | 17.9 ± 12.0 | 6.70 ± 3.42 | 31.2 ± 22.7 | 0.001 | - | - |
| K | 784 ± 53.6 | 1964 ± 102.4 | 2128 ± 161.9 | 0.002 | - | - |
| Si | 1.29 ± 0.48 | 2.04 ± 1.42 | 1.30 ± 0.73 | 0.002 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Jeong, C.; Kim, M.; Nam, J.; Shim, C.; Shin, J. Evaluation of the Impact of Activated Biochar-Manure Compost Pellet Fertilizer on Volatile Organic Compound Emissions and Heavy Metal Saturation. Int. J. Environ. Res. Public Health 2022, 19, 12405. https://doi.org/10.3390/ijerph191912405
Kim M, Jeong C, Kim M, Nam J, Shim C, Shin J. Evaluation of the Impact of Activated Biochar-Manure Compost Pellet Fertilizer on Volatile Organic Compound Emissions and Heavy Metal Saturation. International Journal of Environmental Research and Public Health. 2022; 19(19):12405. https://doi.org/10.3390/ijerph191912405
Chicago/Turabian StyleKim, Minsoo, Changyoon Jeong, Minjeong Kim, Joohee Nam, Changki Shim, and Joungdu Shin. 2022. "Evaluation of the Impact of Activated Biochar-Manure Compost Pellet Fertilizer on Volatile Organic Compound Emissions and Heavy Metal Saturation" International Journal of Environmental Research and Public Health 19, no. 19: 12405. https://doi.org/10.3390/ijerph191912405
APA StyleKim, M., Jeong, C., Kim, M., Nam, J., Shim, C., & Shin, J. (2022). Evaluation of the Impact of Activated Biochar-Manure Compost Pellet Fertilizer on Volatile Organic Compound Emissions and Heavy Metal Saturation. International Journal of Environmental Research and Public Health, 19(19), 12405. https://doi.org/10.3390/ijerph191912405







