Direct Oral Anticoagulants versus Vitamin K Antagonists in Individuals Aged 80 Years and Older: An Overview in 2021
Abstract
1. Introduction
2. Methods
2.1. Design of the Study
2.2. Subjects
2.3. Collected Data
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Population Par Âge—Tableaux de l’économie Française | Insee. Available online: https://www.insee.fr/fr/statistiques/3303333?sommaire=3353488 (accessed on 30 August 2022).
- Arai, H.; Ouchi, Y.; Yokode, M.; Ito, H.; Uematsu, H.; Eto, F.; Oshima, S.; Ota, K.; Saito, Y.; Sasaki, H.; et al. Toward the Realization of a Better Aged Society: Messages from Gerontology and Geriatrics: Realization of a Better Aged Society. Geriatr. Gerontol. Int. 2012, 12, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-J.; Wolf, P.A.; Kelly-Hayes, M.; Beiser, A.S.; Kase, C.S.; Benjamin, E.J.; D’Agostino, R.B. Stroke Severity in Atrial Fibrillation. Stroke 1996, 27, 1760–1764. [Google Scholar] [CrossRef] [PubMed]
- ISTH Steering Committee for World Thrombosis Day. Thrombosis: A Major Contributor to the Global Disease Burden. J. Thromb. Haemost. 2014, 12, 1580–1590. [Google Scholar] [CrossRef]
- Ageno, W.; Gallus, A.S.; Wittkowsky, A.; Crowther, M.; Hylek, E.M.; Palareti, G. Oral Anticoagulant Therapy. Chest 2012, 141, e44S–e88S. [Google Scholar] [CrossRef] [PubMed]
- Loriot, M.-A.; Beaune, P. La Vitamine K Epoxyde Réductase: Du Sang Neuf dans les Traitements Anticoagulants Oraux. Rev. Med. Interne 2006, 27, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Galanaud, J.-P.; Laroche, J.-P.; Righini, M. The History and Historical Treatments of Deep Vein Thrombosis. J. Thromb. Haemost. 2013, 11, 402–411. [Google Scholar] [CrossRef]
- EAFT (European Atrial Fibrillation Trial) Study Group. Secondary Prevention in Non-Rheumatic Atrial Fibrillation after Transient Ischaemic Attack or Minor Stroke. Lancet 1993, 342, 1255–1262. [Google Scholar] [CrossRef]
- Les Anticoagulants Oraux. Available online: https://www.has-sante.fr/jcms/c_2851086/fr/les-anticoagulants-oraux (accessed on 31 August 2022).
- Aronis, K.N.; Hylek, E.M. Evidence Gaps in the Era of Non–Vitamin K Oral Anticoagulants. J. Am. Heart. Assoc. 2018, 7, e007338. [Google Scholar] [CrossRef]
- Chen, A.; Stecker, E.; Warden, B.A. Direct Oral Anticoagulant Use: A Practical Guide to Common Clinical Challenges. J. Am. Heart. Assoc. 2020, 9, e017559. [Google Scholar] [CrossRef]
- Hoffmann, C.; Leven, C.; Le Mao, R.; De Moreuil, C.; Lacut, K. Anticoagulants Oraux Directs: Dans Quelles Indications? Lequel Prescrire? Pour ou Contre chez les Personnes Fragiles et dans les Situations Atypiques? Quelle Surveillance et Gestion des Accidents Hémorragiques? Rev. Meéd. Interne 2020, 41, 598–606. [Google Scholar] [CrossRef]
- Tektonidou, M.G.; Andreoli, L.; Limper, M.; Amoura, Z.; Cervera, R.; Costedoat-Chalumeau, N.; Cuadrado, M.J.; Dörner, T.; Ferrer-Oliveras, R.; Hambly, K.; et al. EULAR Recommendations for the Management of Antiphospholipid Syndrome in Adults. Ann. Rheum. Dis. 2019, 78, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.-C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the Management of Atrial Fibrillation Developed in Collaboration with EACTS. Eur. Heart. J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef] [PubMed]
- Salazar, C.A.; del Aguila, D.; Cordova, E.G. Direct Thrombin Inhibitors versus Vitamin K Antagonists for Preventing Cerebral or Systemic Embolism in People with Non-valvular Atrial Fibrillation. Cochrane. Database. Syst. Rev. 2014, 2014, CD009893. [Google Scholar] [CrossRef]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the Efficacy and Safety of New Oral Anticoagulants with Warfarin in Patients with Atrial Fibrillation: A Meta-Analysis of Randomised Trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef] [PubMed]
- van der Hulle, T.; Kooiman, J.; den Exter, P.L.; Dekkers, O.M.; Klok, F.A.; Huisman, M.V. Effectiveness and Safety of Novel Oral Anticoagulants as Compared with Vitamin K Antagonists in the Treatment of Acute Symptomatic Venous Thromboembolism: A Systematic Review and Meta-Analysis. J. Thromb. Haemost. 2014, 12, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, R.P. Non-Vitamin K Antagonist Oral Anticoagulants in Older and Frail Patients with Atrial Fibrillation. Eur. Heart. J. Suppl. 2022, 24 (Suppl. A), A1–A10. [Google Scholar] [CrossRef]
- Manckoundia, P.; Nuemi, G.; Hacquin, A.; Menu, D.; Rosay, C.; Vovelle, J.; Nuss, V.; Baudin-Senegas, C.; Barben, J.; Putot, A. Direct Oral Anticoagulants versus Vitamin K Antagonists in Patients Aged 80 Years and Older. Int. J. Environ. Res. Public Health 2021, 18, 4443. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. Corrigendum to: 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Developed with the Special Contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 4194. [Google Scholar]
- ICD-10 Version 2016. Available online: https://icd.who.int/browse10/2016/en (accessed on 31 August 2022).
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 31 August 2022).
- Yamashita, T.; Suzuki, S.; Inoue, H.; Akao, M.; Atarashi, H.; Ikeda, T.; Okumura, K.; Koretsune, Y.; Shimizu, W.; Tsutsui, H.; et al. Two-Year Outcomes of More than 30 000 Elderly Patients with Atrial Fibrillation: Results from the All Nippon AF In the Elderly (ANAFIE) Registry. Eur. Heart J. Qual. Care Clin. Outcomes 2022, 8, 202–213. [Google Scholar] [CrossRef]
- Hanon, O.; Jeandel, C.; Jouanny, P.; Paccalin, M.; Puisieux, F.; Krolak-Salmon, P.; Berrut, G. Anticoagulant Treatment in Elderly Patients with Atrial Fibrillation: A Position Paper. Geriatr. Psychol. Neuropsychiatr. Vieil. 2019, 17, 341–354. [Google Scholar]
- Martinez, K.A.; Hurwitz, H.M.; Rothberg, M.B. Qualitative Analysis of Patient–Physician Discussions Regarding Anticoagulation for Atrial Fibrillation. JAMA. Intern. Med. 2022, 182, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Toorop, M.M.A.; van Rein, N.; Nierman, M.C.; Vermaas, H.W.; Huisman, M.V.; van der Meer, F.J.M.; Cannegieter, S.C.; Lijfering, W.M. Switching from Vitamin K Antagonists to Direct Oral Anticoagulants: Treatment Satisfaction and Patient Concerns. J. Thromb. Haemost. 2020, 18, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Drouin, L.; Gegu, M.; Mahe, J.; de Decker, L.; Berrut, G.; Chevalet, P. Observance du Traitement Anticoagulant Oral chez le Sujet Âgé à l’ère des anticoagulants Oraux Directs. Ann. Cardiol. Angeiol. (Paris) 2017, 66, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [PubMed]
- Lip, G.Y.H.; Keshishian, A.; Kang, A.; Dhamane, A.D.; Luo, X.; Klem, C.; Rosenblatt, L.; Mardekian, J.; Jiang, J.; Yuce, H.; et al. Effectiveness and Safety of Oral Anticoagulants among Non-Valvular Atrial Fibrillation Patients with Polypharmacy. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 405–414. [Google Scholar] [CrossRef]
- Lutz, J.; Jurk, K.; Schinzel, H. Direct Oral Anticoagulants in Patients with Chronic Kidney Disease: Patient Selection and Special Considerations. Int. J. Nephrol. Renovasc. Dis. 2017, 10, 135–143. [Google Scholar] [CrossRef]
- Parker, K.; Thachil, J. The Use of Direct Oral Anticoagulants in Chronic Kidney Disease. Br. J. Haematol. 2018, 183, 170–184. [Google Scholar] [CrossRef]
- Poli, D.; Antonucci, E.; Zanazzi, M.; Grifoni, E.; Testa, S.; Ageno, W.; Palareti, G. Impact of Glomerular Filtration Estimate on Bleeding Risk in Very Old Patients Treated with Vitamin K Antagonists: Results of EPICA Study on the Behalf of FCSA (Italian Federation of Anticoagulation Clinics). Thromb. Haemost. 2012, 107, 1100–1106. [Google Scholar] [CrossRef]
- Vitale, C.; Uchmanowicz, I. Frailty in Patients with Heart Failure. Eur. Heart. J. Suppl. 2019, 21, L12–L16. [Google Scholar] [CrossRef]
- Hoffman, M. Coagulation in Liver Disease. Clin. Gastroenterol. Hepatol. 2015, 41, 447–454. [Google Scholar] [CrossRef]
- Ballestri, S.; Capitelli, M.; Fontana, M.C.; Arioli, D.; Romagnoli, E.; Graziosi, C.; Lonardo, A.; Marietta, M.; Dentali, F.; Cioni, G. Direct Oral Anticoagulants in Patients with Liver Disease in the Era of Non-Alcoholic Fatty Liver Disease Global Epidemic: A Narrative Review. Adv. Ther. 2020, 37, 1910–1932. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Chan, Y.; Chang, S.; Tu, H.; Chen, S.; Yeh, Y.; Wu, L.; Kuo, C.; Kuo, C.; See, L. Effectiveness and Safety of Non–Vitamin K Antagonist Oral Anticoagulant and Warfarin in Cirrhotic Patients With Nonvalvular Atrial Fibrillation. J. Am. Heart Assoc. 2019, 8, e011112. [Google Scholar] [CrossRef] [PubMed]
- Steuber, T.D.; Howard, M.L.; Nisly, S.A. Direct Oral Anticoagulants in Chronic Liver Disease. Ann. Pharmacother. 2019, 53, 1042–1049. [Google Scholar] [CrossRef]
- European Medicines Agency. Eliquis®—Summary of Product Characteristics. 2014. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002148/WC500107728.pdf (accessed on 31 August 2022).
- Turco, L.; de Raucourt, E.; Valla, D.-C.; Villa, E. Anticoagulation in the Cirrhotic Patient. JHEP Rep. 2019, 1, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.A.; Trankle, C.R.; Eubanks, G.; Schumann, C.; Thompson, P.; Wallace, R.L.; Gottiparthi, S.; Ruth, B.; Kramer, C.M.; Salerno, M.; et al. Off-Label Use of Direct Oral Anticoagulants Compared With Warfarin for Left Ventricular Thrombi. JAMA. Cardiol. 2020, 5, 685. [Google Scholar] [CrossRef]
- Grossmann, K. Direct Oral Anticoagulants: A New Therapy against Alzheimer’s Disease? Neural. Regen. Res. 2021, 16, 1556. [Google Scholar] [CrossRef] [PubMed]
- Castrejón-Pérez, R.C.; Gutiérrez-Robledo, L.M.; Cesari, M.; Pérez-Zepeda, M.U. Diabetes Mellitus, Hypertension and Frailty: A Population-Based, Cross-Sectional Study of Mexican Older Adults. Geriatr. Gerontol. Int. 2017, 17, 925–930. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in Elderly People. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Kim, D.H.; Pawar, A.; Gagne, J.J.; Bessette, L.G.; Lee, H.; Glynn, R.J.; Schneeweiss, S. Frailty and Clinical Outcomes of Direct Oral Anticoagulants versus Warfarin in Older Adults with Atrial Fibrillation: A Cohort Study. Ann. Intern. Med. 2021, 174, 1214–1223. [Google Scholar] [CrossRef]
- Burnett, A.E.; Mahan, C.E.; Vazquez, S.R.; Oertel, L.B.; Garcia, D.A.; Ansell, J. Guidance for the Practical Management of the Direct Oral Anticoagulants (DOACs) in VTE Treatment. J. Thromb. Thrombolysis 2016, 41, 206–232. [Google Scholar] [CrossRef]
- Sindet-Pedersen, C.; Pallisgaard, J.L.; Staerk, L.; Berger, J.S.; Lamberts, M.; Torp-Pedersen, C.; Gislason, G.H.; Olesen, J.B. Temporal Trends in Initiation of VKA, Rivaroxaban, Apixaban and Dabigatran for the Treatment of Venous Thromboembolism—A Danish Nationwide Cohort Study. Sci. Rep. 2017, 7, 3347. [Google Scholar] [CrossRef] [PubMed]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace 2021, 23, 1612–1676. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Polymeris, A.A.; Macha, K.; Paciaroni, M.; Wilson, D.; Koga, M.; Cappellari, M.; Schaedelin, S.; Zietz, A.; Peters, N.; Seiffge, D.J.; et al. Oral Anticoagulants in the Oldest Old with Recent Stroke and Atrial Fibrillation. Ann. Neurol. 2022, 91, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Turpin, M.; Gregory, P. Direct Oral Anticoagulant Use and Risk of Diverticular Hemorrhage: A Systematic Review of the Literature. Can. J. Gastroenterol. Hepatol. 2019, 2019, 9851307. [Google Scholar] [CrossRef]
- Villa Zapata, L.; Hansten, P.D.; Panic, J.; Horn, J.R.; Boyce, R.D.; Gephart, S.; Subbian, V.; Romero, A.; Malone, D.C. Risk of Bleeding with Exposure to Warfarin and Nonsteroidal Anti-Inflammatory Drugs: A Systematic Review and Meta-Analysis. Thromb. Haemost. 2020, 120, 1066–1074. [Google Scholar] [CrossRef]
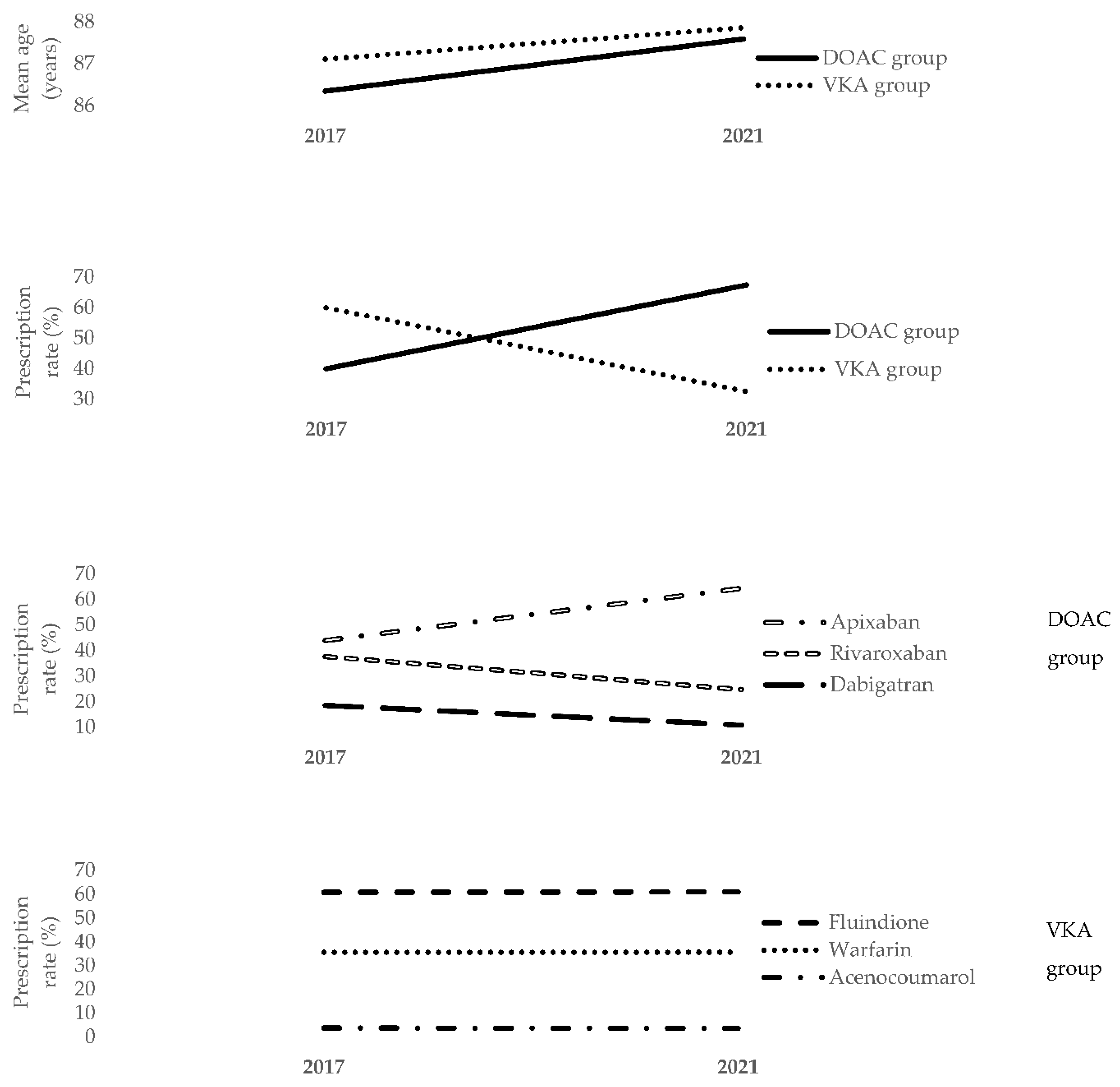
| Variables | DOAC Group (N = 2883) | VKA Group (N = 1392) | OR [95% CI] | p | |
|---|---|---|---|---|---|
| Mean ± SD or % (N) | Mean ± SD or % (N) | ||||
| Mean age (years) | 87.58 ± 4.52 | 87.85 ± 4.64 | 0.98 [0.97–1.00] | 0.075 | |
| Age range (years) | 80–84 | 28.27 (815) | 27.37 (381) | Reference | |
| 85–89 | 38.64 (1114) | 35.99 (501) | 1.04 [0.88–1.22] | 0.186 | |
| 90–94 | 25.42 (733) | 27.8 (387) | 0.89 [0.75–1.05] | 0.105 | |
| 95–99 | 7.18 (207) | 8.12 (113) | 0.86 [0.66–1.11] | 0.303 | |
| >100 | 0.49 (14) | 0.72 (10) | 0.65 [0.29–1.49] | 0.462 | |
| Sex | Women | 54.98 (1585) | 46.98 (654) | Reference | |
| Men | 45.02 (1298) | 53.02 (738) | 0.73 [0.64–0.83] | <0.001 | |
| RCD | No RCD | 8.53 (246) | 6.32 (88) | Reference | |
| ≥1 RCD | 91.47 (2637) | 93.68 (1304) | 0.72 [0.56–0.93] | 0.014 | |
| Mean number of RCD | 1.78 ± 1.12 | 2.05 ± 1.21 | 0.82 [0.78–0.86] | <0.001 | |
| Mean number of drugs/prescriptions | 7.40 ± 2.804 | 7.69 ± 2.98 | 0.97 [0.94–0.99] | 0.002 | |
| Anticoagulant duration | Initiation | 66.11 (1906) | 19.61 (273) | Reference | |
| Refill | 33.89 (977) | 80.39 (1119) | 0.12 [0.11–0.15] | <0.001 | |
| Prescriber specialty | General Practitioner | 86.78 (2502) | 92.03 (1281) | 0.57 [0.46–0.71] | <0.001 |
| Other specialties | 13.22 (381) | 7.97 (111) | Reference | ||
| Anticoagulant indication | AF | 41.55 (1198) | 47.20 (657) | 0.80 [0.70–0.90] | 0.001 |
| VTE | 2.53 (73) | 6.75 (94) | 0.36 [0.26–0.49] | <0.001 | |
| Deceased | 8.60 (248) | 9.41 (131) | 0.91 [0.73–1.13] | 0.389 | |
| Anticoagulation switch | 8.68 (246) | 1.14 (16) | 8.02 [4.83–13.36] | <0.001 | |
| Variables | DOAC Group (N = 2883) | VKA Group (N = 1392) | OR [95% CI] | p | |
|---|---|---|---|---|---|
| % (N) | % (N) | ||||
| RCD | Alzheimer’s disease | 7.49 (216) | 4.31 (60) | 0.56 [0.41–0.75] | <0.001 |
| Severe heart failure or heart rhythm disorders | 56.50 (1629) | 68.03 (947) | 1.64 [1.43–1.87] | <0.001 | |
| Severe hypertension | 17.31 (499) | 21.62 (301) | 1.32 [1.12–1.55] | 0.001 | |
| Severe chronic nephropathy and/or PNS | 1.38 (40) | 3.59 (50) | 2.65 [1.74–4.03] | <0.001 | |
| Severe chronic respiratory failure | 2.77 (80) | 4.17 (58) | 1.52 [1.08–2.15] | 0.021 | |
| Illnesses not on the list | 8.91 (257) | 14.08 (196) | 1.67 [1.37–2.04] | <0.001 | |
| Drug | Furosemide | 48.53 (1399) | 55.75 (776) | 1.34 [1.18–1.52] | <0.001 |
| Digoxin | 9.23 (266) | 12.07 (168) | 1.35 [1.10–1.66] | 0.005 | |
| Other antiarrhythmic drugs | 14.08 (406) | 11.71 (163) | 0.81 [0.67–0.98] | 0.035 | |
| Proton pump inhibitors | 43.95 (1267) | 39.44 (549) | 0.83 [0.73–0.95] | 0.006 | |
| Fibrates | 2.32 (67) | 3.88 (54) | 1.70 [1.18–2.44] | 0.006 | |
| Antirheumatics | 1.60 (46) | 0.65 (9) | 0.40 [0.20–0.82] | 0.009 | |
| Statins | 26.99 (778) | 30.60 (426) | 1.19 [1.04–1.37] | 0.015 | |
| Nitrate derivatives | 4.65 (134) | 6.39 (89) | 1.40 [1.06–1.85] | 0.019 | |
| Calcium channel blockers | 22.06 (636) | 24.86 (346) | 1.17 [1.01–1.36] | 0.044 | |
| Variables | OR [95% CI] | p |
|---|---|---|
| Male sex | 0.80 [0.69–0.93] | 0.003 |
| Refill prescriptions | 0.12 [0.15–0.11] | <0.001 |
| General practitioner as prescriber | 0.72 [0.56–0.94] | 0.014 |
| Atrial fibrillation | 1.30 [1.06–1.60] | 0.011 |
| VTE | 0.34 [0.23–0.50] | <0.001 |
| Severe heart failure or heart rhythm disorders | 0.68 [0.55–0.86] | 0.001 |
| Alzheimer’s disease | 1.83 [1.30–2.57] | 0.001 |
| RCD | 0.96 [0.86–0.99] | 0.036 |
| Severe chronic nephropathy and/or PNS | 0.31 [0.19–0.50] | <0.001 |
| Anticoagulation switching | 2.36 [1.34–4.16] | 0.003 |
| Beta-blockers | 1.12 [0.96–1.30] | 0.149 |
| Other antiarrhythmic drugs | 1.20 [0.96–1.49] | 0.112 |
| Furosemide | 0.76 [0.66–0.89] | 0.001 |
| Nitrate derivatives | 0.78 [0.57–1.08] | 0.135 |
| Fibrates | 0.54 [0.36–0.83] | 0.004 |
| Other cholesterol and triglyceride regulator | 0.58 [0.31–1.09] | 0.089 |
| Calcium channel blockers | 0.85 [0.71–1.01] | 0.065 |
| PPI | 1.21 [1.04–1.40] | 0.015 |
| Heparins | 0.51 [0.30–0.88] | 0.012 |
| Antirheumatics | 2.59 [1.19–5.61] | 0.016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azzoug, C.; Nuémi, G.; Menu, D.; De Maistre, E.; Boulin, M.; Putot, A.; Manckoundia, P. Direct Oral Anticoagulants versus Vitamin K Antagonists in Individuals Aged 80 Years and Older: An Overview in 2021. Int. J. Environ. Res. Public Health 2023, 20, 1448. https://doi.org/10.3390/ijerph20021448
Azzoug C, Nuémi G, Menu D, De Maistre E, Boulin M, Putot A, Manckoundia P. Direct Oral Anticoagulants versus Vitamin K Antagonists in Individuals Aged 80 Years and Older: An Overview in 2021. International Journal of Environmental Research and Public Health. 2023; 20(2):1448. https://doi.org/10.3390/ijerph20021448
Chicago/Turabian StyleAzzoug, Chana, Gilles Nuémi, Didier Menu, Emmanuel De Maistre, Mathieu Boulin, Alain Putot, and Patrick Manckoundia. 2023. "Direct Oral Anticoagulants versus Vitamin K Antagonists in Individuals Aged 80 Years and Older: An Overview in 2021" International Journal of Environmental Research and Public Health 20, no. 2: 1448. https://doi.org/10.3390/ijerph20021448
APA StyleAzzoug, C., Nuémi, G., Menu, D., De Maistre, E., Boulin, M., Putot, A., & Manckoundia, P. (2023). Direct Oral Anticoagulants versus Vitamin K Antagonists in Individuals Aged 80 Years and Older: An Overview in 2021. International Journal of Environmental Research and Public Health, 20(2), 1448. https://doi.org/10.3390/ijerph20021448







