Impact of Prescribed Exercise on the Physical and Cognitive Health of Adults with Down Syndrome: The MinDSets Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Measurements
2.4. Data Acquisition
2.5. Statistical Analysis
3. Results
3.1. Baseline
3.2. Intervention Outcomes
3.2.1. Physical Fitness
3.2.2. Cognitive Responses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patterson, D.; Costa, A. Down syndrome and genetics—A case of linked histories. Nat. Rev. Genet. 2005, 6, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Tsou, A.Y.; Bulova, P.; Capone, G.; Chicoine, B.; Gelaro, B.; Harville, T.O.; Martin, B.A.; McGuire, D.E.; McKelvey, K.D.; Peterson, M.; et al. Medical care of adults with Down syndrome: A clinical guideline. Jama 2020, 324, 1543–1556. [Google Scholar] [CrossRef] [PubMed]
- Guerra, M.; Llorens, N.; Fernhall, B. Chronotropic incompetence in persons with down syndrome. Arch. Phys. Med. Rehabil. 2003, 84, 1604–1608. [Google Scholar] [CrossRef]
- Klotzbier, T.J.; Holfelder, B.; Schott, N. Associations of motor performance and executive functions: Comparing children with Down syndrome to chronological and mental age-matched controls. Children 2022, 9, 73. [Google Scholar] [CrossRef]
- Danopoulos, S.; Deutsch, G.H.; Dumortier, C.; Mariani, T.J.; Al Alam, D. Lung disease manifestations in Down syndrome. Am. J. Physiol. Lung Cell. Mol. 2021, 321, L892–L899. [Google Scholar] [CrossRef]
- Ram, G.; Chinen, J. Infections and immunodeficiency in Down syndrome. Clin. Exp. Immunol. 2011, 164, 9–16. [Google Scholar] [CrossRef]
- Tungate, A.S.; Conners, F.A. Executive function in Down syndrome: A meta-analysis. Res. Dev. Disabil. 2021, 108, 103802. [Google Scholar] [CrossRef]
- Barr, M.; Shields, N. Identifying the barriers and facilitators to participation in physical activity for children with Down syndrome. J. Intellect. Disabil. Res. 2011, 55, 1020–1033. [Google Scholar] [CrossRef]
- Oreskovic, N.M.; Cottrell, C.; Torres, A.; Patsiogiannis, V.; Santoro, S.; Nichols, D.; Moore, C.; Skotko, B.G. Physical activity patterns in adults with Down Syndrome. J. Appl. Res. Intellect. Disabil. 2020, 33, 1457–1464. [Google Scholar] [CrossRef]
- Mahy, J.; Shields, N.; Taylor, N.F.; Dodd, K.J. Identifying facilitators and barriers to physical activity for adults with Down syndrome. J. Intellect. Disabil. Res. 2010, 54, 795–805. [Google Scholar] [CrossRef]
- Cugusi, L.; Carta, M.G. Conventional exercise interventions for adults with intellectual disabilities: A systematic review and meta-analysis. Transl. Sports Med. 2021, 4, 6–20. [Google Scholar] [CrossRef]
- Hassan, N.M.; Landorf, K.B.; Shields, N.; Munteanu, S.E. Effectiveness of interventions to increase physical activity in individuals with intellectual disabilities: A systematic review of randomised controlled trials. J. Intellect. Disabil. Res. 2019, 63, 168–191. [Google Scholar] [CrossRef] [PubMed]
- Andriolo, R.B.; El Dib, R.; Ramos, L.; Atallah, Á.N.; da Silva, E.M. Aerobic exercise training programmes for improving physical and psychosocial health in adults with Down syndrome. Cochrane Database Syst. Rev. 2005, 12, CD005176. [Google Scholar]
- Cotman, C.W.; Berchtold, N.C. Exercise: A behavioural intervention to enhance brain health and plasticity. Trends Neurosci. 2002, 25, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Lista, I.; Sorrentino, G. Biological mechanisms of physical activity in preventing cognitive decline. Cell. Mol. Neurobiol. 2010, 30, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Fiocco, A.J.; Lindquist, K.; Vittinghoff, E.; Simonsick, E.M.; Newman, A.B.; Satterfield, S.; Rosano, C.; Rubin, S.M.; Ayonayon, H.N.; et al. Predictors of maintaining cognitive function in older adults: The Health ABC study. Neurology 2009, 72, 2029–2035. [Google Scholar] [CrossRef]
- Diamond, A.B. The cognitive benefits of exercise in youth. Curr. Sports Med. Rep. 2015, 14, 320–326. [Google Scholar] [CrossRef]
- Fernandes, J.; Soares, J.C.K.; do Amaral Baliego, L.G.Z.; Arida, R.M. A single bout of resistance exercise improves memory consolidation and increases the expression of synaptic proteins in the hippocampus. Hippocampus 2016, 26, 1096–1103. [Google Scholar] [CrossRef]
- Fernandes, J.; Arida, R.M.; Gomez-Pinilla, F. Physical exercise as an epigenetic modulator of brain plasticity and cognition. Neurosci. Biobehav. Rev. 2017, 80, 443–456. [Google Scholar] [CrossRef]
- Ludyga, S.; Gerber, M.; Brand, S.; Holsboer-Trachsler, E.; Pühse, U. Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: A meta-analysis. Psychophysiology 2016, 53, 1611–1626. [Google Scholar] [CrossRef]
- Drollette, E.S.; Scudder, M.R.; Raine, L.B.; Moore, R.D.; Saliba, B.J.; Pontifex, M.B.; Hillman, C.H. Acute exercise facilitates brain function and cognition in children who need it most: An ERP study of individual differences in inhibitory control capacity. Dev. Cognit. Neurosci. 2014, 7, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Numminen, H.; Service, E.; Ahonen, T.; Ruoppila, I. Working memory and everyday cognition in adults with Down’s syndrome. J. Intellect. Disabil. Res. 2001, 45, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Heller, T.; Hsieh, K.; Rimmer, J.H. Attitudinal and psychosocial outcomes of a fitness and health education program on adults with Down syndrome. Am. J. Ment. Retard. 2004, 109, 175–185. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. Jama 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Tsimaras, V.; Giagazoglou, P.; Fotiadou, E.; Christoulas, K.; Angelopoulou, N. Jog-walk training in cardiorespiratory fitness of adults with Down syndrome. Percept. Mot. Skills 2003, 96 (Suppl. S3), 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Kane, S.P. ClinCalc.com. Available online: https://clincalc.com/ (accessed on 1 February 2023).
- Chen, C.C.; Hunt, L.M.; Ringenbach, S.D.R. Exploring Associations with 6-Minute Walk Test Performance in Adolescents and Young Adults with Down Syndrome: A Pilot Study. J. Dev. Phys. Disabil. 2018, 30, 783–792. [Google Scholar] [CrossRef]
- Enright, P.L.; Sherrill, D.L. Reference equations for the six-minute walk in healthy adults. Am. J. Respir. Crit. Care Med. 1998, 158, 1384–1387. [Google Scholar] [CrossRef]
- Foster, C.; Porcari, P.J.; Ault, S.; Doro, K.; Dubiel, J.; Engen, M.; Kolman, D.; Xiong, S. Exercise prescription when there is no exercise test: The Talk Test. Kinesiology 2018, 50 (Suppl. S1), 33–48. [Google Scholar]
- Stoet, G. PsyToolkit: A software package for programming psychological experiments using Linux. Behav. Res. Methods 2010, 42, 1096–1104. [Google Scholar] [CrossRef]
- Stoet, G. PsyToolkit: A novel web-based method for running online questionnaires and reaction-time experiments. Teach. Psychol. 2017, 44, 24–31. [Google Scholar] [CrossRef]
- Picorelli, A.M.A.; Pereira, L.S.M.; Pereira, D.S.; Felício, D.; Sherrington, C. Adherence to exercise programs for older people is influenced by program characteristics and personal factors: A systematic review. J. Physiotherapy 2014, 60, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Balic, M.; Oviedo, G.R.; Javierre, C.; Fortuño, J.; Barnet-López, S.; Niño, O.; Alamo, J.; Fernhall, B. Reliability and validity of the 6-min walk test in adults and seniors with intellectual disabilities. Res. Dev. Disabil. 2015, 47, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Hamacher, D.; Herold, F.; Wiegel, P.; Hamacher, D.; Schega, L. Brain activity during walking: A systematic review. Neurosci. Biobehav. Rev. 2015, 57, 310–327. [Google Scholar] [CrossRef] [PubMed]
- La Fougere, C.; Zwergal, A.; Rominger, A.; Förster, S.; Fesl, G.; Dieterich, M.; Brandt, T.; Strupp, M.; Bartenstein, P.; Jahn, K. Real versus imagined locomotion: A [18F]-FDG PET-fMRI comparison. Neuroimage 2010, 50, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Montagne, G.; Buekers, M.; Camachon, C.; De Rugy, A.; Laurent, M. The learning of goal-directed locomotion: A perception-action perspective. Q. J. Exp. Psychol. Sec. A 2003, 56, 551–567. [Google Scholar] [CrossRef]
- Rigoldi, C.; Galli, M.; Mainardi, L.; Crivellini, M.; Albertini, G. Postural control in children, teenagers and adults with Down syndrome. Res. Dev. Disabil. 2011, 32, 170–175. [Google Scholar] [CrossRef]
- Jackson, J.D.; Balota, D.A. Mind-wandering in younger and older adults: Converging evidence from the Sustained Attention to Response Task and reading for comprehension. Psychol. Aging 2012, 27, 106. [Google Scholar] [CrossRef]
- Tiernan, B.N.; Mutter, S.A. The effects of age and uncertainty in the Stroop priming task. Psychol. Aging 2021, 36, 452. [Google Scholar] [CrossRef]
- Ivanoff, J.; Branning, P.; Marois, R. fMRI evidence for a dual process account of the speed-accuracy tradeoff in decision-making. PLoS ONE 2008, 3, e2635. [Google Scholar] [CrossRef]
- Grießbach, E.; Raßbach, P.; Herbort, O.; Cañal-Bruland, R. Embodied decisions during walking. J. Neurophysiol. 2022, 128, 1207–1223. [Google Scholar] [CrossRef]
- McGlinchey, E.; McCarron, M.; Holland, A.; McCallion, P. Examining the effects of computerised cognitive training on levels of executive function in adults with Down syndrome. J. Intellect. Disabil. Res. 2019, 63, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Vandierendonck, A.; Kemps, E.; Fastame, M.C.; Szmalec, A. Working memory components of the Corsi blocks task. Br. J. Psychol. 2004, 95, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Schaller, A.; Rudolf, K.; Dejonghe, L.; Grieben, C.; Froboese, I. Influencing factors on the overestimation of self-reported physical activity: A cross-sectional analysis of low back pain patients and healthy controls. Biomed Res. Int. 2016, 2016, 1497213. [Google Scholar] [CrossRef]
- McMorris, T.; Hale, B.J. Differential effects of differing intensities of acute exercise on speed and accuracy of cognition: A meta-analytical investigation. Brain Cogn. 2012, 80, 338–351. [Google Scholar] [CrossRef]
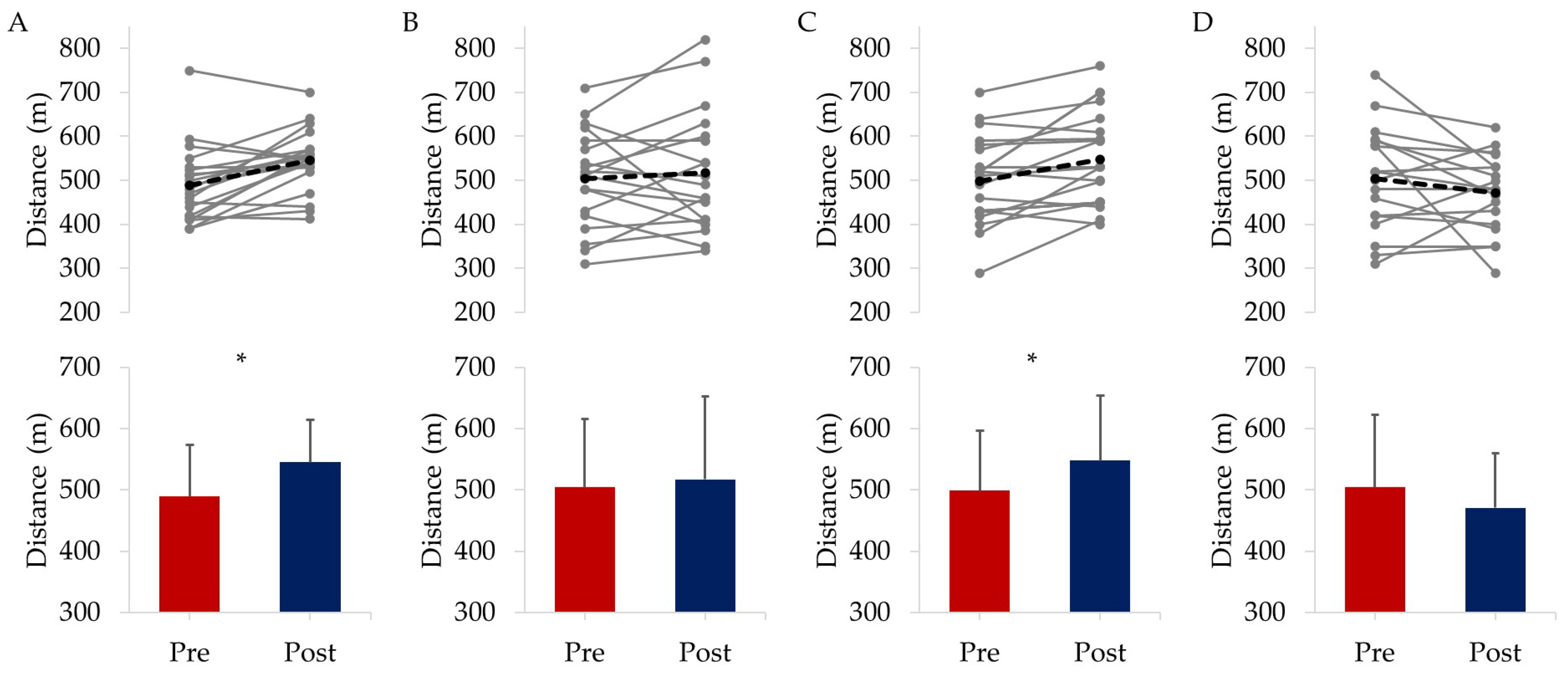
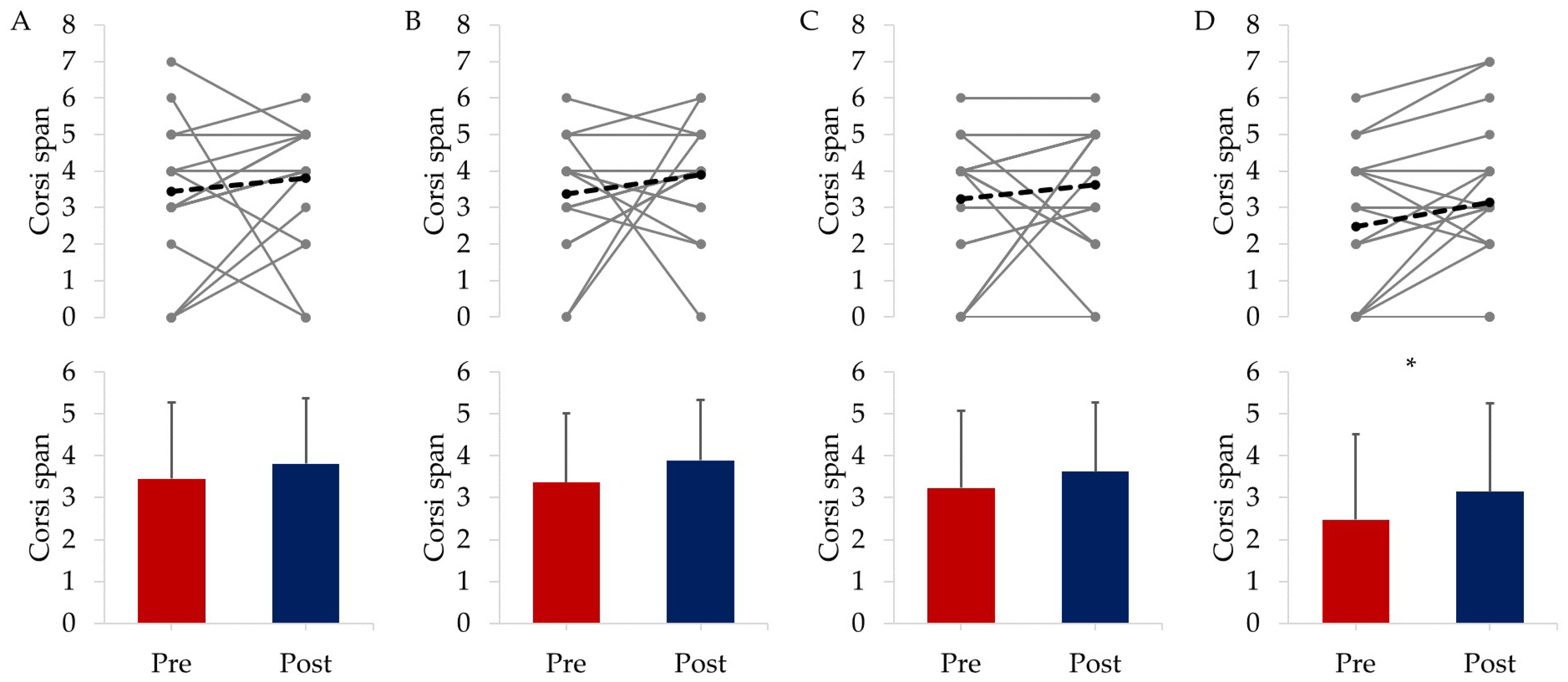
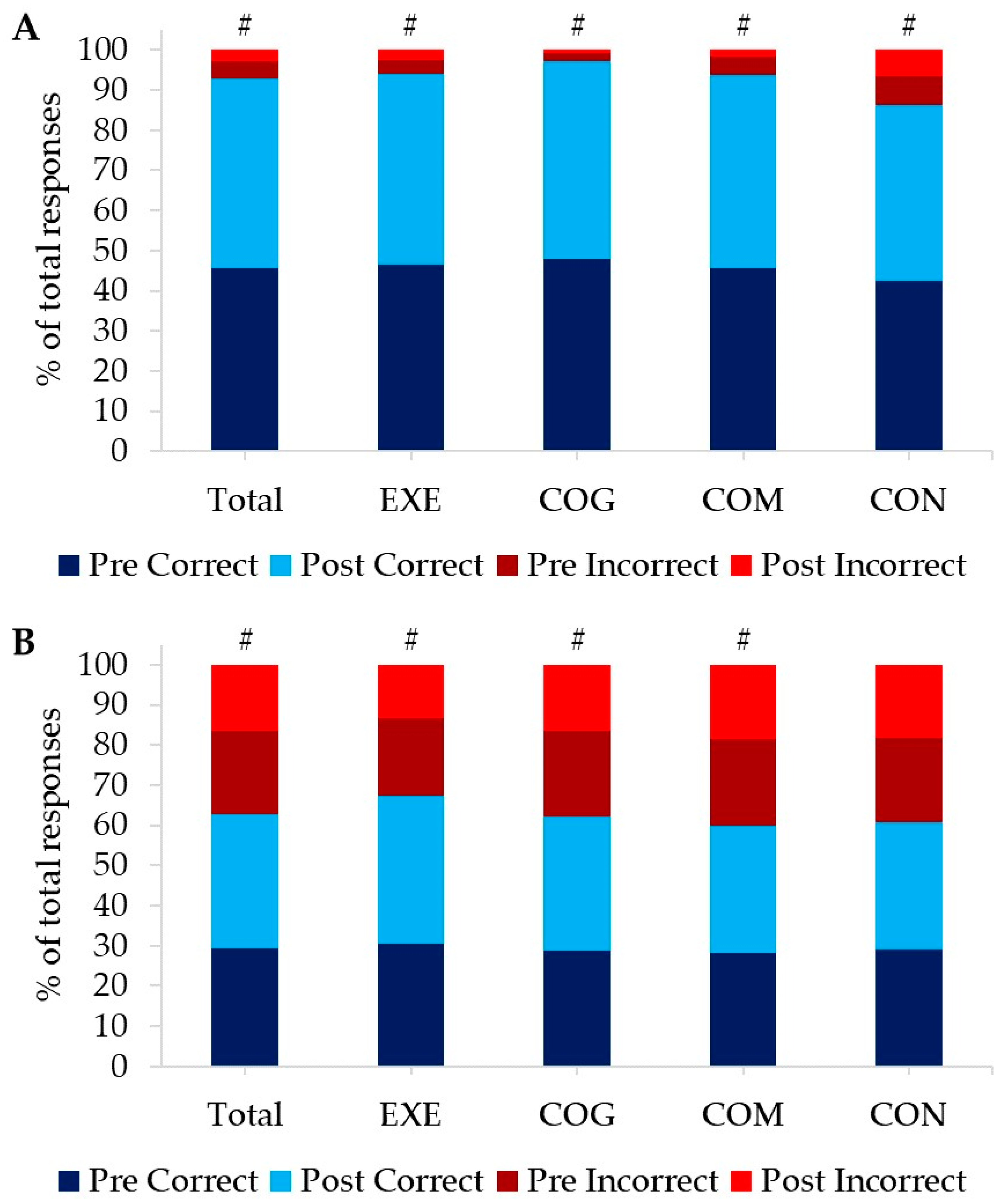
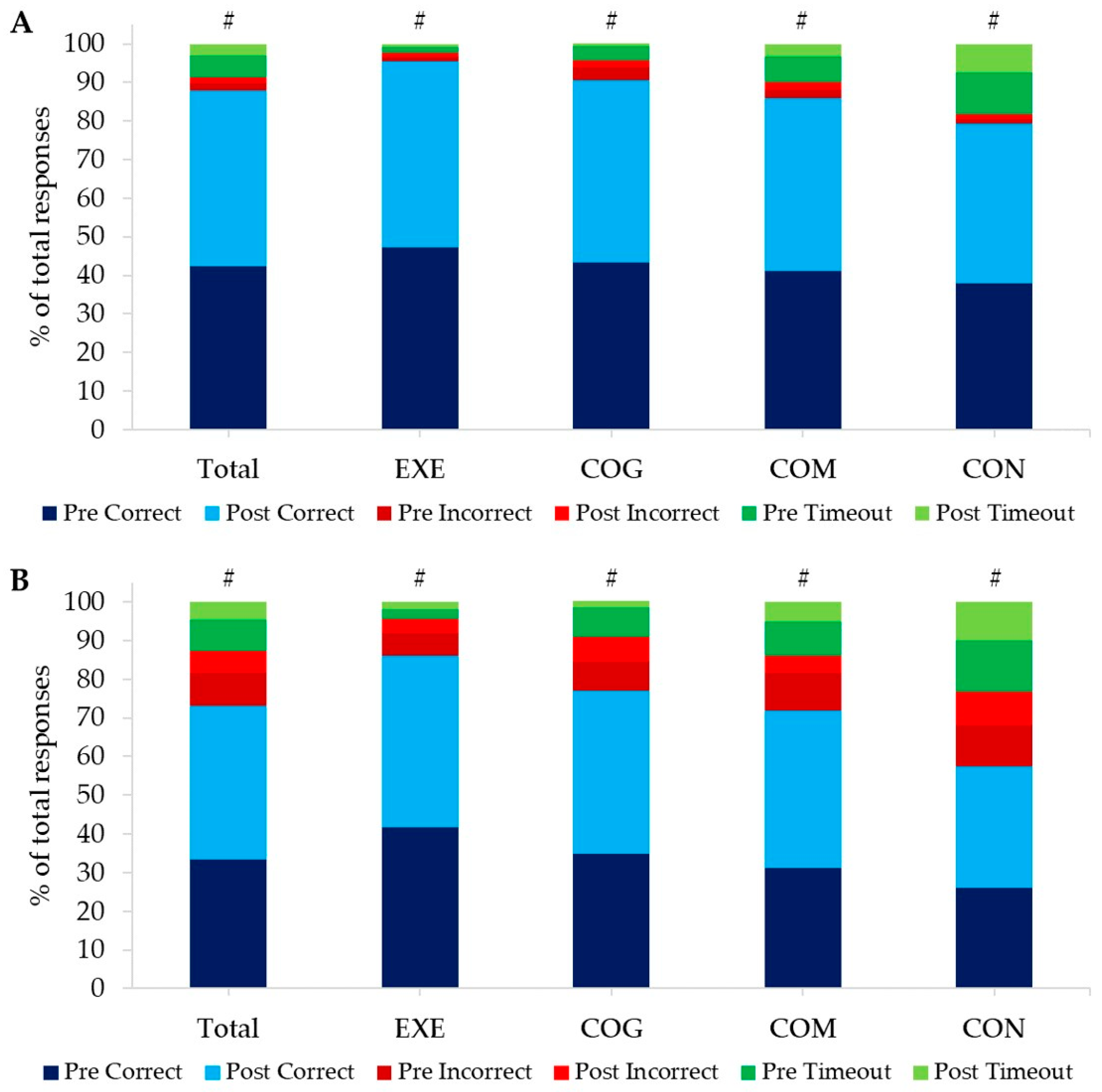
| Total | EXE | COG | COM | CON | |
|---|---|---|---|---|---|
| Number of participants | 83 | 22 | 19 | 21 | 21 |
| Males | 43 | 9 | 4 | 14 | 16 |
| Females | 40 | 13 | 15 | 7 | 5 |
| Age (years) | 27.1 ± 8.0 | 25.0 ± 7.1 | 26.2 ± 6.1 | 28.9 ± 9.2 | 28.2 ± 9.0 |
| Countries | Canada (53) Finland (1) Germany (1) Ireland (2) Myanmar (1) New Zealand (1) South Africa (5) Thailand (1) UK (4) USA (14) | Canada (14) Finland (1) Ireland (1) South Africa (1) Thailand (1) UK (1) USA (3) | Canada (12) Ireland (1) Myanmar (1) South Africa (2) UK (1) USA (2) | Canada (13) Germany (1) New Zealand (1) South Africa (1) UK (1) USA (4) | Canada (14) South Africa (1) UK (1) USA (5) |
| Visual impairment (VI) * | 24 Amblyopia (2) Astigmatism (10) Cortical visual impairment (1) Essential iris atrophy (1) Hyperopia (8) Keratoconus (1) Left eye blindness (1) Myopia (8) Nystagmus (2) Strabismus (3) | 6 Amblyopia (1) Astigmatism (2) Cortical visual impairment (1) Essential iris atrophy (1) Hyperopia (2) Left eye blindness (1) Myopia (2) Nystagmus (1) Strabismus (1) | 4 Astigmatism (1) Hyperopia (2) Keratoconus (1) Myopia (1) Strabismus (1) | 7 Amblyopia (1) Astigmatism (3) Hyperopia (1) Myopia (4) | 7 Astigmatism (4) Hyperopia (3) Myopia (1) Nystagmus (1) Strabismus (1) |
| Auditory impairment (AI) | 18 | 4 | 4 | 4 | 6 |
| Double impairment (VI and AI) | 12 | 2 | 2 | 3 | 5 |
| Raven’s Advanced Progressive Matrices—Set 1 | 4.9 ± 2.8 | 4.4 ± 2.8 | 4.8 ± 3.1 | 5.4 ± 3.0 | 4.7 ± 2.2 |
| Total (n = 83) | EXE (n = 22) | COG (n = 19) | COM (n = 21) | CON (n = 21) | |
|---|---|---|---|---|---|
| Time spent sitting or reclining on a typical day | |||||
| Time (min) | 425.8 ± 214.8 | 418.6 ± 184.8 | 435.8 ± 189.3 | 462.9 ± 287.5 | 387.1 ± 187.8 |
| Vigorous PA in day-to-day life (e.g., carrying/lifting heavy loads, household chores, etc.) | |||||
| No. of yes responses | 14 | 1 | 2 | 4 | 7 |
| How many days per week? (days) | 4.4 ± 1.4 | 4.0 | 4.5 ± 2.1 | 4.3 ± 1.5 | 4.4 ± 1.6 |
| How much time per day? (min) | 39.6 ± 41.7 | 15.0 | 45.0 ± 21.2 | 30.0 ± 0 | 47.1 ± 58.8 |
| How much time per week? (min) | 199.3 ± 308.6 | 60.0 | 180.0 ± 0.0 | 127.5 ± 45.0 | 265.7 ± 439.7 |
| Moderate PA in day-to-day life (e.g., brisk walking, carrying light loads, etc.) | |||||
| No. of yes responses | 52 | 16 | 9 | 14 | 13 |
| How many days per week? (days) | 4.5 ± 1.5 | 4.3 ± 1.4 | 3.9 ± 1.5 | 4.9 ± 1.3 | 4.8 ± 1.6 |
| How much time per day? (min) | 83.2 ± 171.8 | 94.1 ± 216.7 | 70.0 ± 87.6 | 55.7 ± 46.3 | 108.5 ± 239.8 |
| How much time per week? (min) | 404.6 ± 866.5 | 454.1 ± 1090.4 | 295.6 ± 455.1 | 295.7 ± 278.8 | 536.5 ± 1199.1 |
| Walk or use a bicycle to get to and from places for at least 10 min continuously | |||||
| No. of yes responses | 41 | 13 | 6 | 10 | 12 |
| How many days per week? (days) | 4.5 ± 1.4 | 4.7 ± 1.8 | 3.7 ± 1.2 | 5.1 ± 1.6 | 4.2 ± 1.1 |
| How much time per day? (min) | 43.8 ± 39.6 | 52.5 ± 60.7 | 30.8 ± 18.0 | 42.5 ± 21.0 | 42.2 ± 32.2 |
| How much time per week? (min) | 186.4 ± 127.4 | 192.1 ± 102.9 | 122.5 ± 105.2 | 233.5 ± 173.1 | 168.9 ± 107.8 |
| Participation in sports, fitness or recreational (leisure) activities | |||||
| No. of yes responses | 65 | 16 | 15 | 17 | 17 |
| Type of activities (no. of responses) | Aquatic and paddle sports (e.g., canoeing, diving, kayaking, water polo, swimming etc.) (27), Athletics (track and field) (10), Ball-over-net game (e.g., badminton, tennis, volleyball, table tennis, basketball etc.) (16), Bat-and-ball (e.g., baseball, cricket, softball etc.) (6), Bodyweight exercises (e.g., squats, push-ups, lunges, pull-ups etc.) (21), Bocce (1), Catching games (e.g., dodgeball) (1), Cycling (off-road) (7), Cycling (road) (10), Dancing (28), Equestrian sport (6), Fitness cardio machines (e.g., treadmill, cross-trainer, rowing machine, cycle ergometer) (12), Flying disc sports (e.g., frisbee) (1), Golf (6), Gymnastics (4), Ice sports (e.g., ice hockey, speed skating etc.) (7), Martial arts/combat sports (3), Pilates (2), Powerlifting (1), Running (6), Shooting sports (1), Snow sports (9), Soccer (8), Stick and ball games (e.g., hockey, lacrosse) (3), Target sports (e.g., archery, bowling, curling) (1), Trampoline (1), Walking (40), Weight training (e.g., exercise with heavy weights) (7), Yoga (15), Other: Playing violin (for hours at a time) (1) | ||||
| Vigorous intensity sport, fitness or recreational (leisure) activities for at least 10 min continuously | |||||
| No. of yes responses | 39 | 8 | 8 | 12 | 11 |
| How many days per week? (days) | 3.1 ± 1.5 | 3.0 ± 1.3 | 4.1 ± 1.7 | 2.4 ± 1.3 | 3.0 ± 1.4 |
| How much time per day? (min) | 51.8 ± 31.5 | 58.1 ± 14.9 | 63.8 ± 33.8 | 40.0 ± 15.3 | 50.0 ± 48.1 |
| How much time per week? (min) | 177.3 ± 185.8 | 176.3 ± 101.7 | 240.0 ± 133.2 | 116.4 ± 93.5 | 195.0 ± 313.4 |
| Moderate intensity sport, fitness or recreational (leisure) activities for at least 10 min continuously | |||||
| No. of yes responses | 51 | 13 | 11 | 12 | 15 |
| How many days per week? (days) | 3.4 ± 1.8 | 3.5 ± 2.1 | 2.8 ± 1.2 | 3.9 ± 1.7 | 3.1 ± 1.8 |
| How much time per day? (min) | 53.8 ± 50.1 | 54.2 ± 62.0 | 68.6 ± 46.6 | 38.8 ± 23.8 | 54.7 ± 57.6 |
| How much time per week? (min) | 159.6 ± 141.4 | 175.4 ± 213.9 | 182.7 ± 135.8 | 157.5 ± 125.3 | 130.7 ± 71.6 |
| Adherence | EXE (n = 22) | COG (n = 19) | COM (n = 21) | |
|---|---|---|---|---|
| Walks | Total walks completed | 26.0 ± 5.2 | 26.5 ± 4.1 | |
| % of target number (out of 24) | 108.1 ± 21.5 | 110.3 ± 16.9 | ||
| Average walks per week | 3.3 ± 0.6 | 3.3 ± 0.5 | ||
| Cognitive training | Total levels completed | 435.5 ± 61.9 | 443.1 ± 73.3 | |
| % of target levels (out of 384) | 113.4 ± 16.1 | 115.4 ± 19.1 | ||
| Average levels per week | 53.2 ± 7.0 | 56.5 ± 7.9 | ||
| Sessions completed | 45.8 ± 4.8 | 48.4 ± 5.9 | ||
| % of target sessions completed (out of 48) | 95.5 ± 10.1 | 100.8 ± 12.4 | ||
| GO-Trials | Total | EXE | COG | COM | CON | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Correct response time (ms) | 295.0 ± 79.2 * | 270.5 ± 91.6 | 298.8 ± 84.4 | 289.1 ± 101.0 | 261.5 ± 74.1 | 241.5 ± 99.2 | 293.7 ± 75.3 | 256.3 ± 77.3 | 322.8 ± 75.9 | 291.3 ± 83.7 |
| Number correct (/200) | 182.3 ± 27.8 # | 188.8 ± 23.9 | 186.1± 12.4 # | 190.5 ± 13.3 | 191.7 ± 8.2 # | 197.5 ± 4.1 | 182.0 ± 29.1 # | 193.8 ± 8.9 | 170.3 ± 43.0 # | 174.2 ± 41.7 |
| Number incorrect (/200) | 17.7 ± 27.8 # | 11.2 ± 23.9 | 13.9 ± 12.4 # | 9.5 ± 13.3 | 8.3 ± 8.2 # | 2.5 ± 4.1 | 18.0 ± 29.1 # | 6.2 ± 8.9 | 29.7 ± 43.0 # | 25.8 ± 41.7 |
| NO-GO-Trials | Total | EXE | COG | COM | CON | |||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Incorrect response time (ms) | 286.5 ± 142.0 * | 222.6 ± 117.2 | 278.0 ± 126.3 * | 210.7 ± 121.4 | 233.4 ± 146.2 | 193.8 ± 98.8 | 274.5 ± 113.5 * | 216.4 ± 104.3 | 343.8 ± 163.9 | 264.1 ± 135.5 |
| Number correct (/25) | 14.6 ± 5.8 # | 16.7 ± 5.7 | 15.3 ± 6.6 # | 18.4 ± 5.4 | 14.5 ± 5.7 # | 16.7 ± 5.7 | 14.1 ± 6.0 # | 15.8 ± 5.5 | 14.5 ± 5.4 | 15.9 ± 6.1 |
| Number incorrect (/25) | 10.4 ± 5.8 # | 8.3 ± 5.7 | 9.7 ± 6.6 # | 6.6 ± 5.4 | 10.5 ± 5.7 # | 8.3 ± 5.7 | 10.9 ± 6.0 # | 9.2 ± 5.5 | 10.5 ± 5.4 | 9.1 ± 6.1 |
| Compatible | Total | EXE | COG | COM | CON | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| No. of trials | 59.5 ± 5.3 | 60.1 ± 1.2 | 58.4 ± 6.2 | 60.0 ± 0.0 | 60.3 ± 4.8 | 60.2 ± 2.2 | 60.0 ± 3.3 | 60.0 ± 0.0 | 58.9 ± 6.1 | 60.3 ± 1.3 |
| Response time (ms) | 1096.2 ± 232.2 * | 1053.3 ± 224.0 | 1011.5 ± 180.9 § | 1007.2 ± 204.5 | 1013.8 ± 181.5 § | 927.5 ± 180.9 | 1140.6 ± 270.6 | 1099.6 ± 166.0 | 1221.1 ± 227.1 | 1174.9 ± 267.3 |
| Per cent correct § | 84.9 ± 22.0 # | 90.8 ± 16.3 | 94.7 ± 8.7 # | 96.4 ± 6.3 | 86.3 ± 22.8 # | 94.7 ± 9.7 | 82.3 ± 23.0 # | 89.4 ± 21.1 | 79.7 ± 20.4 # | 84.0 ± 19.9 |
| Per cent incorrect | 3.9 ± 9.8 # | 2.9 ± 6.2 | 2.3 ± 6.4 # | 1.9 ± 4.8 | 6.5 ± 14.0 # | 3.8 ± 8.1 | 4.8 ± 12.5 # | 3.8 ± 8.0 | 2.5 ± 2.7 # | 2.2 ± 2.9 |
| Per cent timeouts § | 11.2 ± 18.8 # | 6.3 ± 12.8 | 3.1 ± 3.8 # | 1.7 ± 3.0 | 7.2 ± 13.4 # | 1.5 ± 3.0 | 13.0 ± 19.8 # | 6.8 ± 14.2 | 17.8 ± 19.2 # | 13.8 ± 18.2 |
| Incompatible | Total | EXE | COG | COM | CON | |||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| No. of trials | 180.5 ± 5.3 | 179.9 ± 1.2 | 181.6 ± 6.2 | 180.0 ± 0.0 | 179.7 ± 4.8 | 179.8 ± 2.2 | 180.0 ± 3.3 | 180.0 ± 0.0 | 181.2 ± 6.1 | 179.7 ± 1.3 |
| Response time (ms) | 1188.1 ± 241.8 | 1159.0 ± 226.1 | 1119.7 ± 190.3 § | 1141.3 ± 209.0 | 1127.4 ± 188.7 | 1047.8 ± 197.5 | 1194.2 ± 313.7 | 1189.4 ± 186.8 | 1318.5 ± 215.1 | 1252.7 ± 271.7 |
| Per cent correct § | 67.1 ± 34.3 # | 79.1 ± 26.9 | 83.5 ± 25.0 # | 88.7 ± 19.0 | 69.5 ± 33.6 # | 84.2 ± 23.9 | 62.6 ± 34.2 # | 81.1 ± 22.6 | 54.8 ± 36.7 # | 65.2 ± 32.6 |
| Per cent incorrect | 16.9 ± 23.6 # | 11.4 ± 18.4 | 11.5 ± 23.3 # | 7.4 ± 16.7 | 15.2 ± 19.0 # | 12.7 ± 22.6 | 19.7 ± 26.2 # | 8.8 ± 11.4 | 22.6 ± 25.4 # | 16.3 ± 21.3 |
| Per cent timeouts § | 16.0 ± 21.5 # | 9.4 ± 14.7 | 5.0 ± 5.1 # | 4.0 ± 4.3 | 15.3 ± 23.7 # | 3.1 ± 4.9 | 17.7 ± 20.7 # | 10.1 ± 15.6 | 22.7 ± 20.3 # | 18.5 ± 19.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merzbach, V.; Ferrandino, M.; Gernigon, M.; Marques Pinto, J.; Scruton, A.; Gordon, D. Impact of Prescribed Exercise on the Physical and Cognitive Health of Adults with Down Syndrome: The MinDSets Study. Int. J. Environ. Res. Public Health 2023, 20, 7121. https://doi.org/10.3390/ijerph20237121
Merzbach V, Ferrandino M, Gernigon M, Marques Pinto J, Scruton A, Gordon D. Impact of Prescribed Exercise on the Physical and Cognitive Health of Adults with Down Syndrome: The MinDSets Study. International Journal of Environmental Research and Public Health. 2023; 20(23):7121. https://doi.org/10.3390/ijerph20237121
Chicago/Turabian StyleMerzbach, Viviane, Michael Ferrandino, Marie Gernigon, Jorge Marques Pinto, Adrian Scruton, and Dan Gordon. 2023. "Impact of Prescribed Exercise on the Physical and Cognitive Health of Adults with Down Syndrome: The MinDSets Study" International Journal of Environmental Research and Public Health 20, no. 23: 7121. https://doi.org/10.3390/ijerph20237121
APA StyleMerzbach, V., Ferrandino, M., Gernigon, M., Marques Pinto, J., Scruton, A., & Gordon, D. (2023). Impact of Prescribed Exercise on the Physical and Cognitive Health of Adults with Down Syndrome: The MinDSets Study. International Journal of Environmental Research and Public Health, 20(23), 7121. https://doi.org/10.3390/ijerph20237121







