Effectiveness Evaluation of Repetitive Transcranial Magnetic Stimulation Therapy Combined with Mindfulness-Based Stress Reduction for People with Post-Stroke Depression: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- Group A: real rTMS stimulation and MBSR (rTMS–MBSR);
- Group B: sham rTMS stimulation and MBSR (sham rTMS–MBSR);
- Group C: sham rTMS stimulation and general psychological care.
2.2. Inclusion and Exclusion Criteria
2.3. Sample Size Estimation
2.4. Intervention
2.4.1. Repetitive Transcranial Magnetic Stimulation
2.4.2. Mindfulness-Based Stress Reduction
2.4.3. General Psychological Care
2.5. Data Collection
2.6. Outcome Measures
Clinical Assessment
2.7. Statistical Analysis
2.8. Ethics Statement
3. Results
3.1. Depressive State
3.2. Cognitive Function
3.3. Activities of Daily Living
3.4. Sleep Quality
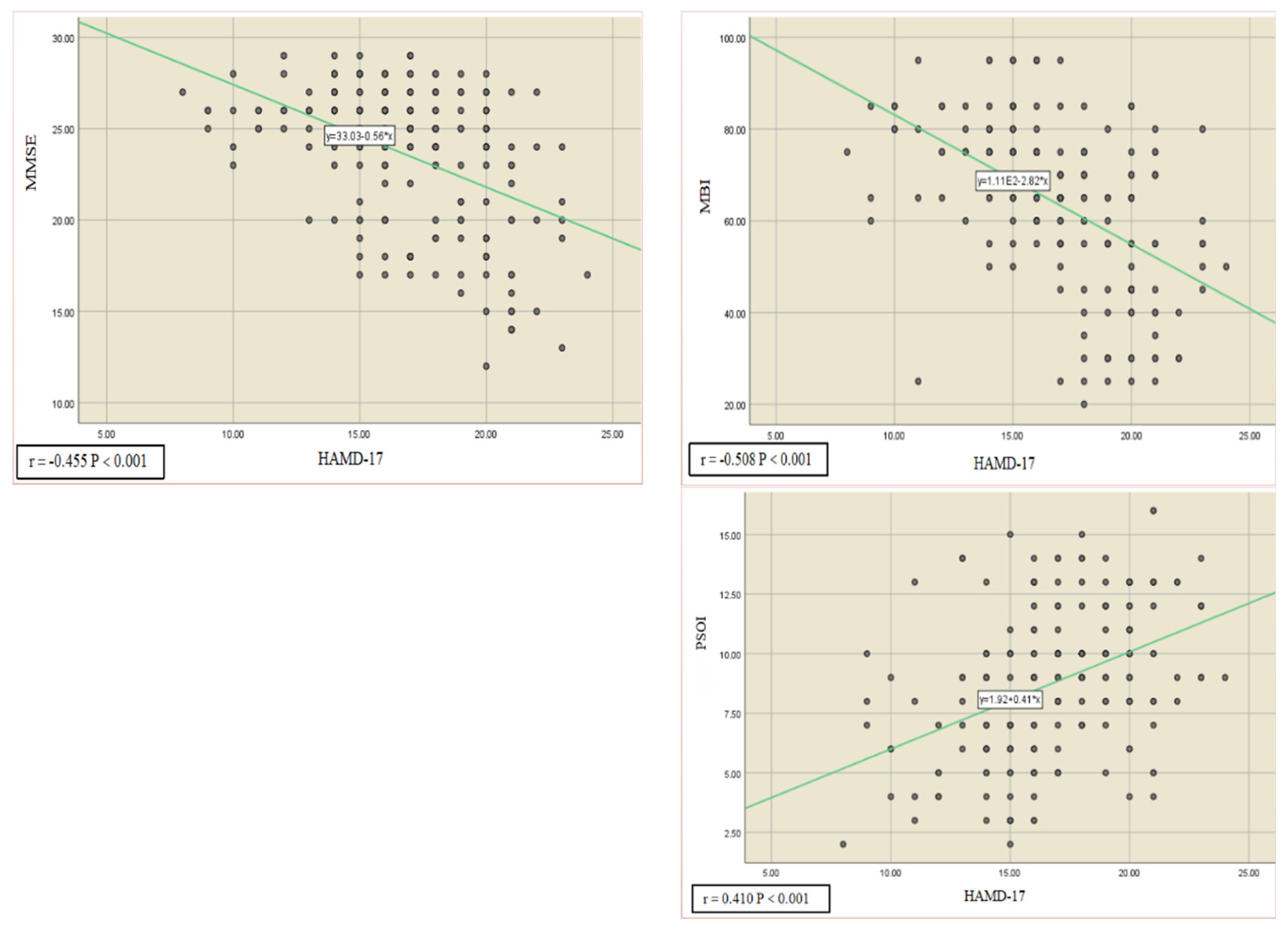
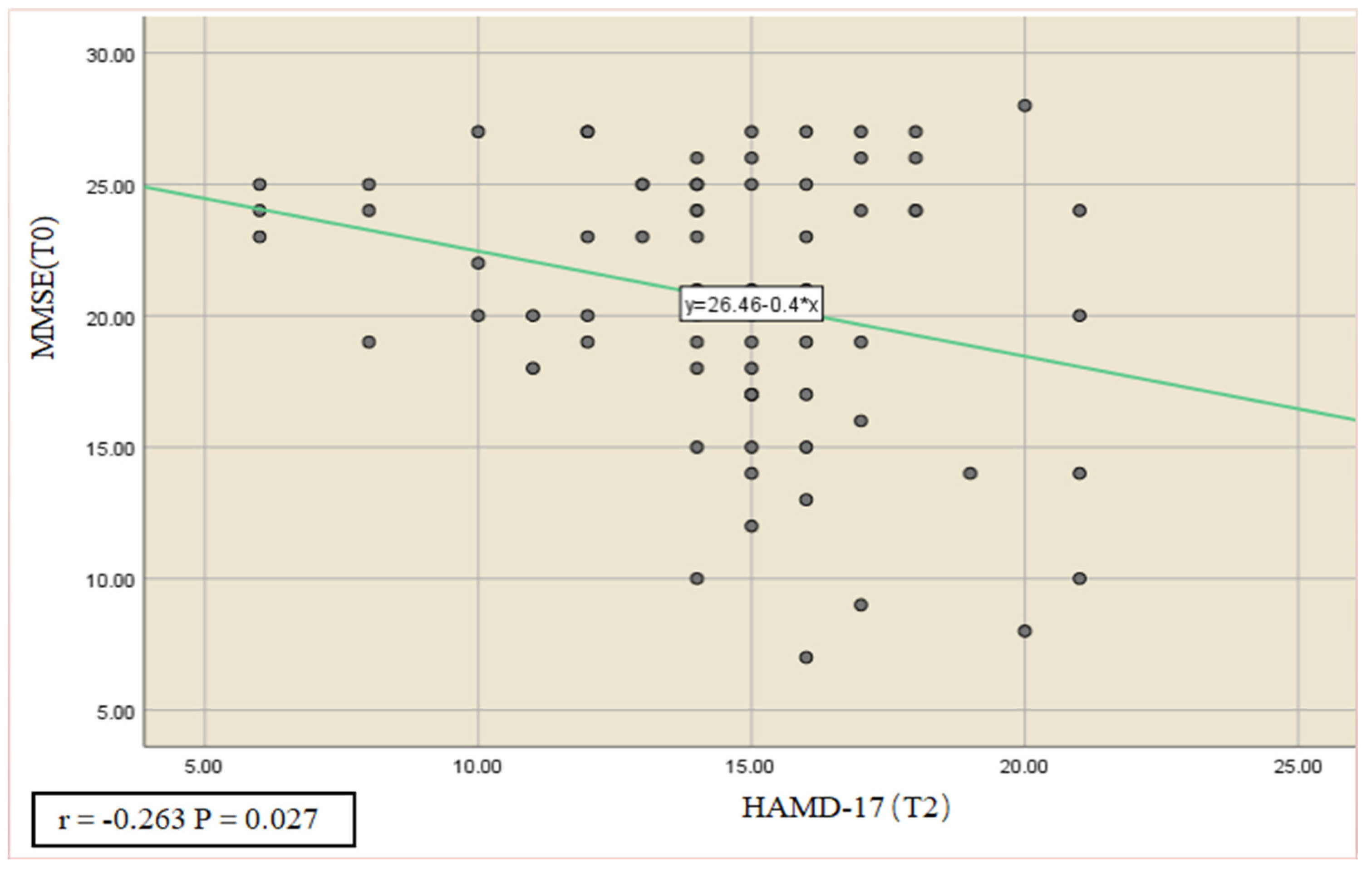
3.5. Correlation and Predictive Role of Cognitive Function, Activities of Daily Living, and Sleep Quality with Depression
4. Discussion
5. Limitations and Further Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sang, S.; Chu, C.; Zhang, T.; Chen, H.; Yang, X. The global burden of disease attributable to ambient fine particulate matter in 204 countries and territories, 1990–2019: A systematic analysis of the Global Burden of Disease Study 2019. Ecotoxicol. Environ. Saf. 2022, 238, 113588. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.Y.; Ford, A.; Kutlubaev, M.A.; Almeida, O.P.; Mead, G.E. Depression, Anxiety, and Suicide After Stroke: A Narrative Review of the Best Available Evidence. Stroke 2022, 53, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.G.; Starr, L.B.; Kubos, K.L.; Price, T.R. A two-year longitudinal study of post-stroke mood disorders: Findings during the initial evaluation. Stroke 1983, 14, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Ytterberg, C.; Cegrell, L.; von Koch, L.; Wiklander, M. Depression symptoms 6 years after stroke are associated with higher perceived impact of stroke, limitations in ADL and restricted participation. Sci. Rep. 2022, 12, 7816. [Google Scholar] [CrossRef]
- Robinson, R.G.; Jorge, R.E. Post-Stroke Depression: A Review. Am. J. Psychiatry 2016, 173, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Volz, M.; Möbus, J.; Letsch, C.; Werheid, K. The influence of early depressive symptoms, social support and decreasing self-efficacy on depression 6 months post-stroke. J. Affect. Disord. 2016, 206, 252–255. [Google Scholar] [CrossRef]
- Pinter, D.; Fandler-Höfler, S.; Fruhwirth, V.; Berger, L.; Bachmaier, G.; Horner, S.; Eppinger, S.; Kneihsl, M.; Enzinger, C.; Gattringer, T. Relevance of Cognition and Emotion for Patient-Reported Quality of Life After Stroke in Working Age: An Observational Cohort Study. Front. Neurol. 2022, 13, 869550. [Google Scholar] [CrossRef]
- Frey, J.; Najib, U.; Lilly, C.; Adcock, A. Novel TMS for Stroke and Depression (NoTSAD): Accelerated Repetitive Transcranial Magnetic Stimulation as a Safe and Effective Treatment for Post-stroke Depression. Front. Neurol. 2020, 11, 788. [Google Scholar] [CrossRef]
- Blumberger, D.M.; Maller, J.J.; Thomson, L.; Mulsant, B.H.; Rajji, T.K.; Maher, M.; Brown, P.E.; Downar, J.; Vila-Rodriguez, F.; Fitzgerald, P.B.; et al. Unilateral and bilateral MRI-targeted repetitive transcranial magnetic stimulation for treatment-resistant depression: A randomized controlled study. J. Psychiatry Neurosci. 2016, 41, E58–E66. [Google Scholar] [CrossRef]
- Blumberger, D.M.; Vila-Rodriguez, F.; Thorpe, K.E.; Feffer, K.; Noda, Y.; Giacobbe, P.; Knyahnytska, Y.; Kennedy, S.H.; Lam, R.W.; Daskalakis, Z.J.; et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): A randomised non-inferiority trial. Lancet 2018, 391, 1683–1692. [Google Scholar] [CrossRef]
- Meng, R.; Lei, C. Research progress on effect and mechanism of repetitive transcranial magnetic stimulation on post-stroke depression. Chin. J. Phys. Med. Rehabil. 2020, 4, 367–371. [Google Scholar]
- Liu, C.; Wang, M.; Liang, X.; Xue, J.; Zhang, G. Efficacy and Safety of High-Frequency Repetitive Transcranial Magnetic Stimulation for Poststroke Depression: A Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2019, 100, 1964–1975. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Liu, Y.; Zhang, L.; Zheng, H.; Peng, L.; Ai, Y.; Luo, J.; Hu, X. Effects of rTMS Treatment on Cognitive Impairment and Resting-State Brain Activity in Stroke Patients: A Randomized Clinical Trial. Front. Neural Circuits 2020, 14, 563777. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chen, A.; Zhu, S.; Yang, L.; Zhou, J.; Pan, S.; Shao, M.; Zhao, L. Repetitive transcranial magnetic stimulation for depression after basal ganglia ischaemic stroke: Protocol for a multicentre randomised double-blind placebo-controlled trial. BMJ Open 2018, 8, e018011. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, X.; Liang, H.; Yan, N.; Wang, Y. Repetitive Transcranial Magnetic Stimulation for Treatment of Post—Stroke Depression of Liver—Qi Stagnation Type in 33 case. Rehabil. Med. 2016, 26, 14–18, 23. [Google Scholar] [CrossRef]
- Bishop, S.R. Mindfulness: A Proposed Operational Definition. Clin. Psychol. Sci. Pract. 2010, 11, 230–241. [Google Scholar] [CrossRef]
- Disner, S.G.; Beevers, C.G.; Haigh, E.A.; Beck, A.T. Neural mechanisms of the cognitive model of depression. Nat. Rev. Neurosci. 2011, 12, 467–477. [Google Scholar] [CrossRef]
- Tang, Y.Y.; Hölzel, B.K.; Posner, M.I. The neuroscience of mindfulness meditation. Nat. Rev. Neurosci. 2015, 16, 213–225. [Google Scholar] [CrossRef]
- Allen, M.; Dietz, M.; Blair, K.S.; van Beek, M.; Rees, G.; Vestergaard-Poulsen, P.; Lutz, A.; Roepstorff, A. Cognitive-affective neural plasticity following active-controlled mindfulness intervention. J. Neurosci. 2012, 32, 15601–15610. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Wang, C.; Lv, J. The effects of mindfulness-based intervention on quality of life and poststroke depression in patients with spontaneous intracerebral hemorrhage in China. Int. J. Geriatr. Psychiatry 2020, 35, 572–580. [Google Scholar] [CrossRef]
- Baylan, S.; Haig, C.; MacDonald, M.; Stiles, C.; Easto, J.; Thomson, M.; Cullen, B.; Quinn, T.J.; Stott, D.; Mercer, S.W.; et al. Measuring the effects of listening for leisure on outcome after stroke (MELLO): A pilot randomized controlled trial of mindful music listening. Int. J. Stroke 2020, 15, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Sasaki, J.E.; Zeng, N.; Wang, C.; Sun, L. A Systematic Review With Meta-Analysis of Mindful Exercises on Rehabilitative Outcomes Among Poststroke Patients. Arch. Phys. Med. Rehabil. 2018, 99, 2355–2364. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.A. Living the Full Catastrophe: A Mindfulness-Based Program to Support Recovery from Stroke. Healthcare 2020, 8, 498. [Google Scholar] [CrossRef] [PubMed]
- Regier, D.A.; Narrow, W.E.; Kuhl, E.A.; Kupfer, D.J. The conceptual development of DSM-V. Am. J. Psychiatry 2009, 166, 645–650. [Google Scholar] [CrossRef]
- Xu, W.; Xiong, L. Efficacy observation of repetitive transcranial magnetic stimulation combined with psychological intervention in the treatment of patients with post-stroke depression. Chin. J. Phys. Med. Rehabil. 2022, 44, 348–352. [Google Scholar]
- Desu, M.; Raghavarao, D. Sample Size Methodology; Elsevier: Amsterdam, The Netherlands, 1990; pp. 55–61. [Google Scholar]
- Fleiss, J.L. Design and Analysis of Clinical Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Kirk, R.E. Experimental Design, Brooks; Cole Publishing Co., Wadsworth. Inc.: Belmont, CA, USA, 1982; Volume 94002, p. 534. [Google Scholar]
- Santorelli, S.F.; Kabat-Zinn, J.; Blacker, M.; Meleo-Meyer, F.; Koerbel, L. Mindfulness-Based Stress Reduction (MBSR) Authorized Curriculum Guide; Center for Mindfulness in Medicine, Health Care, and Society (CFM), University of Massachusetts Medical School: Worcester, MA, USA, 2017. [Google Scholar]
- Stahl, B.; Elisha, G. A Mindfulness-Based Stress Reduction Workbook; New Harbinger Publications: Oakland, CA, USA, 2014. [Google Scholar]
- Brach, T. Advance in Contemplative Psychotherapy; Routledge: Boca Raton, FL, USA, 2017; pp. 146–154. [Google Scholar]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef]
- Morriss, R.; Leese, M.; Chatwin, J.; Baldwin, D. Inter-rater reliability of the Hamilton Depression Rating Scale as a diagnostic and outcome measure of depression in primary care. J. Affect. Disord. 2008, 111, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Bech, P.; Engelhardt, N.; Evans, K.R.; Gibertini, M.; Kalali, A.H.; Kobak, K.A.; Lipsitz, J.D.; Williams, J.B.; Pearson, J.D.; Rothman, M. Why the Hamilton Depression Rating Scale endures. Am. J. Psychiatry 2005, 162, 2394–2395. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Carpenter, J.S.; Andrykowski, M.A. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J. Psychosom. Res. 1998, 45, 5–13. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Gao, L.; Xie, J.; Huang, T.; Shang, Y.; Gao, Z. Effects of mindfulness decompression therapy combined with transcranial magnetic stimulation in generalized anxiety disorder. Am. J. Transl. Res. 2021, 13, 6827–6836. [Google Scholar] [PubMed]
- Xie, J.; Geng, X.; Fan, F.; Fu, X.; He, S.; Li, T. The efficacy of therapies for post-stroke depression in aging: An umbrella review. Front. Aging Neurosci. 2022, 14, 993250. [Google Scholar] [CrossRef]
- Starosta, M.; Cichoń, N.; Saluk-Bijak, J.; Miller, E. Benefits from Repetitive Transcranial Magnetic Stimulation in Post-Stroke Rehabilitation. J. Clin. Med. 2022, 11, 2149. [Google Scholar] [CrossRef]
- Saban, K.L.; Tell, D.; De La Pena, P. Nursing Implications of Mindfulness-Informed Interventions for Stroke Survivors and Their Families. Stroke 2022, 53, 3485–3493. [Google Scholar] [CrossRef]
- Bermudo-Gallaguet, A.; Ariza, M.; Dacosta-Aguayo, R.; Agudelo, D.; Camins-Vila, N.; Boldó, M.; Carrera, Ò.; Vidal, S.; Ferrer-Uris, B.; Busquets, A.; et al. Effects and mechanisms of mindfulness training and physical exercise on cognition, emotional wellbeing, and brain outcomes in chronic stroke patients: Study protocol of the MindFit project randomized controlled trial. Front. Aging Neurosci. 2022, 14, 936077. [Google Scholar] [CrossRef]
- Li, Y.; Li, K.; Feng, R.; Li, Y.; Li, Y.; Luo, H.; Yu, Q. Mechanisms of Repetitive Transcranial Magnetic Stimulation on Post-stroke Depression: A Resting-State Functional Magnetic Resonance Imaging Study. Brain Topogr. 2022, 35, 363–374. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, X.; Guo, L.; Yuan, L.; Zhang, C. Clinical effect of mirtazapine combined with repetitive transcranial magnetic stimulation on post-stroke depression. World Latest Med. Inf. 2017, 17, 121+123. [Google Scholar] [CrossRef]
- Kim, J.; Cha, B.; Lee, D.; Kim, J.M.; Kim, M. Effect of Cognition Recovery by Repetitive Transcranial Magnetic Stimulation on Ipsilesional Dorsolateral Prefrontal Cortex in Subacute Stroke Patients. Front. Neurol. 2022, 13, 823108. [Google Scholar] [CrossRef]
- Begemann, M.J.; Brand, B.A.; Ćurčić-Blake, B.; Aleman, A.; Sommer, I.E. Efficacy of non-invasive brain stimulation on cognitive functioning in brain disorders: A meta-analysis. Psychol. Med. 2020, 50, 2465–2486. [Google Scholar] [CrossRef]
- Fredrickson, B.L. What Good Are Positive Emotions? Rev. Gen. Psychol. 1998, 2, 300–319. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.L.; Hanley, A.W.; Goldin, P.R.; Gross, J.J. Testing the mindfulness-to-meaning theory: Evidence for mindful positive emotion regulation from a reanalysis of longitudinal data. PLoS ONE 2017, 12, e0187727. [Google Scholar] [CrossRef] [PubMed]
- Wagle, J.; Farner, L.; Flekkøy, K.; Bruun Wyller, T.; Sandvik, L.; Fure, B.; Stensrød, B.; Engedal, K. Early post-stroke cognition in stroke rehabilitation patients predicts functional outcome at 13 months. Dement. Geriatr. Cogn. Disord. 2011, 31, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Gómez Pérez, L.J.; Cardullo, S.; Cellini, N.; Sarlo, M.; Monteanni, T.; Bonci, A.; Terraneo, A.; Gallimberti, L.; Madeo, G. Sleep quality improves during treatment with repetitive transcranial magnetic stimulation (rTMS) in patients with cocaine use disorder: A retrospective observational study. BMC Psychiatry 2020, 20, 153. [Google Scholar] [CrossRef]
- Xue, X.; Huang, Y.; Li, S.; Chen, Z.; Cao, H.; Wang, J. Effect of mindfulness training on resilience, sleep and quality of life of patients with post-stroke depression. China J. Health Psychol. 2020, 28, 1187–1191. [Google Scholar] [CrossRef]
- LeMoult, J.; Gotlib, I.H. Depression: A cognitive perspective. Clin. Psychol. Rev. 2019, 69, 51–66. [Google Scholar] [CrossRef]
- Lindsay, E.K.; Creswell, J.D. Mindfulness, acceptance, and emotion regulation: Perspectives from Monitor and Acceptance Theory (MAT). Curr. Opin. Psychol. 2019, 28, 120–125. [Google Scholar] [CrossRef]
- Cheng, W.; Rolls, E.T.; Ruan, H.; Feng, J. Functional Connectivities in the Brain That Mediate the Association Between Depressive Problems and Sleep Quality. JAMA Psychiatry 2018, 75, 1052–1061. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, C.; Zhou, D.; Zhao, Y.; Zhang, H.; Jiang, X.; Feng, Y. Construction and validation of a nomogram prediction model for post-stroke depression. J. Nurs. Sci. 2022, 37, 5–8, 22. [Google Scholar]
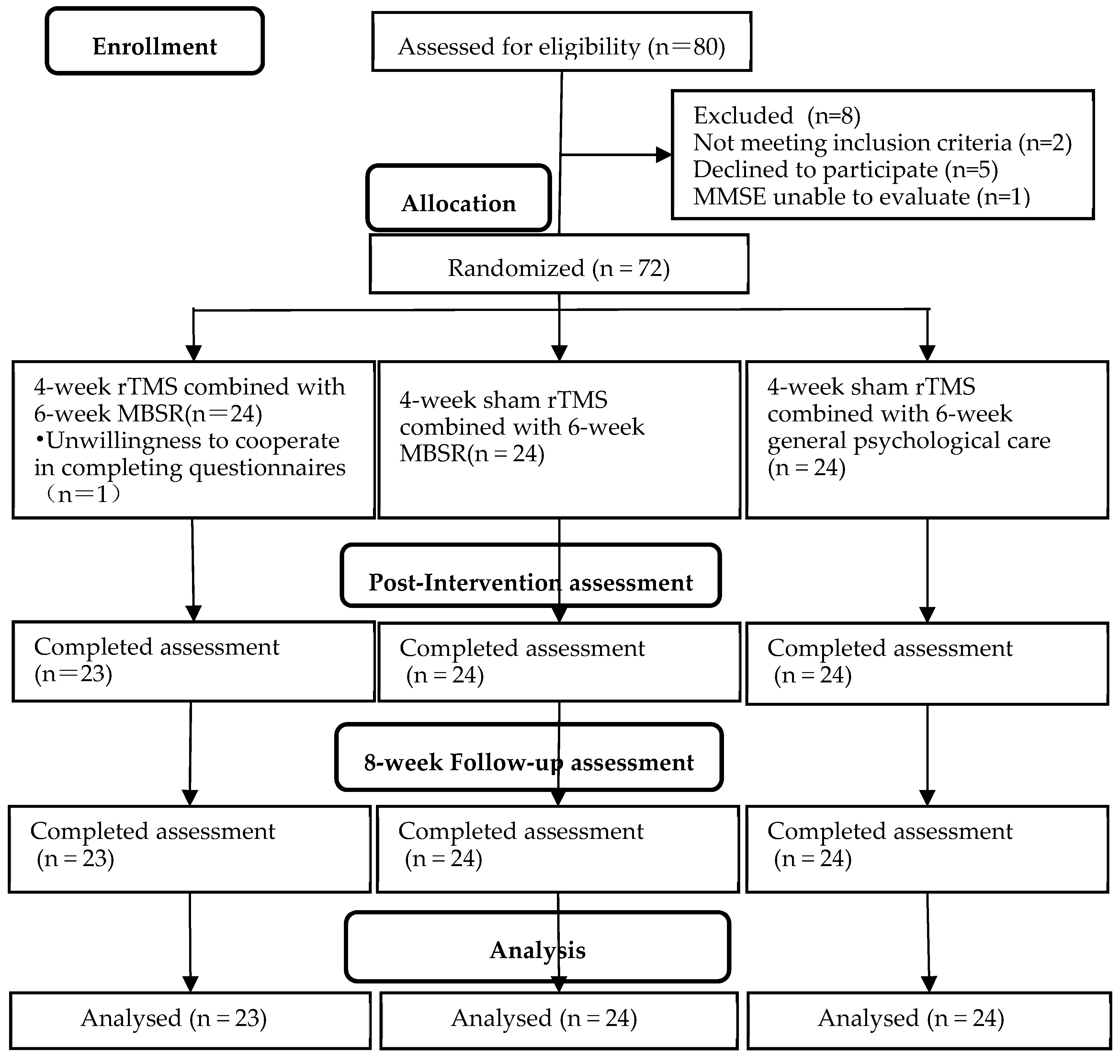
| Parameters | Group A (n = 23) | Group B (n = 24) | Group C (n = 24) | χ2/F Value | p Value |
|---|---|---|---|---|---|
| Gender, n (%) | 0.158 | 0.924 | |||
| Male | 19 (82.6) | 20 (83.3) | 19 (79.2) | ||
| Female | 4 (17.4) | 4 (16.7) | 5 (20.8) | ||
| Age (year) | 58.30 ± 13.06 | 53.63 ± 13.01 | 54.42 ± 14.27 | 0.808 | 0.450 |
| The course of stroke (day) | 72.57 ± 20.62 | 74.00 ± 18.55 | 70.88 ± 20.73 | 0.147 | 0.864 |
| Education, n (%) | 2.971 | 0.563 | |||
| Primary school and below | 6 (26.1) | 11 (45.8) | 8 (33.3) | ||
| Junior high school | 5 (21.7) | 4 (16.7) | 7 (29.2) | ||
| High school and above | 12 (52.2) | 9 (37.5) | 9 (37.5) | ||
| Family annual income, n (%) | 0.599 | 0.741 | |||
| <100,000 | 13 (56.5) | 13 (54.2) | 11 (45.8) | ||
| ≥100,000 | 10 (43.5) | 11 (45.8) | 13 (54.2) | ||
| Marital status, n (%) | 6.275 | 0.180 | |||
| Bereaved spouse | 1 (4.3) | 0 (0) | 2 (8.3) | ||
| Unmarried | 0 (0) | 0 (0) | 2 (8.3) | ||
| Married | 22 (95.7) | 24 (100) | 20 (83.4) | ||
| Occupation, n (%) | 7.572 | 0.109 | |||
| Unemployed | 2 (8.7) | 10 (41.7) | 6 (25) | ||
| Retired | 7 (30.4) | 5 (20.8) | 4 (16.7) | ||
| Employed | 14 (60.9) | 9 (37.5) | 14 (58.3) | ||
| Lesion Location, n (%) | 6.654 | 0.354 | |||
| Brainstem | 3 (13) | 8 (33.3) | 3 (12.5) | ||
| Cerebellar | 3 (13) | 0 (0) | 3 (12.5) | ||
| Cortical–sub-cortical | 8 (34.8) | 8 (33.3) | 9 (37.5) | ||
| Sub-cortical | 9 (39.1) | 8 (33.3) | 9 (37.5) | ||
| Lesion hemisphere, n (%) | 0.544 | 0.797 | |||
| right | 13 (56.5) | 11 (45.8) | 12 (50) | ||
| left | 10 (43.5) | 13 (54.2) | 12 (50) |
| Group | Samples | Pre-Test | Post-Test | 8-Week Follow-Up | F | p |
|---|---|---|---|---|---|---|
| Group A | 23 | 19.04 ± 2.16 | 13.96 ± 2.99 a | 11.96 ± 3.24 a | 80.501 | <0.001 |
| Group B | 24 | 19.13 ± 3.05 | 15.63 ± 2.02 ab | 13.96 ± 2.14 ab | 43.833 | <0.001 |
| Group C | 24 | 20.25 ± 1.39 | 17.38 ± 1.41 abc | 17.54 ± 2.25 abc | 19.082 | <0.001 |
| F | 2.032 | 13.602 | 29.421 | |||
| p | 0.139 | <0.001 | <0.001 |
| Group | Samples | Pre-Test | Post-Test | 8-Week Follow-Up | F | p |
|---|---|---|---|---|---|---|
| Group A | 23 | 21.09 ± 4.33 | 26.48 ± 1.27 a | 27.00 ± 1.28 a | 30.810 | <0.001 |
| Group B | 24 | 20.83 ± 5.59 | 25.46 ± 2.90 a | 25.04 ± 1.63 ab | 28.455 | <0.001 |
| Group C | 24 | 20.04 ± 5.83 | 22.50 ± 3.86 abc | 21.54 ± 3.43 bc | 12.739 | <0.001 |
| F | 0.250 | 12.021 | 33.436 | |||
| p | 0.779 | <0.001 | <0.001 |
| Group | Samples | Pre-Test | Post-Test | 8-Week Follow-Up | F | p |
|---|---|---|---|---|---|---|
| Group A | 23 | 46.52 ± 19.45 | 75.43 ± 9.40 a | 81.96 ± 9.50 a | 97.753 | <0.001 |
| Group B | 24 | 45.00 ± 18.00 | 66.46 ± 9.03 ab | 75.00 ± 7.37 ab | 87.982 | <0.001 |
| Group C | 24 | 43.75 ± 12.87 | 65.83 ± 8.43 ab | 71.46 ± 6.83 ab | 63.619 | <0.001 |
| F | 0.157 | 8.395 | 10.528 | |||
| p | 0.855 | 0.001 | <0.001 |
| Group | Samples | Samples | Pre-Test | 8-Week Follow-Up | F | p |
|---|---|---|---|---|---|---|
| Group A | 23 | 10.87 ± 1.87 | 7.43 ± 1.70 a | 4.35 ± 1.19 a | 104.631 | <0.001 |
| Group B | 24 | 11.79 ± 2.54 | 9.08 ± 2.62 ab | 6.88 ± 2.85 ab | 64.902 | <0.001 |
| Group C | 24 | 10.54 ± 2.34 | 8.67 ± 2.20 a | 8.75 ± 2.94 abc | 25.688 | <0.001 |
| F | 1.952 | 3.508 | 18.688 | |||
| p | 0.150 | 0.035 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, H.; Yan, X.; Meng, S.; Qiu, L.; Zhang, J.; Yang, C.; Liu, S. Effectiveness Evaluation of Repetitive Transcranial Magnetic Stimulation Therapy Combined with Mindfulness-Based Stress Reduction for People with Post-Stroke Depression: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2023, 20, 930. https://doi.org/10.3390/ijerph20020930
Duan H, Yan X, Meng S, Qiu L, Zhang J, Yang C, Liu S. Effectiveness Evaluation of Repetitive Transcranial Magnetic Stimulation Therapy Combined with Mindfulness-Based Stress Reduction for People with Post-Stroke Depression: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2023; 20(2):930. https://doi.org/10.3390/ijerph20020930
Chicago/Turabian StyleDuan, Haoran, Xin Yan, Shifeng Meng, Lixia Qiu, Jiayu Zhang, Chunxia Yang, and Sha Liu. 2023. "Effectiveness Evaluation of Repetitive Transcranial Magnetic Stimulation Therapy Combined with Mindfulness-Based Stress Reduction for People with Post-Stroke Depression: A Randomized Controlled Trial" International Journal of Environmental Research and Public Health 20, no. 2: 930. https://doi.org/10.3390/ijerph20020930
APA StyleDuan, H., Yan, X., Meng, S., Qiu, L., Zhang, J., Yang, C., & Liu, S. (2023). Effectiveness Evaluation of Repetitive Transcranial Magnetic Stimulation Therapy Combined with Mindfulness-Based Stress Reduction for People with Post-Stroke Depression: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 20(2), 930. https://doi.org/10.3390/ijerph20020930





