Impact of Partnered Pharmacist Medication Charting (PPMC) on Medication Discrepancies and Errors: A Pragmatic Evaluation of an Emergency Department-Based Process Redesign
Abstract
1. Introduction
2. Materials and Methods
2.1. Partnered Pharmacist Medication Charting
2.2. Study Design, Population, and Period
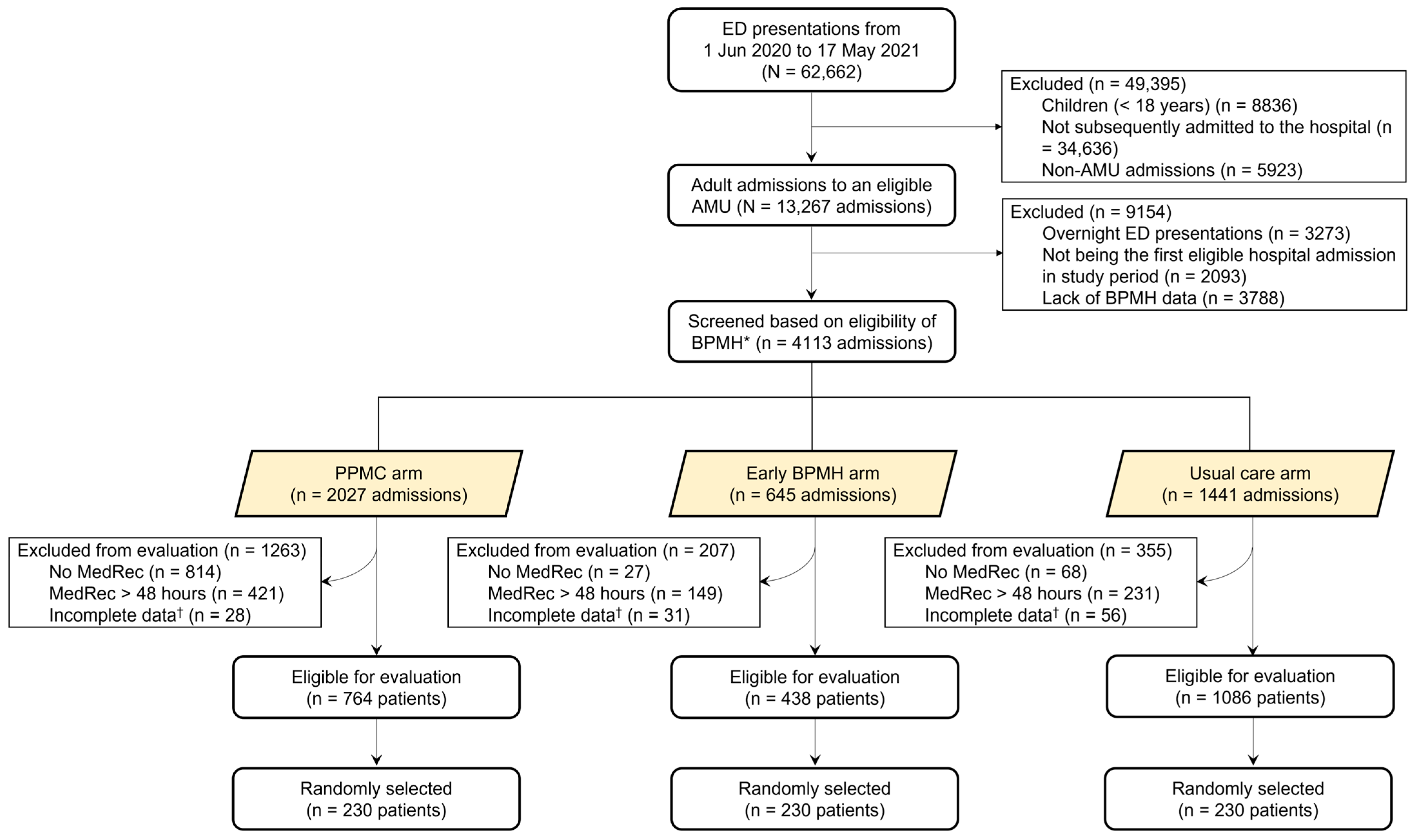
2.3. Assignment of Patients into Study Arms, and Inclusion and Exclusion Criteria
2.4. Study Arms
2.5. Data Collection
2.6. Outcome Measures
2.7. Data Analysis
2.8. Ethical Considerations
2.9. Sample Size Calculation
3. Results
3.1. Patient Characteristics
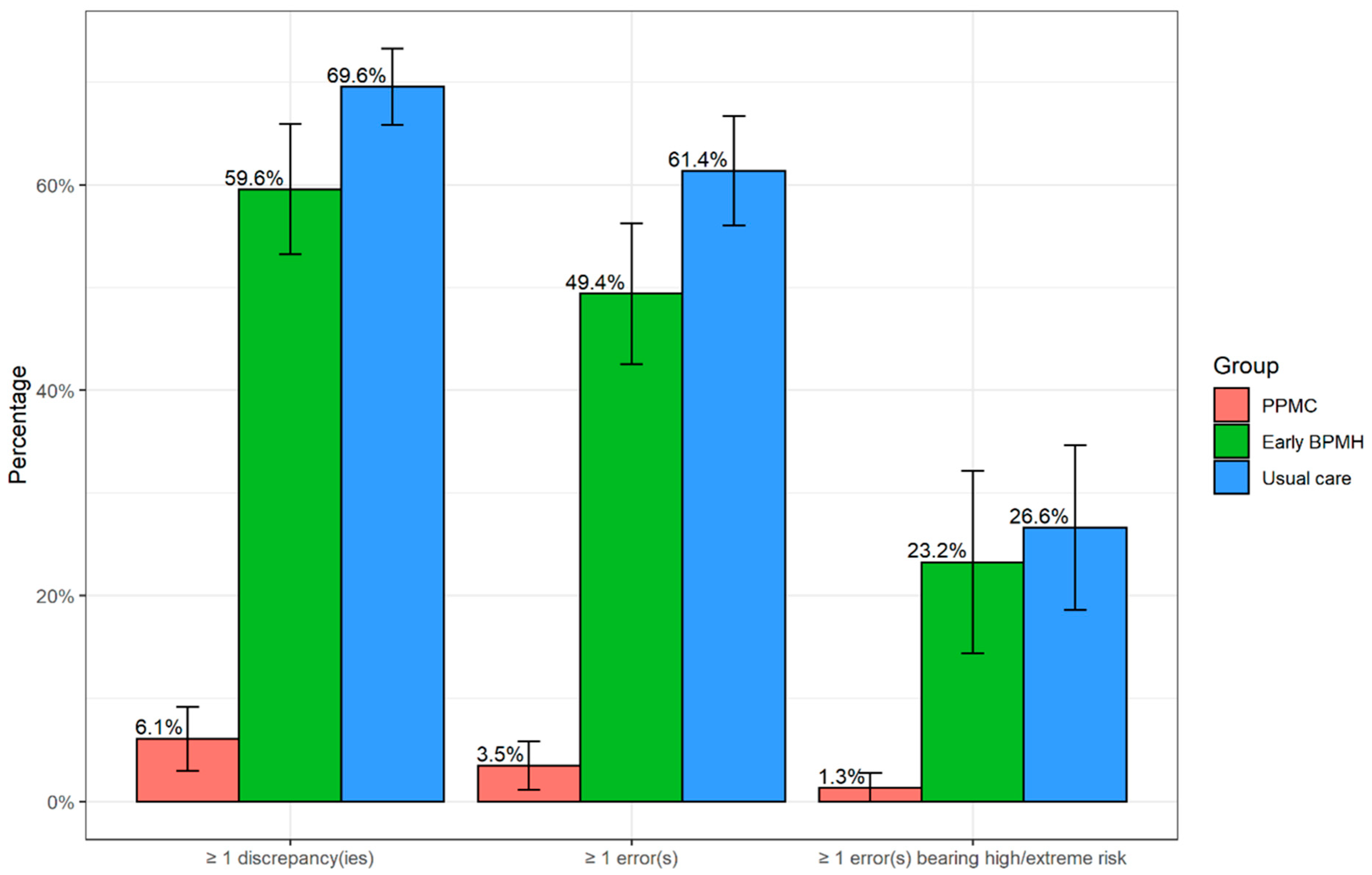
3.2. Prevalence and Types of Medication Discrepancies and Errors
3.3. Patients Having at Least One Error
3.4. Number of Errors per Medication Order
3.5. Errors Involving High-Risk and Time-Critical Medicines
4. Discussion
4.1. Clinical Implications
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Burstin, H. “Crossing the Quality Chasm” in emergency medicine. Acad. Emerg. Med. 2002, 9, 1074–1077. [Google Scholar] [CrossRef] [PubMed]
- Croskerry, P.; Shapiro, M.; Campbell, S.; LeBlanc, C.; Sinclair, D.; Wren, P.; Marcoux, M. Profiles in patient safety: Medication errors in the emergency department. Acad. Emerg. Med. 2004, 11, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Brown, M. Medication safety issues in the emergency department. Crit. Care Nurs. Clin. North Am. 2005, 17, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Cobaugh, D.J.; Schneider, S.M. Medication use in the emergency department: Why are we placing patients at risk? Am. J. Health Syst. Pharm. 2005, 62, 1832–1833. [Google Scholar] [CrossRef]
- National Coordinating Council for Medication Error Reporting and Prevention What is a Medication Error? Available online: https://www.nccmerp.org/about-medication-errors (accessed on 15 September 2021).
- Patanwala, A.E.; Warholak, T.L.; Sanders, A.B.; Erstad, B.L. A prospective observational study of medication errors in a tertiary care emergency department. Ann. Emerg. Med. 2010, 55, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Zeraatchi, A.; Talebian, M.-T.; Nejati, A.; Dashti-Khavidaki, S. Frequency and types of the medication errors in an academic emergency department in Iran: The emergent need for clinical pharmacy services in emergency departments. J. Res. Pharm. Pract. 2013, 2, 118–122. [Google Scholar]
- Shitu, Z.; Aung, M.M.T.; Tuan Kamauzaman, T.H.; Ab Rahman, A.F. Prevalence and characteristics of medication errors at an emergency department of a teaching hospital in Malaysia. BMC Health Serv. Res. 2020, 20, 56. [Google Scholar] [CrossRef]
- Anzan, M.; Alwhaibi, M.; Almetwazi, M.; Alhawassi, T.M. Prescribing errors and associated factors in discharge prescriptions in the emergency department: A prospective cross-sectional study. PLoS ONE 2021, 16, e0245321. [Google Scholar] [CrossRef]
- Rothschild, J.M.; Churchill, W.; Erickson, A.; Munz, K.; Schuur, J.D.; Salzberg, C.A.; Lewinski, D.; Shane, R.; Aazami, R.; Patka, J.; et al. Medication errors recovered by emergency department pharmacists. Ann. Emerg. Med. 2010, 55, 513–521. [Google Scholar] [CrossRef]
- Gleason, K.M.; McDaniel, M.R.; Feinglass, J.; Baker, D.W.; Lindquist, L.; Liss, D.; Noskin, G.A. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: An analysis of medication reconciliation errors and risk factors at hospital admission. J. Gen. Intern. Med. 2010, 25, 441–447. [Google Scholar] [CrossRef]
- Australian Commission on Safety and Quality in Health Care Quality Use of Medicines. Available online: https://www.safetyandquality.gov.au/our-work/medication-safety/quality-use-medicines (accessed on 11 September 2022).
- Egan, N. Medicine Safety Now a National Priority. Available online: https://www.australianageingagenda.com.au/royal-commission/medicine-safety-now-a-national-priority/ (accessed on 5 May 2022).
- World Health Organization. Medication without Harm. Available online: https://www.who.int/initiatives/medication-without-harm (accessed on 1 October 2022).
- Tong, E.Y.; Roman, C.; Mitra, B.; Yip, G.; Gibbs, H.; Newnham, H.; Smit, D.P.; Galbraith, K.; Dooley, M.J. Partnered pharmacist charting on admission in the General Medical and Emergency Short-stay Unit—A cluster-randomised controlled trial in patients with complex medication regimens. J. Clin. Pharm. Ther. 2016, 41, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Department of Health and Human Services Royal Hobart Hospital. Available online: https://www.health.tas.gov.au/hospitals/royal-hobart-hospital/about-royal-hobart-hospital (accessed on 13 September 2022).
- Tong, E.Y.; Roman, C.P.; Smit, D.V.; Newnham, H.; Galbraith, K.; Dooley, M.J. Partnered medication review and charting between the pharmacist and medical officer in the Emergency Short Stay and General Medicine Unit. Australas. Emerg. Nurs. J. 2015, 18, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Society of Hospital Pharmacists of Australia (SHPA) SHPA ClinCAT. Available online: https://www.shpa.org.au/cpd/shpa-clincat#:~:text=The%20ClinCAT%C2%AE%20achievement%20tool,equip%20them%20to%20perform%20evaluations (accessed on 13 August 2022).
- Society of Hospital Pharmacists of Australia (SHPA) Standards of Practice for Clinical Pharmacy Services. Available online: https://www.shpa.org.au/publications-resources/standards-of-practice/standards-of-practice-for-clinical-pharmacy-services (accessed on 14 December 2022).
- Australian Commission on Safety and Quality in Health Care Match Up Medicines: A Guide to Medication Reconciliation. Available online: https://www.safetyandquality.gov.au/publications-and-resources/resource-library/match-medicines-guide-medication-reconciliation (accessed on 10 March 2022).
- Australasian College for Emergency Medicine Policy on the Australasian Triage Scale. Available online: https://acem.org.au/Content-Sources/Advancing-Emergency-Medicine/Better-Outcomes-for-Patients/Triage (accessed on 5 May 2021).
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Australian Commission on Safety and Quality in Health Care (ACSQHC) APINCHS Classification of High Risk Medicines. Available online: https://www.safetyandquality.gov.au/our-work/medication-safety/high-risk-medicines/apinchs-classification-high-risk-medicines (accessed on 13 February 2021).
- Government of Western Australia Department of Health. Guiding Principles for Timely Administration of Medications. 2020. Available online: https://ww2.health.wa.gov.au/~/media/Files/Corporate/Policy-Frameworks/Clinical-Governance-Safety-and-Quality/Policy/High-Risk-Medication-Policy/Supporting/Guiding-Principles-for-Timely-Administration-of-Medications.pdf (accessed on 3 March 2021).
- Lisby, M.; Nielsen, L.P.; Brock, B.; Mainz, J. How are medication errors defined? A systematic literature review of definitions and characteristics. Int. J. Qual. Health Care 2010, 22, 507–518. [Google Scholar] [CrossRef] [PubMed]
- van den Bemt PM, L.A.; Schrieck-de Loos, E.M.; Linden, C.; Theeuwes AM, L.J.; Pol, A.G. Effect of medication reconciliation on unintentional medication discrepancies in acute hospital admissions of elderly adults: A multicenter study. J. Am. Geriatr. Soc. 2013, 61, 1262–1268. [Google Scholar] [CrossRef]
- Baena Parejo, M.I.; Juanes Borrego, A.M.; Altimiras Ruiz, J.; Crespi Monjo, M.; Garcia-Pelaez, M.; Calderon Hernanz, B.; Calleja Hernandez, M.A.; Chinchilla Fernandez, M.I.; Prats Riera, M.; Garcia Sanchez, R.; et al. Medication list assessment in Spanish hospital emergency departments. J. Emerg. Med. 2015, 48, 416–423. [Google Scholar] [CrossRef]
- Mogaka, B.; Clary, D.; Hong, C.; Farris, C.; Perez, S. Medication reconciliation in the emergency department performed by pharmacists. Bayl. Univ. Med. Cent. Proc. 2018, 31, 436–438. [Google Scholar] [CrossRef]
- Koehl, J.; Steffenhagen, A.; Halfpap, J. Implementation and impact of pharmacist-initiated home medication ordering in an emergency department observation unit. J. Pharm. Pract. 2019, 34, 459–464. [Google Scholar] [CrossRef]
- Tong, E.Y.; Mitra, B.; Yip, G.; Galbraith, K.; Dooley, M.J.; Group, P.R. Multi-site evaluation of partnered pharmacist medication charting and in-hospital length of stay. Br. J. Clin. Pharmacol. 2020, 86, 285–290. [Google Scholar] [CrossRef]
- The Society of Hospital Pharmacists of Australia (SHPA), SHPA Standards of Practice for Clinical Pharmacy. J. Pharm. Pract. Res. 2005, 35, 146.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Fleiss, J.L. Measuring nominal scale agreement among many raters. Psychol. Bull. 1971, 76, 378. [Google Scholar] [CrossRef]
- de Los Ángeles Parro Martín, M.; Muñoz García, M.; Delgado Silveira, E.; Martín-Aragón Álvarez, S.; Bermejo Vicedo, T. Intervention study for the reduction of medication errors in elderly trauma patients. J. Eval. Clin. Pract. 2021, 27, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G. Practical Statistics for Medical Research; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Patanwala, A.E.; Sanders, A.B.; Thomas, M.C.; Acquisto, N.M.; Weant, K.A.; Baker, S.N.; Merritt, E.M.; Erstad, B.L. A prospective, multicenter study of pharmacist activities resulting in medication error interception in the emergency department. Ann. Emerg. Med. 2012, 59, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Weeks, G.R.; Ciabotti, L.; Gorman, E.; Abbott, L.; Marriott, J.L.; George, J. Can a redesign of emergency pharmacist roles improve medication management? A prospective study in three Australian hospitals. Res. Soc. Adm. Pharm. 2014, 10, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Atey, T.M.; Peterson, G.M.; Salahudeen, M.S.; Bereznicki, L.R.; Wimmer, B.C. Impact of pharmacist interventions provided in the emergency department on quality use of medicines: A systematic review and meta-analysis. Emerg. Med. J. 2022. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, V.L.; Hitchen, S.A.; Rawlins MD, M.; Tong, E.Y. Validating the Victorian partnered pharmacist charting model in the Western Australian setting. J. Pharm. Pract. Res. 2020, 50, 456–457. [Google Scholar] [CrossRef]
- Taylor, S.; Hale, A.; Lewis, R.; Rowland, J. Collaborative doctor–pharmacist prescribing in the emergency department and admissions unit: A study of accuracy and safety. J. Pharm. Pract. Res. 2019, 49, 176–178. [Google Scholar] [CrossRef]
- Chen, H.H.; Taylor, S.E.; Harding, A.M.; Taylor, D.M. Accuracy of medication information sources compared to the best possible medication history for patients presenting to the emergency department. Emerg. Med. Australas. 2018, 30, 654–661. [Google Scholar] [CrossRef]
- Pham, J.C.; Andrawis, M.; Shore, A.D.; Fahey, M.; Morlock, L.; Pronovost, P.J. Are temporary staff associated with more severe emergency department medication errors? J. Healthc. Qual. 2011, 33, 9–18. [Google Scholar] [CrossRef]
- Westbrook, J.I.; Raban, M.Z.; Walter, S.R.; Douglas, H. Task errors by emergency physicians are associated with interruptions, multitasking, fatigue and working memory capacity: A prospective, direct observation study. BMJ Qual Saf 2018, 27, 655–663. [Google Scholar] [CrossRef]
- Shitu, Z.; Moe Thwe Aung, M.; Tuan Kamauzaman, T.H.; Ab Rahman, A.F. Factors associated with medication errors at a teaching hospital in Malaysia. Hosp. Pharm. 2021, 56, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Nymoen, L.D.; Tran, T.; Walter, S.R.; Lehnbom, E.C.; Tunestveit, I.K.; Øie, E.; Viktil, K.K. Emergency department physicians’ distribution of time in the fast paced-workflow-a novel time-motion study of drug-related activities. Int. J. Clin. Pharm. 2022, 44, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Beks, H.; Namara, K.M.; Manias, E.; Dalton, A.; Tong, E.; Dooley, M. Hospital pharmacists’ experiences of participating in a partnered pharmacist medication charting credentialing program: A qualitative study. BMC Health Serv. Res. 2021, 21, 251. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, H.E.; Weiss, D.J. Interrater reliability and agreement of subjective judgments. J. Couns. Psychol. 1975, 22, 358. [Google Scholar] [CrossRef]

| Variables | Study Group | p-Value | |||
|---|---|---|---|---|---|
| PPMC (N = 230 Patients) | Early BPMH (N = 230 Patients) | Usual Care (N = 588 Patients) | Overall | Pairwise | |
| Sex female, n (%) | 122 (53%) | 111 (48%) | 310 (53%) | 0.47 * | |
| Age in years, median (IQR) | 79 (68, 87) | 72 (59, 82) | 72 (62, 81) | <0.001 † | <0.001 ‡,§, 0.93 ¶ |
| <65 years, n (%) | 44 (19%) | 80 (35%) | 189 (32%) | <0.001 * | |
| ≥65 years, n (%) | 186 (81%) | 150 (65%) | 399 (68%) | ||
| CCI, median (IQR) | 5 (4, 6) | 4 (3, 6) | 4 (3, 5.3) | <0.001 † | <0.001 ‡,§, 0.93 ¶ |
| ATS, median (IQR) | 3 (3, 4) | 3 (2, 4) | 3 (2, 3) | 0.002 † | 0.003 ‡, 0.45 §, 0.03 ¶ |
| Medicines, median (IQR) | |||||
| Preadmission medicines | 10 (7, 14) | 9 (5, 13) | 9 (6, 13) | 0.02 † | 0.02 ‡, 0.04 §, 0.95 ¶ |
| Initially charted medicines | 10 (8, 14) | 10 (7, 12) | 9 (6, 12) | <0.001 † | <0.001 ‡, 0.02 §, 0.03 ¶ |
| Acute admission units, n (%) | <0.001 ‖ | ||||
| Cardiology | 0 (0%) | 16 (7.0%) | 67 (11%) | ||
| Emergency Medicine | 27 (12%) | 40 (17%) | 112 (19%) | ||
| General Medicine | 203 (88%) | 123 (53%) | 220 (37%) | ||
| Psychiatry | 0 (0%) | 2 (0.9%) | 15 (2.6%) | ||
| Respiratory Medicine | 0 (0%) | 8 (3.5%) | 56 (9.5%) | ||
| Stroke | 0 (0%) | 13 (5.7%) | 67 (11%) | ||
| Others # | 0 (0%) | 28 (12%) | 51 (8.7%) | ||
| Time from ED arrival (hours) | |||||
| BPMH, median (IQR) | 4 (3, 6) | 4 (3, 6) | 25 (20, 42) | <0.001† | <0.001‡,¶, 0.79 § |
| MedRec, median (IQR) | 24 (21, 29) | 21 (11, 30) | 27 (21, 44) | <0.001† | <0.001‡,§,¶ |
| Outcome | Stud Group | ||
|---|---|---|---|
| PPMC (N = 230 Patients) | Early BPMH (N = 230 Patients) | Usual Care (N = 588 Patients) | |
| Total discrepancies | 22 | 360 | 1042 |
| Discrepancy types, n (% of discrepancies) | |||
| Omitted drug * | 18 (81.8%) | 247 (68.6%) | 673 (64.6%) |
| Different drug | 0 (0%) | 11 (3.1%) | 20 (1.9%) |
| Added drug | 0 (0%) | 11 (3.1%) | 38 (3.6%) |
| Different dose † | 2 (9.1%) | 44 (12.2%) | 160 (15.4%) |
| Different frequency † | 2 (9.1%) | 32 (8.9%) | 105 (10.1%) |
| Different route | 0 (0%) | 0 (0%) | 3 (0.3%) |
| Different dosage form | 0 (0%) | 0 (0%) | 10 (1.0%) |
| Incomplete order | 0 (0%) | 15 (4.2%) | 33 (3.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atey, T.M.; Peterson, G.M.; Salahudeen, M.S.; Bereznicki, L.R.; Simpson, T.; Boland, C.M.; Anderson, E.; Burgess, J.R.; Huckerby, E.J.; Tran, V.; et al. Impact of Partnered Pharmacist Medication Charting (PPMC) on Medication Discrepancies and Errors: A Pragmatic Evaluation of an Emergency Department-Based Process Redesign. Int. J. Environ. Res. Public Health 2023, 20, 1452. https://doi.org/10.3390/ijerph20021452
Atey TM, Peterson GM, Salahudeen MS, Bereznicki LR, Simpson T, Boland CM, Anderson E, Burgess JR, Huckerby EJ, Tran V, et al. Impact of Partnered Pharmacist Medication Charting (PPMC) on Medication Discrepancies and Errors: A Pragmatic Evaluation of an Emergency Department-Based Process Redesign. International Journal of Environmental Research and Public Health. 2023; 20(2):1452. https://doi.org/10.3390/ijerph20021452
Chicago/Turabian StyleAtey, Tesfay Mehari, Gregory M. Peterson, Mohammed S. Salahudeen, Luke R. Bereznicki, Tom Simpson, Camille M. Boland, Ed Anderson, John R. Burgess, Emma J. Huckerby, Viet Tran, and et al. 2023. "Impact of Partnered Pharmacist Medication Charting (PPMC) on Medication Discrepancies and Errors: A Pragmatic Evaluation of an Emergency Department-Based Process Redesign" International Journal of Environmental Research and Public Health 20, no. 2: 1452. https://doi.org/10.3390/ijerph20021452
APA StyleAtey, T. M., Peterson, G. M., Salahudeen, M. S., Bereznicki, L. R., Simpson, T., Boland, C. M., Anderson, E., Burgess, J. R., Huckerby, E. J., Tran, V., & Wimmer, B. C. (2023). Impact of Partnered Pharmacist Medication Charting (PPMC) on Medication Discrepancies and Errors: A Pragmatic Evaluation of an Emergency Department-Based Process Redesign. International Journal of Environmental Research and Public Health, 20(2), 1452. https://doi.org/10.3390/ijerph20021452











