Comparison of Epidemiological Data of Complex Regional Pain Syndrome (CRPS) Patients in Relation to Disease Severity—A Retrospective Single-Center Study
Abstract
1. Introduction
Objectives
2. Materials and Methods
2.1. Budapest Clinical Diagnostic Criteria for CRPS
- Persistent pain, which is disproportionate to the initial trauma.
- Patients must report at least one symptom in four of the following categories:
- Sensory—hyperaesthesia and/or allodynia.
- Vasomotor—temperature asymmetry and/or skin color changes and/or skin color asymmetry.
- Sudomotor/oedema—oedema and/or sweating changes and/or sweating asymmetry.
- Motor/trophic—decreased range of motion and/or motor dysfunction (weakness, tremor, or dystonia) and/or trophic changes (hair, nail, or skin).
- Patients must display at least one sign at the time of clinical evaluation in two or more of the following categories:
- Sensory—hyperalgesia and/or allodynia.
- Vasomotor—temperature asymmetry (>1 °C) and/or skin color changes and/or asymmetry.
- Sudomotor/oedema—oedema and/or sweating changes and/or sweating asymmetry.
- Motor/trophic—decreased range of motion and/or motor dysfunction (weakness or tremor) and/or trophic changes (hair or nail).
- There is no other diagnosis that better explains the signs and symptoms [16].
2.2. Statistical Analysis
3. Results
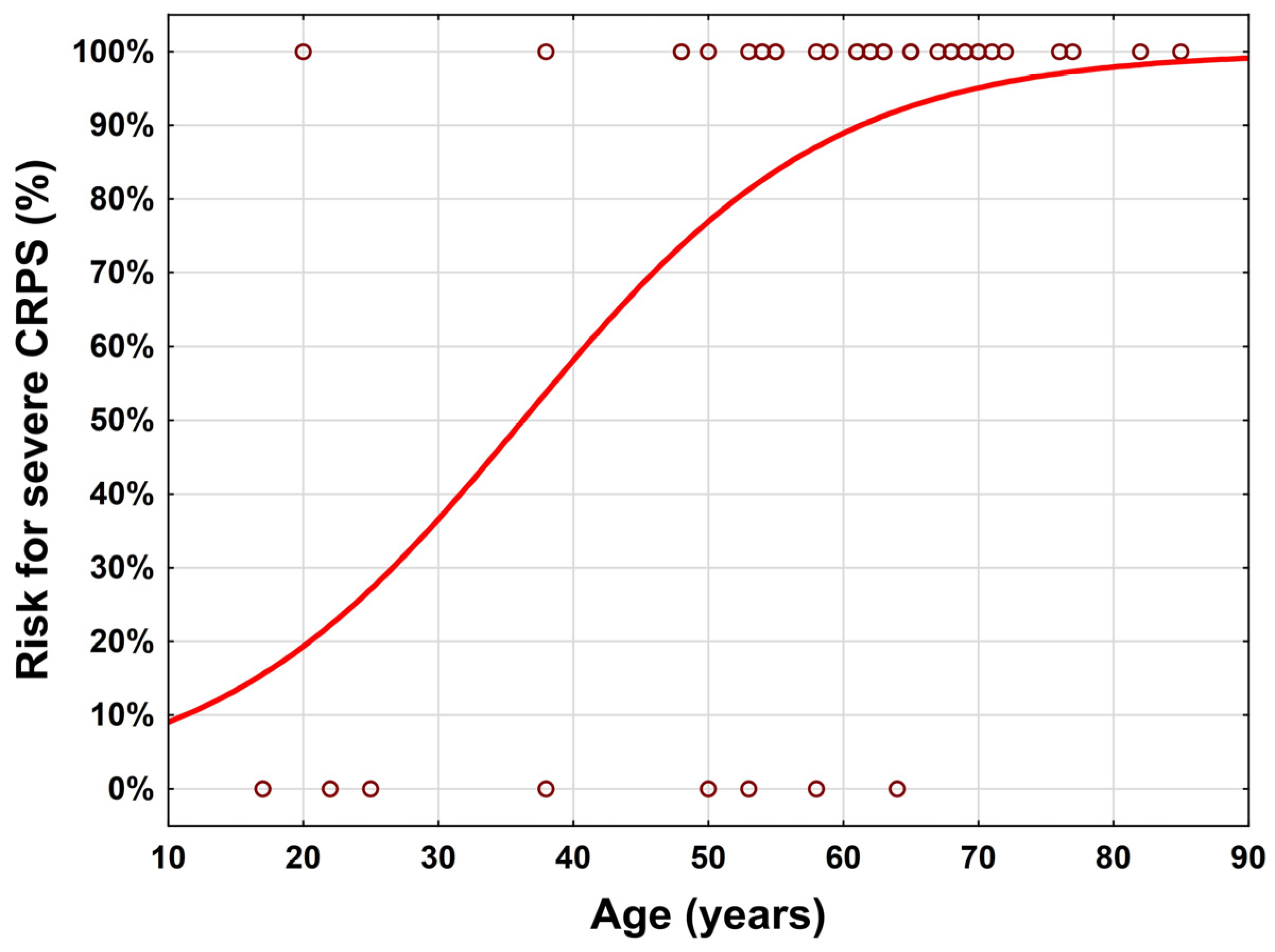
3.1. Study Population
3.2. Anatomical Region
3.3. Trigger Events
3.4. Reduction Maneuvers of Fractures at the Wrist
3.5. Immobilization in Relation to the Entire Collective
3.6. Budapest Criteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shim, H.; Rose, J.; Halle, S.; Shekane, P. Complex regional pain syndrome: A narrative review for the practising clinician. Br. J. Anaesth. 2019, 123, e424–e433. [Google Scholar] [CrossRef] [PubMed]
- Urits, I.; Shen, A.H.; Jones, M.R.; Viswanath, O.; Kaye, A.D. Complex Regional Pain Syndrome, Current Concepts and Treatment Options. Curr. Pain Headache Rep. 2018, 22, 10. [Google Scholar] [CrossRef] [PubMed]
- de Mos, M.; de Bruijn, A.G.; Huygen, F.J.; Dieleman, J.P.; Stricker, C.B.; Sturkenboom, M.C. The incidence of complex regional pain syndrome: A population-based study. Pain 2007, 129, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Crijns, T.J.; van der Gronde, B.A.T.D.; Ring, D.; Leung, N. Complex Regional Pain Syndrome After Distal Radius Fracture Is Uncommon and Is Often Associated With Fibromyalgia. Clin. Orthop. Relat. Res. 2018, 476, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.B.; Mikkelsen, K.L.; Lauritzen, J.B.; Krogsgaard, M.R. Risk Factors for Post-treatment Complex Regional Pain Syndrome (CRPS): An Analysis of 647 Cases of CRPS from the Danish Patient Compensation Association. Pain Pract. 2018, 18, 341–349. [Google Scholar] [CrossRef]
- Bickerstaff, D.R.; Kanis, J.A. Algodystrophy: An Under-recognized complication of minor trauma. Rheumatology 1994, 33, 240–248. [Google Scholar] [CrossRef]
- Field, J.; Protheroe, D.L.; Atkins, R.M. Algodystrophy after Colles fractures is associated with secondary tightness of casts. J. Bone Joint Surg. Br. 1994, 76, 901–905. [Google Scholar] [CrossRef]
- Tileston, K.R.; Griffin, A.; Wagner, J.F.; O’Day, M.N.; Krane, E.J. Team Approach: Complex Regional Pain Syndrome in Children and Adolescents. JBJS Rev. 2020, 8, e0174. [Google Scholar] [CrossRef]
- Kessler, A.; Yoo, M.; Calisoff, R. Complex regional pain syndrome: An updated comprehensive review. Neurorehabilitation 2020, 47, 253–264. [Google Scholar] [CrossRef]
- Merskey, H.; Bogduk, N. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986, 3, S1–S226. [Google Scholar]
- Harden, N.R.; Bruehl, S.; Perez, R.S.; Birklein, F.; Marinus, J.; Maihofner, C.; Lubenow, T.R.; Buvanendran, A.; Mackey, S.; Graciosa, J.R.; et al. Validation of proposed diagnostic criteria (the “Budapest Criteria”) for Complex Regional Pain Syndrome. Pain 2010, 150, 268–274. [Google Scholar] [CrossRef] [PubMed]
- ldufani, J.; Elahmer, N.; Blaise, G. A medical mystery of complex regional pain syndrome. Heliyon 2020, 6, e03329. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.-S.; Noor, N.; Urits, I.; Paladini, A.; Sandhu, M.S.; Gibb, C.; Carlson, T.; Myrcik, D.; Varrassi, G.; Viswanath, O. Complex Regional Pain Syndrome: A Comprehensive Review. Pain Ther. 2021, 10, 875–892. [Google Scholar] [CrossRef]
- Dutton, L.K.; Rhee, P.C. Complex Regional Pain Syndrome and Distal Radius Fracture. Hand Clin. 2021, 37, 315–322. [Google Scholar] [CrossRef]
- Harden, N.R.; Bruehl, S.; Galer, B.S.; Saltz, S.; Bertram, M.; Backonja, M.; Gayles, R.; Rudin, N.; Bhugra, M.K.; Stanton-Hicks, M. Complex regional pain syndrome: Are the IASP diagnostic criteria valid and sufficiently comprehensive? Pain 1999, 83, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Guthmiller, K.B.; Varacallo, M. Complex Regional Pain Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Pak, T.J.; Martin, G.M.; Magness, J.L.; Kavanaugh, G.J. Reflex sympathetic dystrophy. Review of 140 cases. Minn. Med. 1970, 53, 507–512. [Google Scholar]
- Lee, H.-J.; Lee, C.-S.; Yoo, Y.; Noh, J.M.; Yu, J.H.; Kim, Y.-C.; Moon, J.Y. Complex regional pain syndrome in the young male population: A retrospective study of 200 Korean young male patients. Korean J. Pain 2019, 32, 292–300. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Y.; Gao, M.; Li, Y.; Zhao, T.; Zhao, Y. Animal Models of Complex Regional Pain Syndrome Type I. J. Pain Res. 2021, 14, 3711–3721. [Google Scholar] [CrossRef]
- Allen, G.; Galer, B.S.; Schwartz, L. Epidemiology of complex regional pain syndrome: A retrospective chart review of 134 patients. Pain 1999, 80, 539–544. [Google Scholar] [CrossRef]
- Maves, S. Pain Behaviors and Sensory Alterations Following Immobilization of the Rat Hindpaw. In Proceedings of the 8th World Congress on Pain, Vancouver, BC, Canada, 17–22 August 1996; IASP Press: Seattle, WA, USA, 1996; Volume 118. [Google Scholar]
- Jo, Y.-H.; Kim, K.; Lee, B.-G.; Kim, J.-H.; Lee, C.-H.; Lee, K.-H. Incidence of and Risk Factors for Complex Regional Pain Syndrome Type 1 after Surgery for Distal Radius Fractures: A Population-based Study. Sci. Rep. 2019, 9, 4871. [Google Scholar] [CrossRef]
- Guo, T.-Z.; Wei, T.; Li, W.-W.; Li, X.-Q.; Clark, J.D.; Kingery, W.S. Immobilization Contributes to Exaggerated Neuropeptide Signaling, Inflammatory Changes, and Nociceptive Sensitization After Fracture in Rats. J. Pain 2014, 15, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Wasner, G.; Schattschneider, J.; Baron, R. Skin temperature side differences—A diagnostic tool for CRPS? Pain 2002, 98, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, P.U.; Groothoff, J.W.; Duis, H.J.T.; Geertzen, J.H. Incidence of complex regional pain syndrome type I after fractures of the distal radius. Eur. J. Pain 2003, 7, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Atkins, R.; Duckworth, T.; Kanis, J. Features of algodystrophy after Colles’ fracture. J. Bone Jt. Surg. Br. 1990, 72-B, 105–110. [Google Scholar] [CrossRef] [PubMed]
| Source of CRPS (n = 159) | |||||
|---|---|---|---|---|---|
| Trauma (n = 120, 75.5%) | No Trauma (n = 34, 21.4%) | Other (n = 5, 3.1%) | |||
| Surgery | Non-Operative Treatment | Surgery | Other | Idiopathic | Missing Documentation |
| n = 69 (57.5%) | n = 51 (42.5%) | n = 25 (73.5%) | n = 9 (26.5%) | n = 3 (60%) | n = 2 (40%) |
| Amount of Closed Reductions | 0 | 1 | 2 | 3 | 4 | Total |
|---|---|---|---|---|---|---|
| Operative Treatment | 0 (0%) | 19 (65.5%) | 6 (20.7%) | 3 (10.3%) | 1 (3.4%) | 29 (100%) |
| Non-operative Treatment | 2 (9.5%) | 15 (71.4%) | 1 (5%) | 0 (0%) | 0 (0%) | 21 (100%) |
| Incomplete Documentation | 3 (100%) |
| Patients with Cast Immobilization (n = 108) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Operative Treatment (n = 64) | ||||||||||
| Cast Immobilization (weeks) | 11 | 9 | 8 | 7 | 6 | 5 | 4 | 3 | 2 | 1 |
| Number of Patientsn (%) | 0 (0.0) | 1 (1.6) | 1 (1.6) | 1 (1.6) | 23 (35.9) | 11 (17.2) | 16 (25.0) | 4 (6.3) | 5 (7.8) | 2 (3.1) |
| Non-operative Treatment (n = 44) | ||||||||||
| Cast Immobilization (weeks) | 11 | 9 | 8 | 7 | 6 | 5 | 4 | 3 | 2 | 1 |
| Number of Patients (n) (%) | 1 (2.3) | 1 (2.3) | 0 (0.0) | 2 (4.5) | 12 (27.3) | 17 (38.6) | 7 (15.9) | 2 (4.5) | 0 (0.0) | 2 (4.5) |
| Budapest Criteria | 3 | 5 | 6 | 7 | 8 | 9 | 11 | 13 |
|---|---|---|---|---|---|---|---|---|
| Patients (n) | 2 | 13 | 1 | 69 | 1 | 45 | 26 | 2 |
| Percent (%) | 1.3 | 8.2 | 0.6 | 43.3 | 0.6 | 28.3 | 16.4 | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diepold, J.; Deininger, C.; Von Amelunxen, B.-C.; Deluca, A.; Siegert, P.; Freude, T.; Wichlas, F. Comparison of Epidemiological Data of Complex Regional Pain Syndrome (CRPS) Patients in Relation to Disease Severity—A Retrospective Single-Center Study. Int. J. Environ. Res. Public Health 2023, 20, 946. https://doi.org/10.3390/ijerph20020946
Diepold J, Deininger C, Von Amelunxen B-C, Deluca A, Siegert P, Freude T, Wichlas F. Comparison of Epidemiological Data of Complex Regional Pain Syndrome (CRPS) Patients in Relation to Disease Severity—A Retrospective Single-Center Study. International Journal of Environmental Research and Public Health. 2023; 20(2):946. https://doi.org/10.3390/ijerph20020946
Chicago/Turabian StyleDiepold, Julian, Christian Deininger, Berndt-Christian Von Amelunxen, Amelie Deluca, Paul Siegert, Thomas Freude, and Florian Wichlas. 2023. "Comparison of Epidemiological Data of Complex Regional Pain Syndrome (CRPS) Patients in Relation to Disease Severity—A Retrospective Single-Center Study" International Journal of Environmental Research and Public Health 20, no. 2: 946. https://doi.org/10.3390/ijerph20020946
APA StyleDiepold, J., Deininger, C., Von Amelunxen, B.-C., Deluca, A., Siegert, P., Freude, T., & Wichlas, F. (2023). Comparison of Epidemiological Data of Complex Regional Pain Syndrome (CRPS) Patients in Relation to Disease Severity—A Retrospective Single-Center Study. International Journal of Environmental Research and Public Health, 20(2), 946. https://doi.org/10.3390/ijerph20020946






