Abstract
Background: Phase angle (PhA) has been used as mortality prognostic, but there are no studies about its possible use as a screening tool. Therefore, an assessment of the possible utility of PhA in clinical practice is required. The aim of this systematic review was to explore all recent available evidence of PhA, and its possible utility as a screening tool in clinical practice in subjects with chronic metabolic diseases. Materials and Methods: This systematic review was performed and written as stated in the PRISMA 2020 guidelines. The search was conducted in PubMed, ScienceDirect and SciElo. In order to be considered eligible, within the entire search, only articles involving PhA and their utility in metabolic diseases were included. Results: PhA was associated with hyperuricemia and vitamin D deficiency in obese subjects, and decreased cardiovascular risk and malnutrition in hospitalized patients. Conclusion: PhA may be a potential screening tool in clinical practice to evaluate different biomarkers, cardiovascular risk, and nutritional diagnosis in metabolic diseases in adults.
1. Introduction
In the last three decades, a worldwide increase in the prevalence of chronic metabolic diseases and associated health conditions has been reported [1]. This epidemiological scenery requires identifying subjects at risk in a timely manner to provide appropriate treatment and prevent complications, especially when it comes to healthcare systems with financial limitations and limited resources [1,2]. In this sense, phase angle (PhA) is an indicator of membrane integrity function, and has been suggested as an important prognostic indicator of mortality for critically ill patients and in some aspects that may impact directly: inflammation, and functional disabilities [2,3,4]. In addition, Norman et al. [4] suggested that PhA could be used as a non-invasive and non-expensive screening tool to identify subjects with impaired nutritional status in clinical practice.
According to Iragorri and Spackman [5], several methodological issues must be considered in the cost-effectiveness analysis (CEA) of new screening tools, for example, diagnostic test accuracy (sensitivity and specificity) and modeling false positive and negative results, among others. However, data related to PhA as a screening tool are limited, and a CEA is not always feasible to obtain. Currently, there is no clear information on which biomarkers are associated with PhA, and whether there would be any utility or clinical relevance in chronic metabolic diseases and associated health conditions.
In order to establish an approach to the possible utility of PhA in clinical practice, the aim of this review was to explore all recent available evidence of PhA and its possible usefulness, association or implication in chronic metabolic diseases assessment.
2. Materials and Methods
The systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (Figure 1) [6].
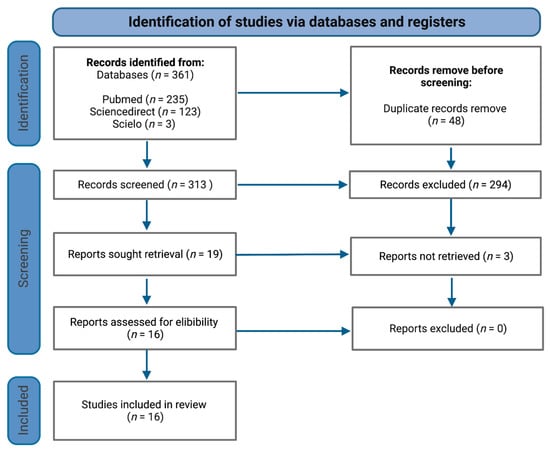
Figure 1.
PRISMA flowchart of study selection process.
2.1. Information Sources and Search Strategy
The steps specified by PRISMA were carried out to implement the systematic review. A search was conducted from August to October 2022. The included electronic databases were PubMed, ScienceDirect and SciElo, from January 2017 to August 2022. No filters were applied for the study design.
As a search strategy, all articles which contained the following keywords, MeSH terms and Boolean operators, were considered: “Phase Angle” AND “Metabolic Diseases” OR “Nutritional and Metabolic Diseases” OR “Metabolic Syndrome” OR “Obesity” OR “Overweight” OR “Dyslipidemias” OR “Diabetes Mellitus” OR “Hypertension” OR “Atherosclerosis” OR “Heart Diseases” OR “Myocardial infarction” OR “Stroke” OR “Renal Insufficiency”.
2.2. Eligibility and Exclusion Criteria
The PICOS strategy was adopted as “P” (participants or problem) of any age and ethnic group, of both sexes with a metabolic disease; “I” (intervention) was considered as PhA measurement in subjects with metabolic diseases; “C” (comparison) was not applicable; “O” (outcomes) was the association or implication of PhA values in metabolic diseases; and “S” (studies) were related only to cross-sectional, cohort studies and clinical trials.
In order to be considered eligible, all studies were required to meet the following inclusion criteria: both sexes, written in English language, the study design of all studies were descriptive observational studies (cross-sectional, prospective and retrospective cohort studies) and clinical trials (multicenter trials). Within the entire search, only studies involving PhA and its utility in metabolic diseases were included. No restriction was applied in relation to sample size.
The exclusion criteria were: articles without full-text availability, opinion pieces, review articles, meta-analysis, editorial letters, records with insufficient information about the involvement of PhA and metabolic diseases, written in Spanish language, and/or did not mention the topic.
2.3. Study Selection and Data Extraction
The protocol for this article was published in the International Prospective Register of Systematic Reviews, PROSPERO, under the register CRD42022381045. Data collection was carried out, beginning in August 2022, and concluding in October 2022. The title and abstracts of publications were updated throughout the screening step, and duplicate research was eliminated. Thereafter, the full text was analyzed to determine its suitability to accomplish a systematic review. All studies were collected and summarized in an electronic database.
Both titles and abstracts were reviewed separately by the authors. The following information was extracted for each selected study: (a) first author name and year of publication, (b) sample characteristics, (c) study design, (d) main outcomes, (e) BIA device and PhA equation, and (f) scientific evidence.
2.4. Certainty of Evidence
The certainty of evidence across studies was assessed using the grading of recommendations, assessment, development and evaluations (GRADE) framework. This framework allows the development and presentation of summaries of evidence, and provides a systematic approach for clinical practice recommendations, summarized in four categories of evidence: “high” (the authors have a large amount of confidence that the true effect is similar to the estimated effect), “moderate” (the authors believe that the true effect is probably close to the estimated effect), “low” (the true effect might be markedly different from the estimated effect), and “very low” (the true effect is probably markedly different from the estimated effect) [7]. Discrepancies were resolved by the three authors through consensus. The GRADE approach takes the study limitations (Risk of Bias), inconsistency of results, indirectness of evidence, imprecision, and publication bias, into consideration.
3. Results
3.1. Search Results
Overall, the initial strategy search retrieved a total of 361 publications (Figure 1), of which 48 were removed as duplicates. Following this, 313 records were screened for titles, of which 294 were excluded because they did not satisfy inclusion criteria. In total, 16 relevant articles/abstracts were reviewed in detail for eligibility and were included for the purposes of this systematic review, namely, nine cross-sectional studies, six cohort studies and one clinical trial (Table 1).

Table 1.
General description of included studies.
3.2. Phase Angle as a Potential Screening Tool in Metabolic Diseases
3.2.1. Overweight and Obesity
According to the included studies, Table 2 and Figure 2, show that PhA may be used as an assessment indicator for the presence of hyperuricemia and vitamin D (25(OH)D) levels in overweight and obese subjects. A low PhA (<5°) was associated with the presence of hyperuricemia (uric acid > 6 mg/dL and > 7 mg/dL in women and men, respectively) in obese subjects (body mass index (BMI) ≥ 35.0 to 64.0 kg/m2) [8]. On the other hand, the lowest values of PhA were significantly associated with obesity and of 25(OH)D deficiency (OR = 0.3, and 0.2 respectively; p < 0.001) [9]. It is important to note that PhA was significantly correlated with several nutritional parameters such as albumin, blood urea nitrogen (BUN), creatinine, uric acid, phosphorus and glucose (r = 0.37, 0.31, 0.50, 0.46, 0.20 and −0.22, respectively; p < 0.05) [10].

Table 2.
Main outcomes of included studies.
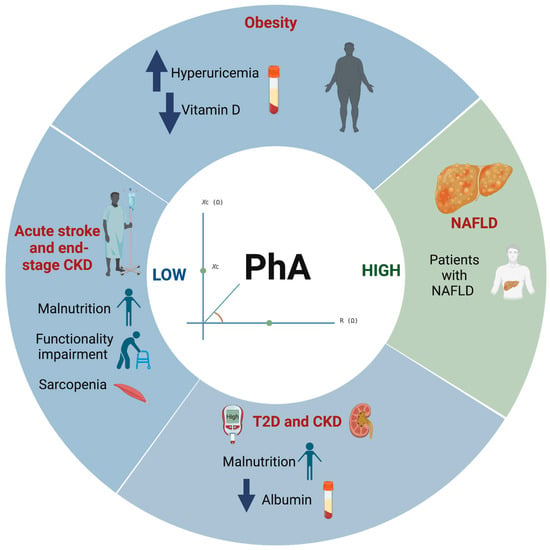
Figure 2.
Graphic summary of reported results in this review, showing the relationship between PhA and metabolic diseases. Abbreviations: PhA: Phase angle; T2D: Type 2 Diabetes; CKD: Chronic kidney disease; NAFLD: Nonalcoholic fatty liver disease.
3.2.2. Malnutrition
In hospitalized patients, PhA could be used as an additional resource to identify subjects at risk of malnutrition and functionality level. Fernandez-Jiménez et al. [11] established that PhA values of ≤5.4° and ≤5.3° in men and women, respectively, admitted to hospital for different causes, were significantly associated with malnutrition or malnutrition-related diseases, as sorted by the malnutrition universal screening tool (MUST) scale.
3.2.3. Cardiovascular and Chronic Kidney Diseases
Regarding functional level, an observational cohort study by Abe et al. [12] reported an independent association between low PhA (men < 5.62° and women < 4.54°) and functional independence measure motor (FIM-motor) in patients with acute stroke. Additionally, in chronic kidney disease (CKD) subjects, low values of PhA were associated with sarcopenia presence [13].
In other studies, it was reported that diabetic CKD stage 5 (DMCKD5) subjects with PhA values less than 4.17° were reported to show GNRI-assessed malnutrition and low albumin levels (3.13 ± 0.52 g/dL) compared with those with PhA ≥ 4.17° [14,15]. Finally, mechanical ventilation time and cardiac operative risk (EuroSCORE) were inversely associated with PhA in patients for elective cardiac surgery. Silva et al. [16] reported that every one point increase in EuroSCORE reduced PhA by 0.22°, and every minute more of mechanical ventilation (MV) reduced PhA by 0.00015°.
3.3. Differences on PhA Values in Several Health Conditions
Studies reported that obese subjects had a higher PhA in comparison with eutrophic subjects (6.9 ± 0.9° vs. 6.5 ± 0.8°; p = 0.003) [17]. Table 1 summarizes data from a multicenter clinical study of 217 individuals, showing a decrease in PhA with respect to age in type 2 diabetes mellitus (T2D) subjects compared with the control group [18]. Furthermore, this decrease was exacerbated and evident in relation to disease duration in patients with T2D [18]. Similarly, it was reported that non-alcoholic fatty liver disease (NAFLD) subjects had a higher PhA in comparison with non-NAFLD subjects (5.53 ± 0.66° vs. 5.43 ± 0.60°; p = 0.04), and PhA was associated with the risk of NAFLD [19]. In end-stage CKD patients, those with PhA < 6° values had higher levels of biomarkers related to arterial stiffness, compared with patients with PhA > 6° values [23].
3.4. Factors That Influence PhA Values
PhA was reported to be modified by various factors such as body mass index (BMI), age and gender. According to Barrea et al. [9], for each unit increase in BMI, PhA decreased 0.54° in subjects with a wide range of BMI. Contrary to these findings, Fu et al. [20] reported that in overweight and obese subjects, as BMI increased, there was an increase in PhA of 0.006°. Similarly, these authors agreed that for each increase in one year of age in subjects with a wide range of BMI, PhA decreased 0.11° and 0.014° in overweight and obese subjects, respectively [9,20]. Regarding sex, it was determined that the difference between men and women in PhA was 0.32° and 0.629°, respectively [9]. In addition, Streb et al. [21] reported that for each increase in the percentage of body fat, PhA decreased 0.66°. Contrarily, PhA was inversely correlated with central adiposity [22] (Table 2).
4. Discussion
It has been reported that obesity involves an inflammatory process that contributes to cardiovascular risk, and one of the mechanisms described is the presence of high uric acid levels that contribute to oxidative stress [24]. In this sense, low levels of vitamin D are also involved in inflammatory processes, mainly in the regulation of cytokine release [25]. In clinical practice, it is not common to assess certain biomarkers, such as vitamin D levels. Therefore, having a potential screening tool to identify possible subjects at risk of hyperuricemia and/or vitamin D deficiency could improve assessment in the clinical scope. According to the results obtained, in subjects living with obesity, a low PhA (<5°) is an indicator of hyperuricemia [8]. Additionally, PhA values are associated with vitamin D levels. Subsequently, this should be confirmed through a blood test, so as to initiate appropriate and timely treatment to prevent cardiovascular events [9]. Therefore, PhA could be a useful tool to assess the status of those biomarkers in subjects with excess adiposity.
In this setting, prompting a screening tool requires, in the first instance, establishing an association between the variable to be measured and the prognostic variable. Although a correlation between PhA and various biomarkers (BUN, creatinine, uric acid, phosphorus, and glucose) had previously been reported in the literature [10], it had not been determined that PhA could predict or be useful as a screening tool for such indicators. In this sense, correlation analysis indicates the intensity of the relationship between variables but not the effect or influence of one on the other.
According to our findings, PhA could be useful to identify subjects at risk of malnutrition in patients admitted to hospital for different causes (≤5.4° and ≤5.3° in men and women, respectively) [11], and functionality impairment in patients with acute stroke (men < 5.62° and women < 4.54°) [12]. These findings suggest that in the hospitalization context, a consideration of PhA to identify subjects with malnutrition and promptly initiate a suitable nutritional therapy should be incorporated to prevent length of stay and associated complications of malnutrition [26].
Additionally, identifying subjects with functional impairment would allow the placement of appropriate medical–nutrition strategies to avoid physical dependence, which could improve the life quality of subjects [27,28]. It is important to note that malnutrition and functionality impairment have a negative impact on healthcare systems and in several scenarios that involve patients, owing to increased hospital expenditures, drug consumption and length of recovery time [29]. Therefore, incorporating PhA into first-line nutritional assessment in the hospital area could show meaningful results [26,27]. In this sense, it would not represent a replacement as part of some step within first-line clinical practice; it solely could be enforced as a non-invasive and non-expensive additional resource in the nutritional scope.
On the other hand, the literature has demonstrated that there are differences in the values of PhA that subjects present under several different health conditions (obesity, diabetes and NAFLD) compared with healthy subjects. As mentioned above, confirmed subjects with diseases present alterations at the tissular level, which is reflected in their PhA values [8,18,19]. Particularly, PhA is influenced by age, BMI and sex, due to the variations in total body water (TBW) content [9,30]. It is well established that PhA is based on the resistance and reactance of the tissues, with TBW being the main component that influences these parameters [31]. It has been reported that TBW decreases with age, increases with the presence of obesity, and women have more liters of TWB compared with men [30,31].
Some limitations should be noted. Most of the selected studies were cross-sectional studies, which limits the power of the results and the possibility of making causal conclusions. In addition, the data were collected using different methods and in different countries, which may have influenced the results, especially given the heterogeneity of health conditions and characteristics of population (sex, ethnic and age group, etc.). None of the studies had the main objective of evaluating or validating PhA as a screening tool; they were secondary findings. Another issue concerns the model, scales, and instruments used to screen participants and the appropriateness of sample selection (different BIA devices), for example, monofrequency and multifrequency BIA.
To date, it is not possible to use phase angle widely in the clinical setting as a potential screening tool, as it requires appropriate validation in different conditions (by sex, ethnic and age group) and association studies. It would be opportune to carry out more studies in this regard, to clearly establish its behavior and utility.
5. Conclusions
In clinical practice phase angle could be a potential screening tool to evaluate different biomarkers, decrease cardiovascular risk and nutritional diagnosis, mainly concerning hyperuricemia and vitamin D deficiency in obese subjects. Additionally, age, sex and BMI influence PhA values. Therefore, more studies are required in this regard to elucidate their association and behavior thereof.
Author Contributions
Conceptualization, S.P.-B. and A.J.M.; methodology, S.P.-B., R.G.-A. and A.J.M.; resources, S.P.-B.; data curation, S.P.-B., R.G.-A. and A.J.M.; writing—original draft preparation, S.P.-B.; writing—review and editing, S.P.-B., R.G.-A., C.A.A.-S. and A.J.M.; supervision, A.J.M. and C.A.A.-S.; project administration, A.J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank the Tecnologico de Monterrey, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán and Unidad de Investigación de Enfermedades Metabólicas, for allowing us to carry out this review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Noncommunicable Diseases. Available online: https://www.who.int/health-topics/noncommunicable-diseases (accessed on 17 November 2022).
- Garlini, L.M.; Alves, F.D.; Ceretta, L.B.; Perry, I.S.; Souza, G.C.; Clausell, N.O. Phase angle and mortality: A systematic review. Eur. J. Clin. Nutr. 2019, 73, 495–508. [Google Scholar] [CrossRef]
- de Borba, E.L.; Ceolin, J.; Ziegelmann, P.K.; Bodanese, L.C.; Gonçalves, M.R.; Cañon-Montañez, W.; Mattiello, R. Phase angle of bioimpedance at 50 kHz is associated with cardiovascular diseases: Systematic review and meta-analysis. Eur. J. Clin. Nutr. 2022, 76, 1366–1373. [Google Scholar] [CrossRef]
- Norman, K.; Stobäus, N.; Pirlich, M.; Bosy-Westphal, A. Bioelectrical phase angle and impedance vector analysis—Clinical relevance and applicability of impedance parameters. Clin. Nutr. 2012, 31, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Iragorri, N.; Spackman, E. Assessing the value of screening tools: Reviewing the challenges and opportunities of cost-effectiveness analysis. Public Health Rev. 2018, 39, 17. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- What Is GRADE?|BMJ Best Practice. Available online: https://bestpractice.bmj.com/info/toolkit/learn-ebm/what-is-grade/ (accessed on 17 November 2022).
- Curvello-Silva, K.; Ramos, L.B.; Sousa, C.; Daltro, C. Phase angle and metabolic parameters in severely obese patients. Nutr. Hosp. 2020, 37, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Muscogiuri, G.; Laudisio, D.; Di Somma, C.; Salzano, C.; Pugliese, G.; De Alteriis, G.; Colao, A.; Savastano, S. Phase Angle: A Possible Biomarker to Quantify Inflammation in Subjects with Obesity and 25(OH)D Deficiency. Nutrients 2019, 11, 1747. [Google Scholar] [CrossRef] [PubMed]
- Shin J ho Kim, C.R.; Park, K.H.; Hwang, J.H.; Kim, S.H. Predicting clinical outcomes using phase angle as assessed by bioelectrical impedance analysis in maintenance hemodialysis patients. Nutrition 2017, 41, 7–13. [Google Scholar] [CrossRef]
- Fernández-Jiménez, R.; Dalla-Rovere, L.; García-Olivares, M.; Abuín-Fernández, J.; Sánchez-Torralvo, F.J.; Doulatram-Gamgaram, V.K.; Hernández-Sanchez, A.M.; García-Almeida, J.M. Phase Angle and Handgrip Strength as a Predictor of Disease-Related Malnutrition in Admitted Patients: 12-Month Mortality. Nutrients 2022, 14, 1851. [Google Scholar] [CrossRef]
- Abe, T.; Yoshimura, Y.; Imai, R.; Yoneoka, Y.; Tsubaki, A.; Sato, Y. Impact of Phase Angle on Physical Function in Patients with Acute Stroke. J. Stroke Cerebrovasc. Dis. 2021, 30, 105941. [Google Scholar] [CrossRef]
- Shin, J.; Hwang, J.H.; Han, M.; Cha, R.H.; Kang, S.H.; An, W.S.; Kim, J.C.; Kim, S.H. Phase angle as a marker for muscle health and quality of life in patients with chronic kidney disease. Clin. Nutr. 2022, 41, 1651–1659. [Google Scholar] [CrossRef]
- Han, B.G.; Lee, J.Y.; Kim, J.S.; Yang, J.W. Clinical Significance of Phase Angle in Non-Dialysis CKD Stage 5 and Peritoneal Dialysis Patients. Nutrients 2018, 10, 1331. [Google Scholar] [CrossRef]
- Han, B.G.; Lee, J.Y.; Kim, J.S.; Yang, J.W. Decreased Bioimpedance Phase Angle in Patients with Diabetic Chronic Kidney Disease Stage 5. Nutrients 2019, 11, 2874. [Google Scholar] [CrossRef]
- Silva TK da Perry, I.D.S.; Brauner, J.S.; Wender, O.C.B.; Souza, G.C.; Vieira, S.R.R. Performance evaluation of phase angle and handgrip strength in patients undergoing cardiac surgery: Prospective cohort study. Aust. Crit. Care 2018, 31, 284–290. [Google Scholar] [CrossRef]
- Oliveira Filho, J.M.; de Bernardes, P.S.; Serpa, G.H.C.; Siquiera, G.D.d.J.; Noll, M.; Venâncio, P.E.M.; Soares, V. Bioelectrical vector analysis in obese adolescents. Rev. Paul. Pediatr. 2020, 38, e2019017. [Google Scholar] [CrossRef] [PubMed]
- Jun, M.H.; Ku, B.; Kim, J.; Kim, K.H.; Kim, J.U. Mediation effect of the duration of diabetes mellitus on the decrease in bioimpedance phase angles in ethnically Korean people: A multicenter clinical study. J. Diabetes Investig. 2021, 12, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Lv, Y.; Ni, W.; Shi, Q.; Xiang, X.; Li, S.; Song, C.; Xiao, M.; Jin, S. Associations between Phase Angle Values Obtained by Bioelectrical Impedance Analysis and Nonalcoholic Fatty Liver Disease in an Overweight Population. Can. J. Gastroenterol. Hepatol. 2020, 2020, e8888405. [Google Scholar] [CrossRef]
- Fu, L.; Ren, Z.; Liu, X.; Wu, N.; Zhao, K.; Luo, G.; Yang, H.; Zhang, Y.; Yan, T.; Liu, Y.; et al. Reference Data of Phase Angle Using Bioelectrical Impedance Analysis in Overweight and Obese Chinese. Front. Endocrinol. 2022, 13, 924199. [Google Scholar] [CrossRef] [PubMed]
- Streb, A.R.; Hansen, F.; Gabiatti, M.P.; Tozetto, W.R.; Del Duca, G.F. Phase angle associated with different indicators of health-related physical fitness in adults with obesity. Physiol. Behav. 2020, 225, 113104. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.d.S.; Barreto Silva, M.I.; da Costa, M.S.; Pontes, K.S.d.S.; Castro, F.G.; Antunes, V.P.; de Carvalho Rosina, K.T.; Menna Barreto, A.P.M.; Souza, E.; Klein, M.R.S.T. High abdominal adiposity and low phase angle in overweight renal transplant recipients. Clin. Transplant 2019, 33, e13654. [Google Scholar] [CrossRef] [PubMed]
- Sarmento-Dias, M.; Santos-Araújo, C.; Poínhos, R.; Oliveira, B.; Sousa, M.; Simões-Silva, L.; Correia, F.; Pestana, M. Phase Angle Predicts Arterial Stiffness and Vascular Calcification in Peritoneal Dialysis Patients. Perit. Dial. Int. 2017, 37, 451–457. [Google Scholar] [CrossRef]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef]
- Yin, K.; Agrawal, D.K. Vitamin D and inflammatory diseases. J. Inflamm. Res. 2014, 7, 69–87. [Google Scholar]
- Nigatu, Y.D.; Gebreyesus, S.H.; Allard, J.P.; Endris, B.S. The effect of malnutrition at admission on length of hospital stay among adult patients in developing country: A prospective cohort study. Clin. Nutr. ESPEN 2021, 41, 217–224. [Google Scholar] [CrossRef]
- Reber, E.; Gomes, F.; Vasiloglou, M.F.; Schuetz, P.; Stanga, Z. Nutritional Risk Screening and Assessment. J. Clin. Med. 2019, 8, 1065. [Google Scholar] [CrossRef] [PubMed]
- Leão, L.L.; Engedal, K.; Monteiro-Junior, R.S.; Tangen, G.G.; Krogseth, M. Malnutrition is associated with impaired functional status in older people receiving home care nursing service. Front. Nutr. 2021, 8, 684438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, W.; Du, Y.; Zhang, J.; Zhang, Y.; Li, W.; Hu, W.; Zong, L.; Liu, Y.; Qin, H.; et al. A simple assessment model based on phase angle for malnutrition and prognosis in hospitalized cancer patients. Clin. Nutr. 2022, 41, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- González-Arellanes, R.; Urquidez-Romero, R.; Rodríguez-Tadeo, A.; Esparza-Romero, J.; Méndez-Estrada, R.O.; Ramírez-López, E.; Robles-Sardin, A.-E.; Pacheco-Moreno, B.-I.; Alemán-Mateo, H. High Hydration Factor in Older Hispanic-American Adults: Possible Implications for Accurate Body Composition Estimates. Nutrients 2019, 11, 2897. [Google Scholar] [CrossRef]
- Ritz, P.; Vol, S.; Berrut, G.; Tack, I.; Arnaud, M.J.; Tichet, J. Influence of gender and body composition on hydration and body water spaces. Clin. Nutr. 2008, 27, 740–746. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).