Associations between Power Training-Induced Changes in Body Composition and Physical Function in Older Men: A Pre-Test–Post-Test Experimental Study
Abstract
:1. Introduction
2. Materials and Methods
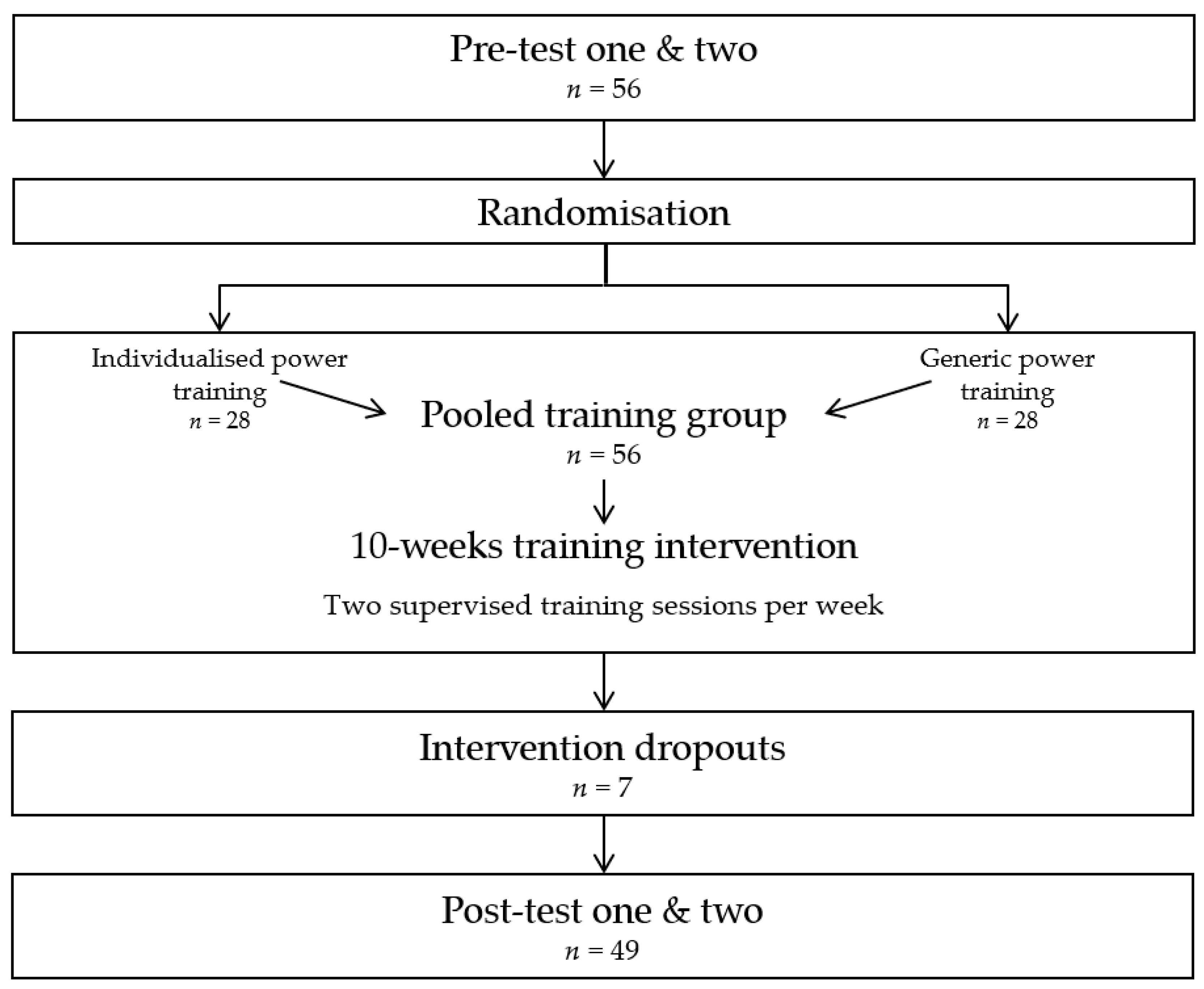
2.1. Study Design
2.2. Study Participants
2.3. Study Intervention
2.4. Study Outcomes
2.5. Ethics Approval and Consent to Participate
2.6. Statistical Analyses
3. Results
3.1. Study Participants
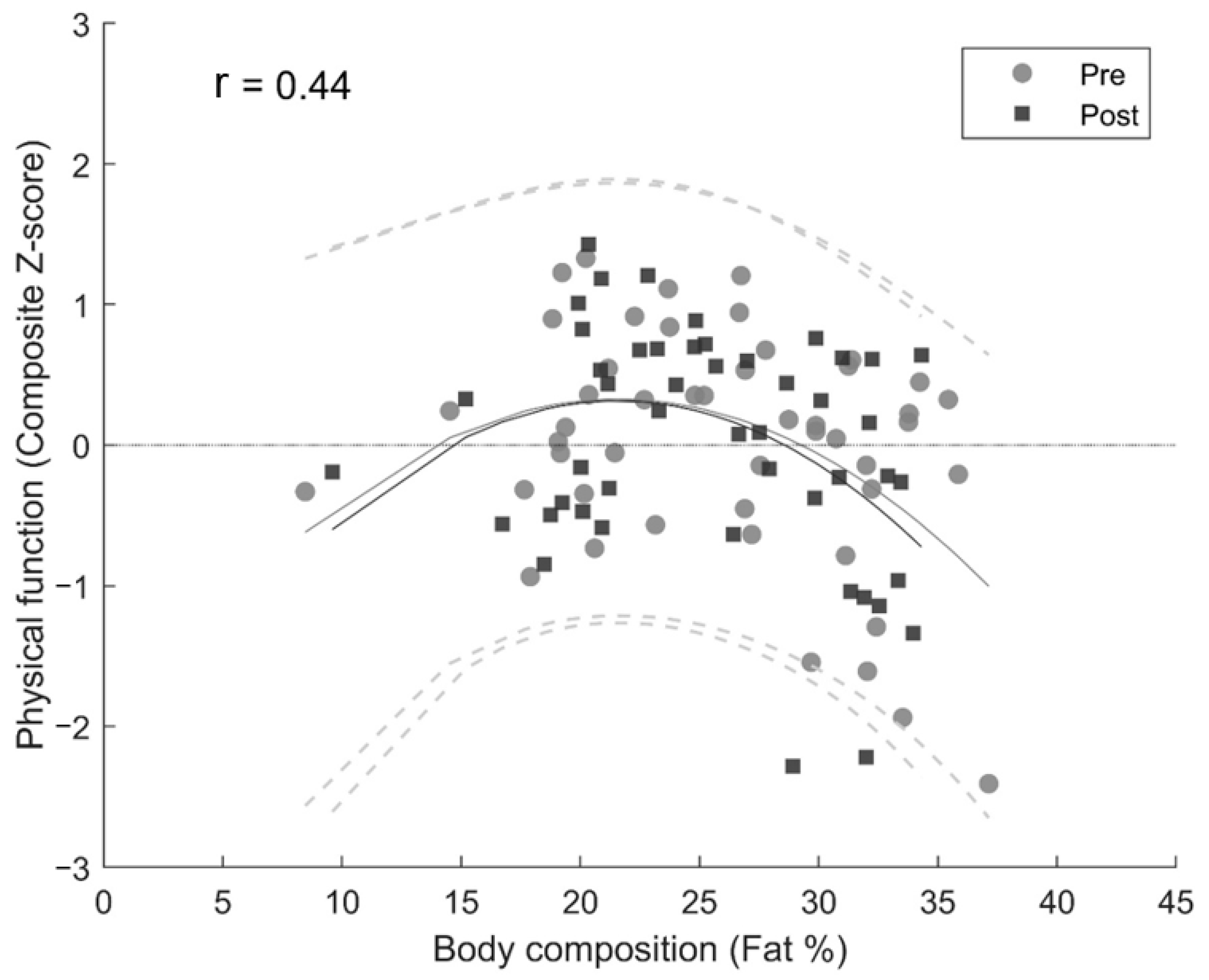
3.2. Cross-Sectional Associations—Body Composition and Physical Function
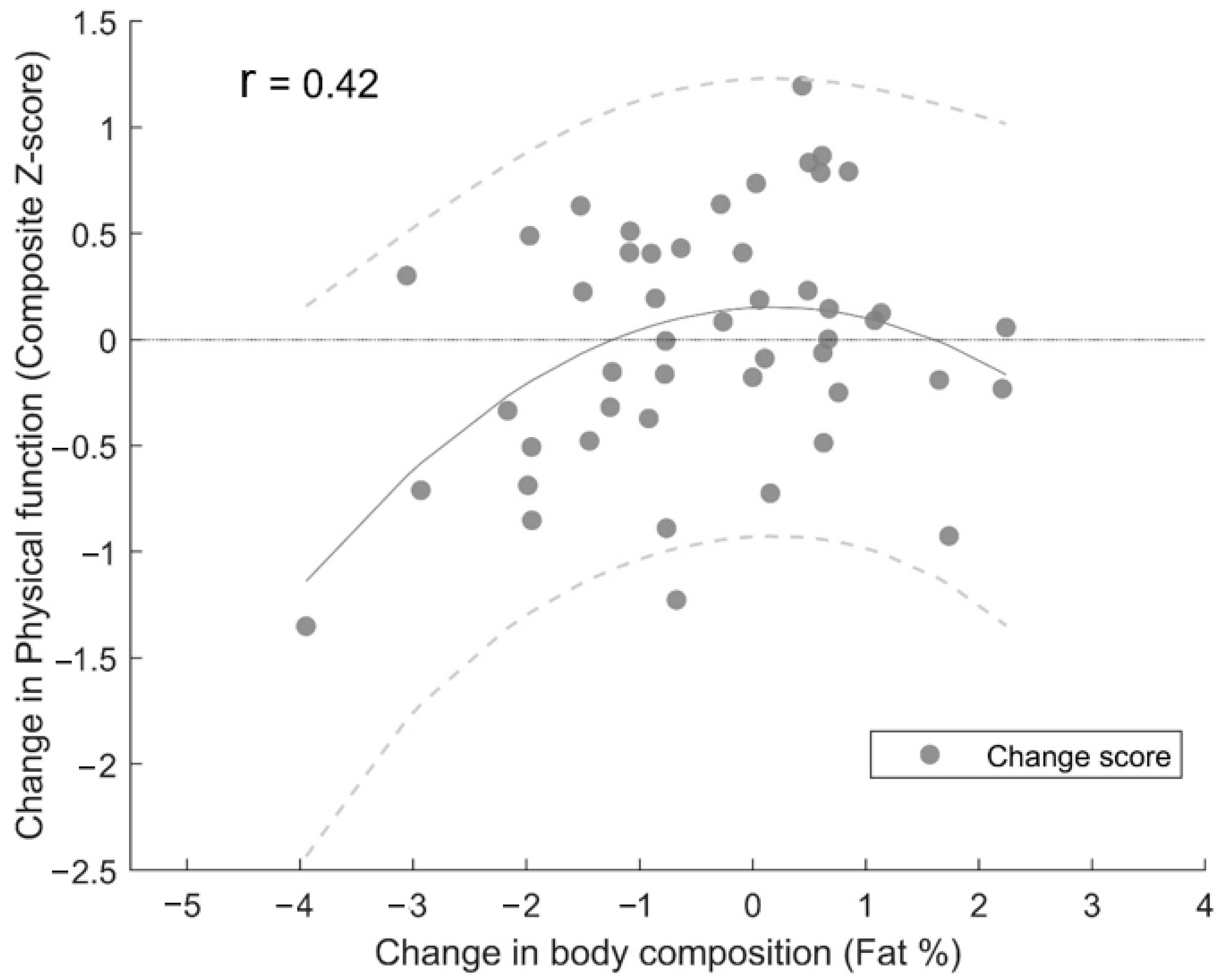
3.3. Power Training-Induced Changes in Body Composition and Physical Function
4. Discussion
Strengths and Limitations
5. Conclusions
Practical Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Original Conference Paper
References
- United Nations, Department of Economic and Social Affair United Nations. Trends in Contraceptive Use Worldwide 2015; ST/ESA/SER. A/349; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M. Sarcopenia: European consensus on definition and diagnosis. Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Painter, P.; Stewart, A.L.; Carey, S. Physical functioning: Definitions, measurement, and expectations. Adv. Ren. Replace. Ther. 1999, 6, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Daniels, N. Global aging and the allocation of health care across the life span. Am. J. Bioeth. 2013, 13, 1–2. [Google Scholar] [CrossRef]
- Waidmann, T.A.; Liu, K. Disability trends among elderly persons and implications for the future. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2000, 55, S298–S307. [Google Scholar] [CrossRef] [PubMed]
- Brady, A.O.; Straight, C.R.; Evans, E.M. Body composition, muscle capacity, and physical function in older adults: An integrated conceptual model. J. Aging Phys. Act. 2014, 22, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Orssatto, L.B.; Bezerra, E.S.; Schoenfeld, B.J.; Diefenthaeler, F. Lean, fast and strong: Determinants of functional performance in the elderly. Clin. Biomech. 2020, 78, 105073. [Google Scholar] [CrossRef]
- Michalakis, K.; Goulis, D.; Vazaiou, A.; Mintziori, G.; Polymeris, A.; Abrahamian-Michalakis, A. Obesity in the ageing man. Metabolism 2013, 62, 1341–1349. [Google Scholar] [CrossRef]
- Visser, M.; Deeg, D.J.; Lips, P.; Harris, T.B.; Bouter, L.M. Skeletal muscle mass and muscle strength in relation to lower-extremity performance in older men and women. J. Am. Geriatr. Soc. 2000, 48, 381–386. [Google Scholar] [CrossRef]
- Reid, K.F.; Naumova, E.N.; Carabello, R.J.; Phillips, E.M.; Fielding, R.A. Lower extremity muscle mass predicts functional performance in mobility-limited elders. J. Nutr. Health Aging 2008, 12, 493. [Google Scholar] [CrossRef]
- Bouchard, D.R.; Beliaeff, S.; Dionne, I.J.; Brochu, M. Fat mass but not fat-free mass is related to physical capacity in well-functioning older individuals: Nutrition as a determinant of successful aging (NuAge)—The Quebec Longitudinal Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 1382–1388. [Google Scholar] [CrossRef]
- Woo, J.; Leung, J.; Kwok, T. BMI, body composition, and physical functioning in older adults. Obesity 2007, 15, 1886–1894. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, C.M.; Gozansky, W.S.; Van Pelt, R.E.; Schenkman, M.L.; Wolfe, P.; Schwartz, R.S.; Kohrt, W.M. Relative contributions of adiposity and muscularity to physical function in community-dwelling older adults. Obesity 2008, 16, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Hardy, R.; Cooper, R.; Aihie Sayer, A.; Ben-Shlomo, Y.; Cooper, C.; Deary, I.J.; Demakakos, P.; Gallacher, J.; Martin, R.M.; McNeill, G. Body mass index, muscle strength and physical performance in older adults from eight cohort studies: The HALCyon programme. PLoS ONE 2013, 8, e56483. [Google Scholar] [CrossRef]
- Yuan, L.; Chang, M.; Wang, J. Abdominal obesity, body mass index and the risk of frailty in community-dwelling older adults: A systematic review and meta-analysis. Age Ageing 2021, 50, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Shi, Y. Body weight status and onset of functional limitations in US middle-aged and older adults. Disabil. Health J. 2015, 8, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Levinger, P.; Nagano, H.; Downie, C.; Hayes, A.; Sanders, K.M.; Cicuttini, F.; Begg, R. Biomechanical balance response during induced falls under dual task conditions in people with knee osteoarthritis. Gait Posture 2016, 48, 106–112. [Google Scholar] [CrossRef]
- Lockhart, T.E.; Frames, C.W.; Soangra, R.; Lieberman, A. Effects of obesity and fall risk on gait and posture of community-dwelling older adults. Int. J. Progn. Health Manag. 2019, 10, 19. [Google Scholar] [CrossRef]
- Alice, A.; Yadav, M.; Verma, R.; Kumari, M.; Arora, S. Effect of obesity on balance: A literature review. Int. J. Health Sci. 2022, 6, 3261–3279. [Google Scholar] [CrossRef]
- Byrne, C.; Faure, C.; Keene, D.J.; Lamb, S.E. Ageing, muscle power and physical function: A systematic review and implications for pragmatic training interventions. Sports Med. 2016, 46, 1311–1332. [Google Scholar] [CrossRef]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance training for older adults: Position statement from the national strength and conditioning association. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef]
- da Rosa Orssatto, L.B.; de la Rocha Freitas, C.; Shield, A.J.; Pinto, R.S.; Trajano, G.S. Effects of resistance training concentric velocity on older adults’ functional capacity: A systematic review and meta-analysis of randomised trials. Exp. Gerontol. 2019, 127, 110731. [Google Scholar] [PubMed]
- Hortobágyi, T.; Lesinski, M.; Gäbler, M.; VanSwearingen, J.M.; Malatesta, D.; Granacher, U. Effects of three types of exercise interventions on healthy old adults’ gait speed: A systematic review and meta-analysis. Sports Med. 2015, 45, 1627–1643. [Google Scholar]
- Bellicha, A.; van Baak, M.A.; Battista, F.; Beaulieu, K.; Blundell, J.E.; Busetto, L.; Carraça, E.V.; Dicker, D.; Encantado, J.; Ermolao, A. Effect of exercise training on weight loss, body composition changes, and weight maintenance in adults with overweight or obesity: An overview of 12 systematic reviews and 149 studies. Obes. Rev. 2021, 22, e13256. [Google Scholar] [CrossRef] [PubMed]
- Perkin, O.J.; McGuigan, P.M.; Thompson, D.; Stokes, K.A. Habitual physical activity levels do not predict leg strength and power in healthy, active older adults. PLoS ONE 2018, 13, e0200089. [Google Scholar]
- Lindberg, K.; Lohne-Seiler, H.; Fosstveit, S.H.; Sibayan, E.E.; Fjeller, J.S.; Løvold, S.; Kolnes, T.; Vårvik, F.T.; Berntsen, S.; Paulsen, G.; et al. Effectiveness of individualized training based on force–velocity profiling on physical function in older men. Scand. J. Med. Sci. Sports 2022, 32, 1013–1025. [Google Scholar] [PubMed]
- Grgic, J.; Schoenfeld, B.J.; Mikulic, P. Effects of plyometric vs. resistance training on skeletal muscle hypertrophy: A review. J. Sport Health Sci. 2021, 10, 530–536. [Google Scholar] [CrossRef]
- Orssatto, L.B.; Bezerra, E.S.; Shield, A.J.; Trajano, G.S. Is power training effective to produce muscle hypertrophy in older adults? A systematic review and meta-analysis. Appl. Physiol. Nutr. Metab. 2020, 45, 1031–1040. [Google Scholar] [CrossRef]
- Chernoff, R. Normal aging, nutrition assessment, and clinical practice. Nutr. Clin. Pract. 2003, 18, 12–20. [Google Scholar] [CrossRef]
- Helms, E.R.; Cronin, J.; Storey, A.; Zourdos, M.C. Application of the repetitions in reserve-based rating of perceived exertion scale for resistance training. Strength Cond. J. 2016, 38, 42. [Google Scholar] [CrossRef]
- Lohne-Seiler, H.; Torstveit, M.K.; Anderssen, S.A. Traditional versus functional strength training: Effects on muscle strength and power in the elderly. J. Aging Phys. Act. 2013, 21, 51–70. [Google Scholar] [CrossRef]
- Schoene, D.; Wu, S.M.S.; Mikolaizak, A.S.; Menant, J.C.; Smith, S.T.; Delbaere, K.; Lord, S.R. Discriminative ability and predictive validity of the timed Up and Go test in identifying older people who fall: Systematic review and meta-analysis. J. Am. Geriatr. Soc. 2013, 61, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; Haff, G.G.; Häkkinen, K.; Newton, R.U. Moderate-load muscular endurance strength training did not improve peak power or functional capacity in older men and women. Front. Physiol. 2017, 8, 743. [Google Scholar]
- Nana, A.; Slater, G.J.; Stewart, A.D.; Burke, L.M. Methodology review: Using dual-energy X-ray absorptiometry (DXA) for the assessment of body composition in athletes and active people. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 198–215. [Google Scholar] [CrossRef] [PubMed]
- Clarys, J.P.; Scafoglieri, A.; Provyn, S.; Louis, O.; Wallace, J.A.; De Mey, J. A macro-quality evaluation of DXA variables using whole dissection, ashing, and computer tomography in pigs. Obesity 2010, 18, 1477. [Google Scholar] [CrossRef] [PubMed]
- Bjørnsen, T.; Salvesen, S.; Berntsen, S.; Hetlelid, K.; Stea, T.; Lohne-Seiler, H.; Rohde, G.; Haraldstad, K.; Raastad, T.; Køpp, U. Vitamin C and E supplementation blunts increases in total lean body mass in elderly men after strength training. Scand. J. Med. Sci. Sports 2016, 26, 755–763. [Google Scholar]
- Hopkins, W.G. Measures of reliability in sports medicine and science. Sports Med. 2000, 30, 1–15. [Google Scholar] [CrossRef]
- W.H.O. Consultation. Obesity: Preventing and managing the global epidemic. World Health Organ. Tech. Rep. Ser. 2000, 894, 1–253. [Google Scholar]
- Buehring, B.; Siglinsky, E.; Krueger, D.; Evans, W.; Hellerstein, M.; Yamada, Y.; Binkley, N. Comparison of muscle/lean mass measurement methods: Correlation with functional and biochemical testing. Osteoporos. Int. 2018, 29, 675–683. [Google Scholar]
- Hayashida, I.; Tanimoto, Y.; Takahashi, Y.; Kusabiraki, T.; Tamaki, J. Correlation between muscle strength and muscle mass, and their association with walking speed, in community-dwelling elderly Japanese individuals. PLoS ONE 2014, 9, e111810. [Google Scholar] [CrossRef]
- Cruz-Jimenez, M. Normal changes in gait and mobility problems in the elderly. Phys. Med. Rehabil. Clin. 2017, 28, 713–725. [Google Scholar] [CrossRef]
- Reed, R.L.; Pearlmutter, L.; Yochum, K.; Meredith, K.E.; Mooradian, A.D. The relationship between muscle mass and muscle strength in the elderly. J. Am. Geriatr. Soc. 1991, 39, 555–561. [Google Scholar] [CrossRef]
- Flegal, K.M.; Shepherd, J.A.; Looker, A.C.; Graubard, B.I.; Borrud, L.G.; Ogden, C.L.; Harris, T.B.; Everhart, J.E.; Schenker, N. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am. J. Clin. Nutr. 2009, 89, 500–508. [Google Scholar] [CrossRef]
- Hortobágyi, T.; Mizelle, C.; Beam, S.; DeVita, P. Old adults perform activities of daily living near their maximal capabilities. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2003, 58, M453–M460. [Google Scholar] [CrossRef]
- Cava, E.; Yeat, N.C.; Mittendorfer, B. Preserving healthy muscle during weight loss. Adv. Nutr. 2017, 8, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Villareal, D.T.; Chode, S.; Parimi, N.; Sinacore, D.R.; Hilton, T.; Armamento-Villareal, R.; Napoli, N.; Qualls, C.; Shah, K. Weight loss, exercise, or both and physical function in obese older adults. N. Engl. J. Med. 2011, 364, 1218–1229. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.L.; Coppinger, T.; McCarthy, A.L. The role of nutrition and physical activity in frailty: A review. Clin. Nutr. ESPEN 2020, 35, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Azzolino, D.; Arosio, B.; Marzetti, E.; Calvani, R.; Cesari, M. Nutritional status as a mediator of fatigue and its underlying mechanisms in older people. Nutrients 2020, 12, 444. [Google Scholar] [CrossRef]
- Waters, D.L.; Ward, A.L.; Villareal, D.T. Weight loss in obese adults 65 years and older: A review of the controversy. Exp. Gerontol. 2013, 48, 1054–1061. [Google Scholar] [CrossRef]
- Tieland, M.; van de Rest, O.; Dirks, M.L.; van der Zwaluw, N.; Mensink, M.; van Loon, L.J.; de Groot, L.C. Protein supplementation improves physical performance in frail elderly people: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2012, 13, 720–726. [Google Scholar] [CrossRef]
- Arnarson, A.; Gudny Geirsdottir, O.; Ramel, A.; Briem, K.; Jonsson, P.; Thorsdottir, I. Effects of whey proteins and carbohydrates on the efficacy of resistance training in elderly people: Double blind, randomised controlled trial. Eur. J. Clin. Nutr. 2013, 67, 821–826. [Google Scholar] [CrossRef]
- Ispoglou, T.; White, H.; Preston, T.; McElhone, S.; McKenna, J.; Hind, K. Double-blind, placebo-controlled pilot trial of L-Leucine-enriched amino-acid mixtures on body composition and physical performance in men and women aged 65–75 years. Eur. J. Clin. Nutr. 2016, 70, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Fosstveit, S.H.; Lindberg, K.; Bjørnsen, T.; Sibayan, E.E.; Fjeller, J.S.; Løvold, S.; Kolnes, T.; Vårvik, F.T.; Berntsen, S.; Lohne-Seiler, H. Is body composition associated with physical function in older men? In Proceedings of the European College of Sport Science, Sevilla, Spain, 31 August–2 September 2022. [Google Scholar]
| Variables | Pre | Post | Change |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Δ% ± SD | |
| Age (yrs) | 67.7 ± 5.3 | ||
| Height (cm) | 178.9 ± 7.0 | ||
| Total mass (kg) | 83.4 ± 10.4 | 83.7 ± 10.4 | 0.5 ± 2.5 |
| Total body fat (kg) | 22.1 ± 7.4 | 21.7 ± 6.8 | −0.7 ± 7.3 |
| Body fat percentage (%) | 26.0 ± 6.4 | 25.5 ± 5.8 | −1.2 ± 5.7 * |
| Total lean mass (kg) | 57.9 ± 5.4 | 58.7 ± 5.7 | 1.3 ± 2.0 * |
| BMI | |||
| Underweight (n) | 0 | 0 | |
| Healthy weight (n) | 18 | 17 | |
| Overweight (n) | 28 | 29 | |
| Obese (n) | 2 | 2 | |
| Physical function | |||
| Sit-to-stand power (W) | 1742 ± 333 | 1731 ± 335 | −0.3 ± 7.7 |
| Timed-up-and-go (s) | 4.13 ± 0.30 | 4.06 ± 0.27 | −1.58 ± 4.90 * |
| Stair climb (s) | 3.74 ± 0.48 | 3.53 ± 0.39 | −5.16 ± 6.66 * |
| Loaded Stair climb (s) | 3.93 ± 0.44 | 3.76 ± 0.46 | −4.07 ± 6.82 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fosstveit, S.H.; Lindberg, K.; Bjørnsen, T.; Sibayan, E.E.; Fjeller, J.S.; Løvold, S.; Kolnes, T.; Vårvik, F.T.; Berntsen, S.; Lohne-Seiler, H. Associations between Power Training-Induced Changes in Body Composition and Physical Function in Older Men: A Pre-Test–Post-Test Experimental Study. Int. J. Environ. Res. Public Health 2023, 20, 7070. https://doi.org/10.3390/ijerph20227070
Fosstveit SH, Lindberg K, Bjørnsen T, Sibayan EE, Fjeller JS, Løvold S, Kolnes T, Vårvik FT, Berntsen S, Lohne-Seiler H. Associations between Power Training-Induced Changes in Body Composition and Physical Function in Older Men: A Pre-Test–Post-Test Experimental Study. International Journal of Environmental Research and Public Health. 2023; 20(22):7070. https://doi.org/10.3390/ijerph20227070
Chicago/Turabian StyleFosstveit, Sindre H., Kolbjørn Lindberg, Thomas Bjørnsen, Erlend E. Sibayan, Joachim S. Fjeller, Sondre Løvold, Tommy Kolnes, Fredrik T. Vårvik, Sveinung Berntsen, and Hilde Lohne-Seiler. 2023. "Associations between Power Training-Induced Changes in Body Composition and Physical Function in Older Men: A Pre-Test–Post-Test Experimental Study" International Journal of Environmental Research and Public Health 20, no. 22: 7070. https://doi.org/10.3390/ijerph20227070
APA StyleFosstveit, S. H., Lindberg, K., Bjørnsen, T., Sibayan, E. E., Fjeller, J. S., Løvold, S., Kolnes, T., Vårvik, F. T., Berntsen, S., & Lohne-Seiler, H. (2023). Associations between Power Training-Induced Changes in Body Composition and Physical Function in Older Men: A Pre-Test–Post-Test Experimental Study. International Journal of Environmental Research and Public Health, 20(22), 7070. https://doi.org/10.3390/ijerph20227070






