Long-Term Symptoms after Mild Coronavirus Disease in Healthy Healthcare Professionals: A 12-Month Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
2.2. Ethical Statement
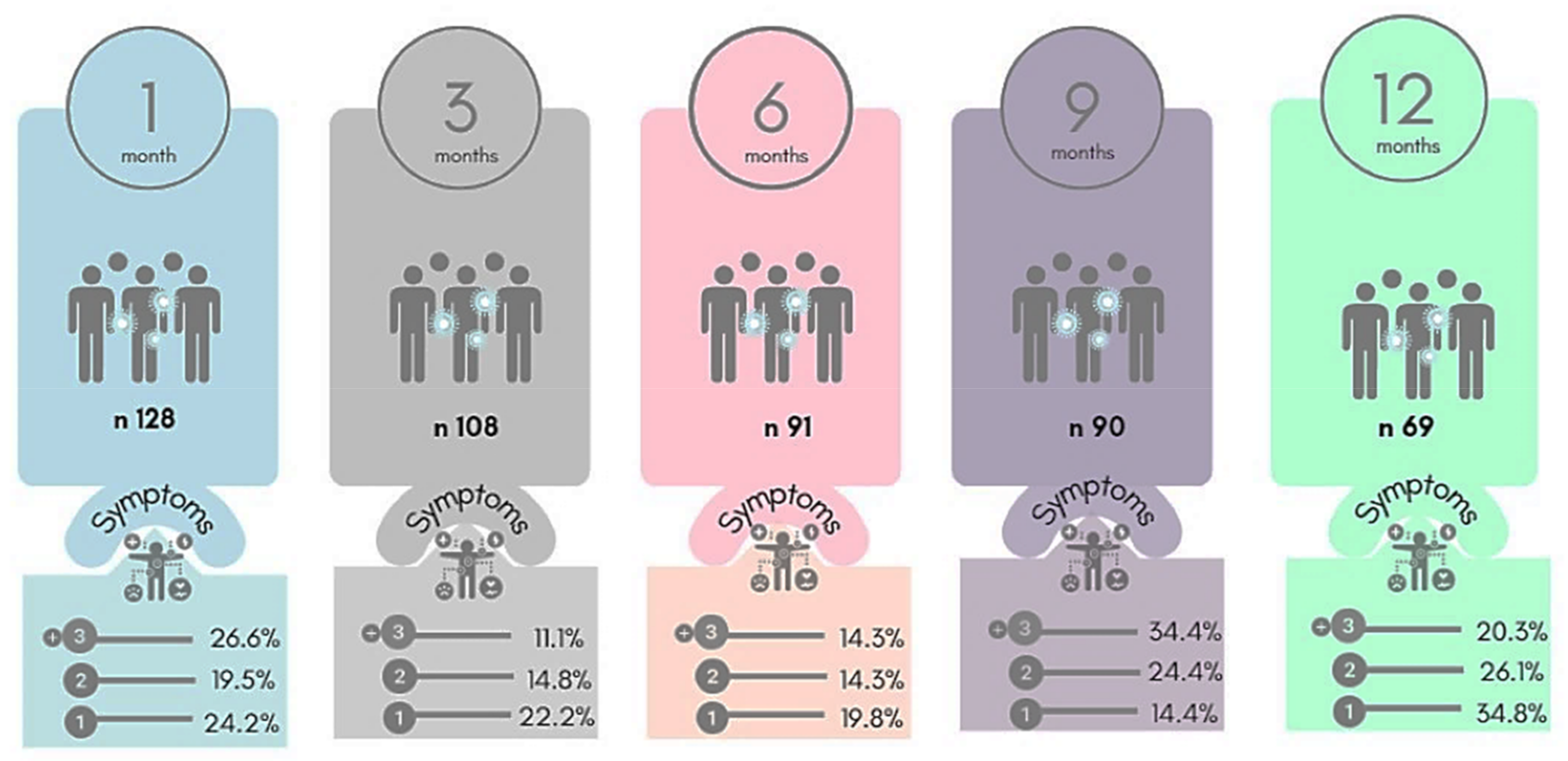
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19). Available online: https://covid19.who.int/ (accessed on 2 June 2022).
- Esakandari, H.; Nabi-Afjadi, M.; Fakkari-Afjadi, J.; Farahmandian, N.; Miresmaeili, S.M.; Bahreini, E. A comprehensive review of COVID-19 characteristics. Biol. Proced. Online 2020, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Ochani, R.; Asad, A.; Yasmin, F.; Shaikh, S.; Khalid, H.; Batra, S.; Sohail, M.R.; Mahmood, S.F.; Ochani, R.; Arshad, M.H.; et al. COVID-19 pandemic: From origins to outcomes. A comprehensive review of viral pathogenesis, clinical manifestations, diagnostic evaluation, and management. Infez. Med. 2021, 29, 20–36. [Google Scholar]
- To, K.K.-W.; Sridhar, S.; Chiu, K.H.-Y.; Hung, D.L.-L.; Li, X.; Hung, I.F.-N.; Tam, A.R.; Chung, T.W.-H.; Chan, J.F.-W.; Zhang, A.J.-X.; et al. Lessons learned 1 year after SARS-CoV-2 emergence leading to COVID-19 pandemic. Emerg. Microbes Infect. 2021, 10, 507–535. [Google Scholar] [CrossRef]
- Machhi, J.; Herskovitz, J.; Senan, A.M.; Dutta, D.; Nath, B.; Oleynikov, M.D.; Blomberg, W.R.; Meigs, D.D.; Hasan, M.; Patel, M.; et al. The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections. J. Neuroimmune Pharm. 2020, 15, 359–386. [Google Scholar] [CrossRef]
- Van Kessel, S.A.M.; Olde Hartman, T.C.; Lucassen, P.L.B.J.; van Jaarsveld, C.H.M. Post-acute and long-COVID-19 symptoms in patients with mild diseases: A systematic review. Fam. Pract. 2022, 39, 159–167. [Google Scholar] [CrossRef]
- Gavriatopoulou, M.; Korompoki, E.; Fotiou, D.; Ntanasis-Stathopoulos, I.; Psaltopoulou, T.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A. Organ-specific manifestations of COVID-19 infection. Clin. Exp. Med. 2020, 20, 493–506. [Google Scholar] [CrossRef]
- Asselah, T.; Durantel, D.; Pasmant, E.; Lau, G.; Schinazi, R.F. COVID-19: Discovery, diagnostics and drug development. J. Hepatol. 2021, 74, 168–184. [Google Scholar] [CrossRef]
- Ahn, D.-G.; Shin, H.-J.; Kim, M.-H.; Lee, S.; Kim, H.-S.; Myoung, J.; Kim, B.-T.; Kim, S.-J. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19). J. Microbiol. Biotechnol. 2020, 30, 313–324. [Google Scholar] [CrossRef]
- Munblit, D.; Bobkova, P.; Spiridonova, E.; Shikhaleva, A.; Gamirova, A.; Blyuss, O.; Nekliudov, N.; Bugaeva, P.; Andreeva, M.; DunnGalvin, A.; et al. Incidence and risk factors for persistent symptoms in adults previously hospitalized for COVID-19. Clin. Exp. Allergy 2021, 51, 1107–1120. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. COVID-19–Long COVID. Centers for Disease Control and Prevention. Updated 1 September 2022. Available online: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html (accessed on 28 November 2022).
- Desai, A.D.; Lavelle, M.; Boursiquot, B.C.; Wan, E.Y. Long-term complications of COVID-19. Am. J. Physiol. Cell Physiol. 2022, 322, 1–11. [Google Scholar] [CrossRef]
- Mayo Foundation for Medical Education and Research. COVID-19: Long-Term Effects. Available online: https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/coronavirus-long-term-effects/art-20490351/ (accessed on 9 November 2022).
- Rodriguez-Morales, A.J.; Cardona-Ospina, J.A.; Gutiérrez-Ocampo, E.; Villamizar-Peña, R.; Houlguin-Rivera, Y.; Escalera-Antezana, J.P.; Alvarado-Arnez, L.E.; Bonilda-Aldana, D.K.; Franco-Paredes, C.; Henao-Martinez, A.F.; et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2020, 34, 101623. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.A.; Meo, A.S.; Al-Jassir, F.F.; Klonoff, D.C. Omicron SARS-CoV-2 new variant: Global prevalence and biological and clinical characteristics. Eur. Rev. Med. Pharm. Sci. 2021, 25, 8012–8018. [Google Scholar] [CrossRef]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long covid-mechanisms, risk factors, and management. BMJ 2021, 374, 1648. [Google Scholar] [CrossRef]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef] [PubMed]
- Joshee, S.; Vatti, N.; Chang, C. Long-term effects of COVID-19. Mayo Clin. Proc. 2022, 97, 579–599. [Google Scholar] [CrossRef]
- Bonifácio, L.P.; Csizmar, V.N.F.; Barbosa-Júnior, F.; Pereira, A.P.S.; Koenigkam-Santos, M.; Wada, D.T.; Gaspar, G.; Carvalho, F.S.; Bollela, V.R.; Santana, R.C.; et al. Long-term symptoms among COVID-19 survivors in prospective cohort study, Brazil. Emerg. Infect. Dis. 2022, 28, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Fernández-De-Las-Peñas, C.; Gómez-Mayordomo, V.; Cuadrado, M.L.; Palacios-Ceña, D.; Florencio, L.L.; Guerrero, A.L.; García-Azorín, D.; Hernández-Barrera, V.; Arendt-Nielsen, L. The presence of headache at onset in SARS-CoV-2 infection is associated with long-term post-COVID headache and fatigue: A case-control study. Cephalalgia 2021, 41, 1332–1341. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent symptoms in patients after acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Cañas, C.A. The triggering of post-COVID-19 autoimmunity phenomena could be associated with both transient immunosuppression and an inappropriate form of immune reconstitution in susceptible individuals. Med. Hypotheses 2020, 145, 110345. [Google Scholar] [CrossRef]
- Peluso, M.J.; Deveau, T.M.; Munter, S.E.; Ryder, D.; Buck, A.; Lu, S.; Goldberg, S.A.; Hoh, R.; Tai, V.; Torres, L.; et al. Impact of pre-existing chronic viral infection and reactivation on the development of long COVID. MedRxiv 2022, e163669. [Google Scholar] [CrossRef] [PubMed]
- Bellan, M.; Soddu, D.; Balbo, P.E.; Baricich, A.; Zeppegno, P.; Avanzi, G.C.; Baldon, G.; Bartolomei, G.; Battaglia, M.; Battistini, S.; et al. Respiratory and Psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA 2021, 4, e2036142. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long Covid: An overview. Diab. Met. Syndr. Clin. Res. Rev. 2021, 15, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Afrin, L.B.; Weinstock, L.B.; Molderings, G.J. COVID-19 hyperinflammation and post-COVID-19 illness may be rooted in mast cell activation syndrome. Int. J. Infect. Dis. 2020, 100, 327–332. [Google Scholar] [CrossRef]
- Coomes, E.A.; Haghbayan, H. Interleukin-6 in COVID-19: A systematic review and meta-analysis. Rev. Med. Virol. 2020, 30, 1–9. [Google Scholar] [CrossRef]
- Sharifkashani, S.; Bafrani, M.A.; Khaboushan, A.S.; Pirzadeh, M.; Kheirandish, A.; Yavarpour_Bali, H.; Hessami, A.; Saghazadeh, A.; Rezaei, N. Angiotensin-converting enzyme 2 (ACE2) receptor and SARS-CoV-2: Potential therapeutic targeting. Eur. J. Pharm. 2020, 884, 173455. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef]
- Nguyen, B.; Tosti, A. Alopecia in patients with COVID-19: A systematic review and meta-analysis. JAAD Int. 2022, 7, 67–77. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Geng, D.; Mei, N.; Wu, P.-Y.; Huang, C.-C.; Jia, T.; Zhao, Y.; Wang, D.; Xiao, A.; et al. Cerebral micro-structural changes in COVID-19 patients–an MRI-based 3-month follow-up study. EClinicalMedicine 2020, 25, 100484. [Google Scholar] [CrossRef]
- Siemieniuk, R.A.; Bartoszko, J.J.; Ge, L.; Zeraatkar, D.; Izcovich, A.; Kum, E.; Pardo-Hernandez, H.; Qasim, A.; Martinez, J.P.D.; Rochwerg, B.; et al. Drug treatments for covid-19: Living systematic review and network meta-analysis. BMJ 2020, 370, 2980. [Google Scholar] [CrossRef] [PubMed]
- Boschiero, M.N.; Palamim, C.V.C.; Ortega, M.M.; Mauch, R.M.; Marson, F.A.L. One year of coronavirus disease 2019 (COVID-19) in Brazil: A political and social overview. Ann. Glob. Health 2021, 87, 1–27. [Google Scholar] [CrossRef]
- Tanni, S.E.; Bacha, H.A.; Naime, A.; Bernardo, W.M. Use of hydroxychloroquine to prevent SARS-CoV-2 infection and treat mild COVID-19: A systematic review and meta-analysis. J. Bras. Pneumol. 2021, 47, e20210236. [Google Scholar] [CrossRef] [PubMed]
- Younis, N.K.; Zareef, R.O.; Hassan, S.N.A.; Bitar, F.; Eid, A.H.; Arabi, M. Hidroxychloroquine in COVID-19 patients: Pros and cons. Front. Pharm. 2020, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-C.; Wang, Y.-H.; Chen, Y.-L.; Tsai, W.-C.; Lee, C.-H.; Chuang, K.-P.; Chen, Y.-M.; Yuan, C.-H.; Ho, S.-Y.; Yang, M.-H.; et al. Chloroquine and Hydroxycholoroquine: Efficacy in the treatment of the COVID-19. Pathogens 2021, 10, 217. [Google Scholar] [CrossRef]
| Characteristic | Value | % |
|---|---|---|
| Age, mean years (SD) | 38.4 (10.2) | |
| Sex | ||
| Female | 94 | 63.9 |
| Male | 53 | 36.1 |
| Race | ||
| White | 48 | 32.7 |
| Mixed | 78 | 53.1 |
| Black | 17 | 11.6 |
| Asian | 4 | 2.6 |
| Occupation | ||
| Healthcare worker | 110 | 74.8 |
| Safety worker | 18 | 12.3 |
| Others | 19 | 12.9 |
| Comorbidities | ||
| Hypertension | 57 | 38.8 |
| Asthma/bronchitis | 34 | 23.1 |
| Chronic cardiac disease | 14 | 9.5 |
| Chronic renal disease | 8 | 5.4 |
| Diabetes mellitus | 6 | 4.1 |
| Chronic gastrointestinal disease | 2 | 1.4 |
| Chronic liver disease | 1 | 0.7 |
| Human immunodeficiency virus infection | 1 | 0.7 |
| Medicines used | ||
| Azithromycin | 64 | 43.5 |
| Ivermectin | 57 | 38.8 |
| Hydroxychloroquine | 16 | 10.9 |
| Analgesics, antipyretics, and vitamins | 75 | 51.0 |
| Other medicine | 58 | 39.5 |
| Duration of follow-up, median (IQR), days | 290 (57–372) | |
| Duration of SARS-CoV-2 RNA positivity, median (IQR), days | 14 (9–32) |
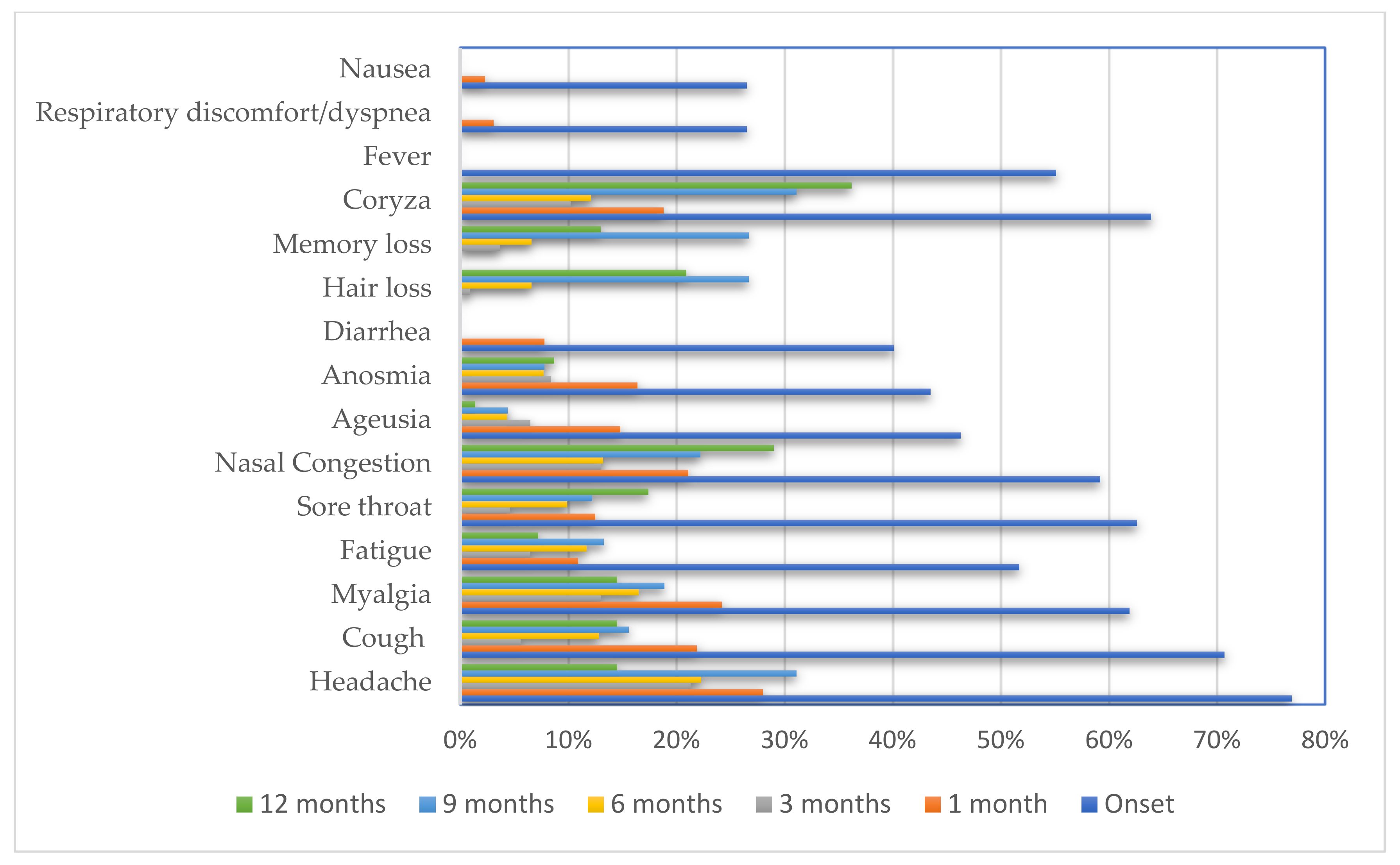
| Characteristic | N | % |
|---|---|---|
| Headache | 113 | 76.9 |
| Cough | 104 | 70.7 |
| Coryza | 94 | 63.9 |
| Sore throat | 92 | 62.6 |
| Myalgia | 91 | 61.9 |
| Nasal congestion | 87 | 59.2 |
| Fatigue | 76 | 51.7 |
| Fever | 81 | 55.1 |
| Ageusia | 68 | 46.3 |
| Anosmia | 64 | 43.5 |
| Diarrhea | 59 | 40.1 |
| Respiratory discomfort/dyspnea | 39 | 26.5 |
| Nausea | 39 | 26.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Costa e Silva, G.R.; Moura, W.É.A.; dos Santos, K.C.; Gomes, D.O.; Bandeira, G.N.; Guimarães, R.A.; Rosso, C.F.W.; Bazilio, G.S.; Leite, V.R.M.C.; Caetano, K.A.A.; et al. Long-Term Symptoms after Mild Coronavirus Disease in Healthy Healthcare Professionals: A 12-Month Prospective Cohort Study. Int. J. Environ. Res. Public Health 2023, 20, 1483. https://doi.org/10.3390/ijerph20021483
da Costa e Silva GR, Moura WÉA, dos Santos KC, Gomes DO, Bandeira GN, Guimarães RA, Rosso CFW, Bazilio GS, Leite VRMC, Caetano KAA, et al. Long-Term Symptoms after Mild Coronavirus Disease in Healthy Healthcare Professionals: A 12-Month Prospective Cohort Study. International Journal of Environmental Research and Public Health. 2023; 20(2):1483. https://doi.org/10.3390/ijerph20021483
Chicago/Turabian Styleda Costa e Silva, Grazielle Rosa, Winny Éveny Alves Moura, Kamila Cardoso dos Santos, Davi Oliveira Gomes, Gabriela Nolasco Bandeira, Rafael Alves Guimarães, Claci Fátima Weirich Rosso, Gabriela Silvério Bazilio, Vanessa Rafaela Milhomem Cruz Leite, Karlla Antonieta Amorim Caetano, and et al. 2023. "Long-Term Symptoms after Mild Coronavirus Disease in Healthy Healthcare Professionals: A 12-Month Prospective Cohort Study" International Journal of Environmental Research and Public Health 20, no. 2: 1483. https://doi.org/10.3390/ijerph20021483
APA Styleda Costa e Silva, G. R., Moura, W. É. A., dos Santos, K. C., Gomes, D. O., Bandeira, G. N., Guimarães, R. A., Rosso, C. F. W., Bazilio, G. S., Leite, V. R. M. C., Caetano, K. A. A., Carneiro, M. A. d. S., & Teles, S. A. (2023). Long-Term Symptoms after Mild Coronavirus Disease in Healthy Healthcare Professionals: A 12-Month Prospective Cohort Study. International Journal of Environmental Research and Public Health, 20(2), 1483. https://doi.org/10.3390/ijerph20021483






