Relationship of Motor Impairment with Cognitive and Emotional Alterations in Patients with Multiple Sclerosis
Abstract
1. Introduction
1.1. Motor Impairment
1.2. Cognitive and Emotional Impairment
2. Materials and Methods
2.1. Study Design
2.2. Sample
2.3. Methodology
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
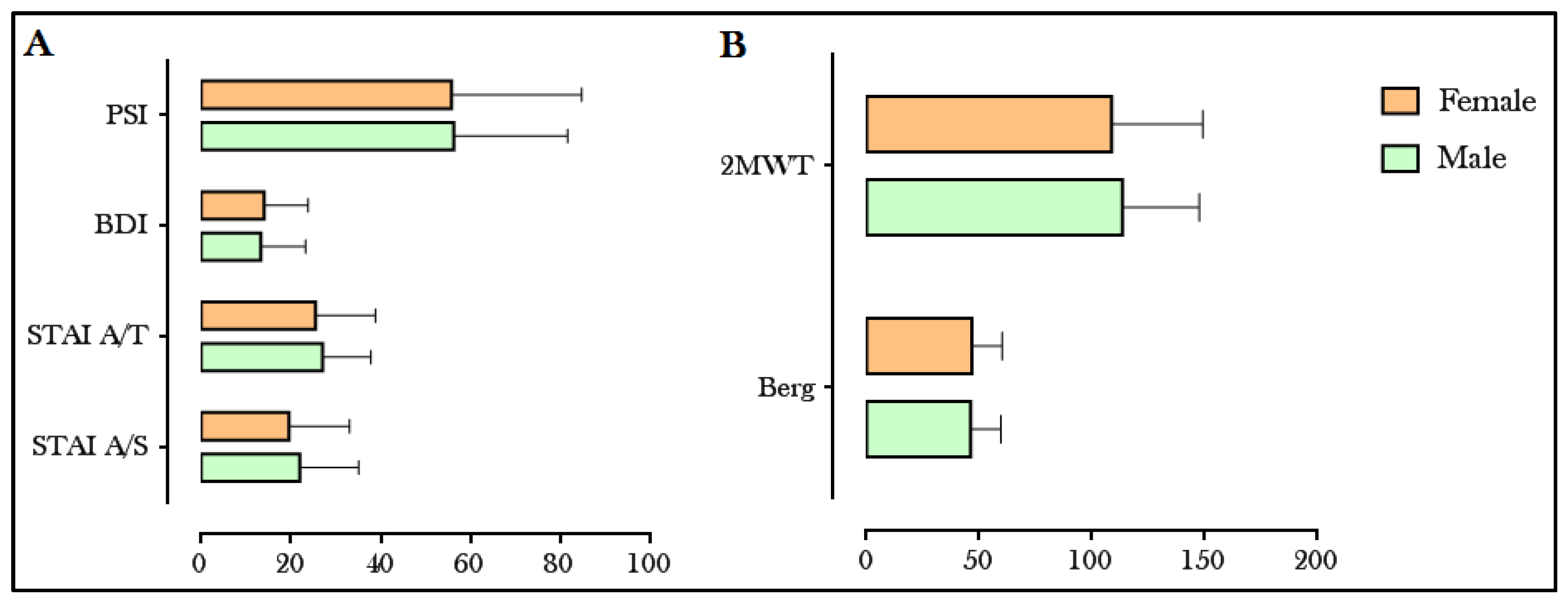
3.1. Motor, Cognitive and Behavioral Activity
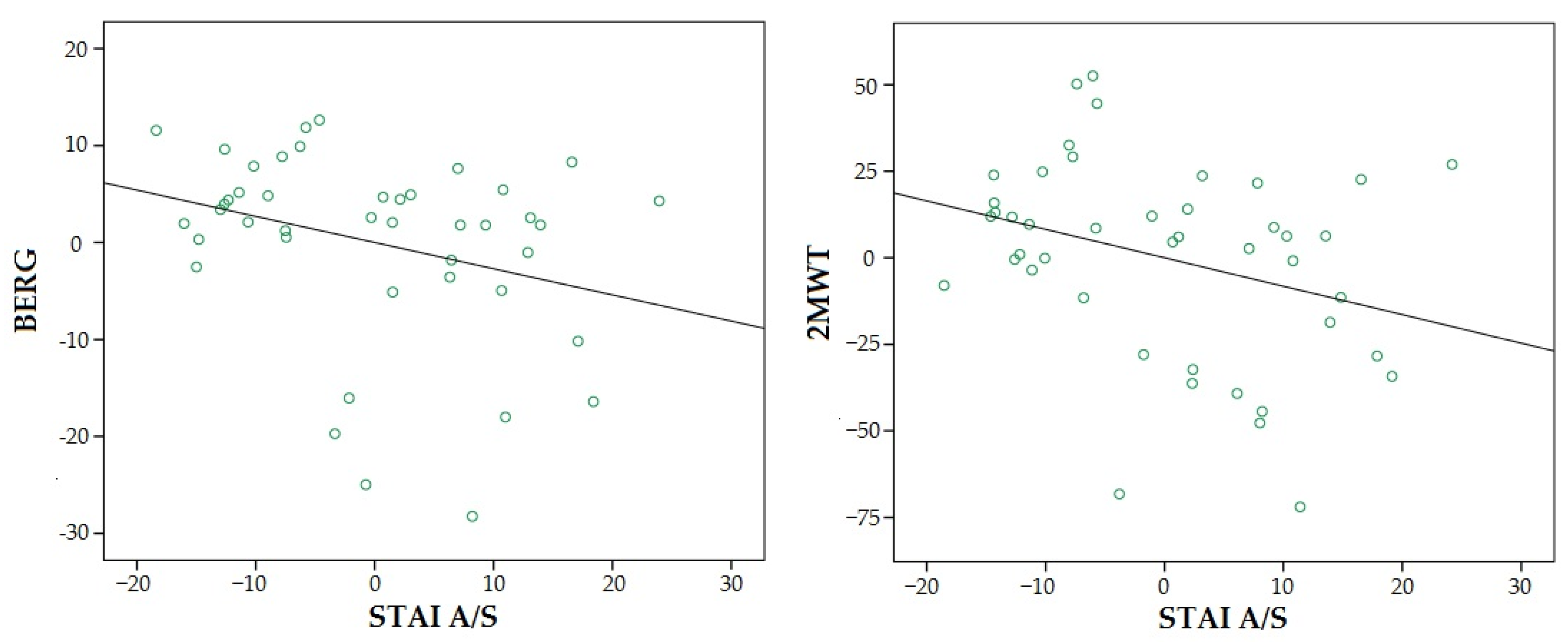
3.2. Relationship between Motor, Cognitive and Emotional Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Male | Female | |||
|---|---|---|---|---|
| M ± SD | M ± SD | t/u | p | |
| Age (years) | 46.5 ± 11.7 | 43.8 ± 11.3 | T | 0.403 |
| EDSS | 4.1 ± 2.1 | 3.1 ± 2 | U | 0.066 |
| STAI A/S | 22 ± 13 | 19.6 ± 13.1 | U | 0.509 |
| STAI A/T | 27 ± 10.8 | 25.5 ± 13.3 | T | 0.682 |
| BDI-II | 13.3 ± 9.9 | 14.07 ± 9.8 | U | 0.822 |
| PSI | 56.3 ± 25.3 | 55.7 ± 29 | T | 0.948 |
| Berg | 46.3 ± 13.3 | 46.7 ± 13.4 | U | 0.479 |
| 2MWT | 113.5 ± 34.1 | 108.8 ± 40.5 | U | 0.753 |
| Muscle mass (%) | 36.8 | 39.9 | T | 0.04 |
| BMI | 25.2 | 25.7 | T | 0.613 |
References
- Garg, N.; Smith, T.W. An update on immunopathogenesis, diagnosis, and treatment of multiple sclerosis. Brain Behav. 2015, 5, e00362. [Google Scholar] [CrossRef]
- Miller, J.R. The importance of early diagnosis of multiple sclerosis. J. Manag. Care Pharm. 2004, 10 (Suppl. B), S4–S11. [Google Scholar]
- Whitacre, C.C.; Reingold, S.C.; O’Looney, P.A.; Blankenhorn, E.; Brinley, F.; Collier, E.; Duquette, P.; Fox, H.; Giesser, B.; Gilmore, W.; et al. A gender gap in autoimmunity. Science 1999, 283, 1277–1278. [Google Scholar] [CrossRef] [PubMed]
- Loleit, V.; Biberacher, V.; Hemmer, B. Current and future therapies targeting the immune system in multiple sclerosis. Curr. Pharm. Biotechnol. 2014, 15, 276–296. [Google Scholar] [CrossRef]
- Motl, R.W.; Goldman, M.D.; Benedict, B. Walking impairment in patients with multiple sclerosis: Exercise training as a treatment option. Neuropsychiatr. Dis. Treat. 2010, 6, 767–774. [Google Scholar] [CrossRef]
- Silveira, S.L.; Cederberg, K.L.J.; Jeng, B.; Sikes, E.M.; Sandroff, B.M.; Jones, C.D.; Motl, R.W. Symptom clusters and quality of life in persons with multiple sclerosis across the lifespan. Qual. Life Res. 2020, 30, 1061–1071. [Google Scholar] [CrossRef]
- Snijders, A.H.; van de Warrenburg, B.P.; Giladi, N.; Bloem, B.R. Neurological gait disorders in elderly people: Clinical approach and classification. Lancet Neurol. 2007, 6, 63–74. [Google Scholar] [CrossRef]
- Sosnoff, J.J.; Boes, M.K.; Sandroff, B.M.; Socie, M.J.; Pula, J.H.; Motl, R.W. Walking and thinking in persons with multiple sclerosis who vary in disability. Arch. Phys. Med. Rehabil. 2011, 92, 2028–2033. [Google Scholar] [CrossRef]
- Eshaghi, A.; Prados, F.; Brownlee, W.J.; Altmann, D.R.; Tur, C.; Cardoso, M.J.; De Angelis, F.; van de Pavert, S.H.; Cawley, N.; De Stefano, N.; et al. Deep gray matter volume loss drives disability worsening in multiple sclerosis. Ann. Neurol. 2018, 83, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Rocca, M.A.; Barkhof, F.; Brück, W.; Chen, J.T.; Comi, G.; DeLuca, G.; de Stefano, N.; Erickson, B.J.; Evangelou, N.; et al. Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol. 2012, 11, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Storm, F.A.; Nair, K.; Clarke, A.J.; Van Der Meulen, J.M.; Mazzà, C. Free-living and laboratory gait characteristics in patients with multiple sclerosis. PLoS ONE 2018, 13, e0196463. [Google Scholar] [CrossRef] [PubMed]
- Spain, R.; George, R.S.; Salarian, A.; Mancini, M.; Wagner, J.; Horak, F.; Bourdette, D. Body-worn motion sensors detect balance and gait deficits in people with multiple sclerosis who have normal walking speed. Gait Posture 2012, 35, 573–578. [Google Scholar] [CrossRef]
- Calabrese, M.; Rinaldi, F.; Grossi, P.; Mattisi, I.; Bernardi, V.; Favaretto, A.; Perini, P.; Gallo, P. Basal ganglia and frontal/parietal cortical atrophy is associated with fatigue in relapsing—Remitting multiple sclerosis. Mult. Scler. J. 2010, 16, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Radetz, A.; Mladenova, K.; Ciolac, D.; Gonzalez-Escamilla, G.; Fleischer, V.; Ellwardt, E.; Krämer, J.; Bittner, S.; Meuth, S.G.; Muthuraman, M.; et al. Linking Microstructural Integrity and Motor Cortex Excitability in Multiple Sclerosis. Front. Immunol. 2021, 12, 748357. [Google Scholar] [CrossRef]
- Calabrese, M.; Agosta, F.; Rinaldi, F.; Mattisi, I.; Grossi, P.; Favaretto, A.; Atzori, M.; Bernardi, V.; Barachino, L.; Rinaldi, L.; et al. Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch. Neurol. 2009, 66, 1144–1150. [Google Scholar] [CrossRef]
- Matias-Guiu, J.A.; Cortés-Martínez, A.; Valles-Salgado, M.; Oreja-Guevara, C.; Pytel, V.; Montero, P.; Moreno-Ramos, T.; Matias-Guiu, J. Functional Components of Cognitive Impairment in Multiple Sclerosis: A Cross-Sectional Investigation. Front. Neurol. 2017, 8, 643. [Google Scholar] [CrossRef]
- Schulz, D.; Kopp, B.; Kunkel, A.; Faiss, J.H. Cognition in the early stage of multiple sclerosis. J. Neurol. 2006, 253, 1002–1010. [Google Scholar] [CrossRef]
- Hankomäki, E.; Multanen, J.; Kinnunen, E.; Hämäläinen, P. The progress of cognitive decline in newly diagnosed MS patients. Acta Neurol. Scand. 2013, 129, 184–191. [Google Scholar] [CrossRef]
- Guimarães, J.; Sá, M.J. Cognitive dysfunction in multiple sclerosis. Front. Neurol. 2012, 3, 74. [Google Scholar] [CrossRef]
- Malygin, V.L.; Boyko, A.N.; Konovalova, O.E.; Pahtusova, E.E.; Dumbrova, E.V.; Tishina, I.A.; Malygin, Y.V. Osobennosti trevozhnykh i depressivnykh rasstroĭstv u bol’nykh rasseiannym sklerozom na razlichnykh étapakh bolezni [Anxiety and depressive psychopathological characteristics of patients with multiple sclerosis at different stages of disease]. Zhurnal Nevrol. Psikhiatrii Im. SS Korsakova 2019, 119, 58–63. [Google Scholar] [CrossRef]
- Marrie, R.A.; Reingold, S.; Cohen, J.; Stuve, O.; Trojano, M.; Sorensen, P.S.; Cutter, G.; Reider, N. The incidence and prevalence of psychiatric disorders in multiple sclerosis: A systematic review. Mult. Scler. J. 2015, 21, 305–317. [Google Scholar] [CrossRef]
- Spielberger, C.D. La ansiedad como estado emocional. In Anxiety: Current Trends in Theory and Research; Spielberger, C.D., Ed.; Prensa Académica: New York, NY, USA, 1972; Volume 1, pp. 23–49. [Google Scholar] [CrossRef]
- Sandi, C.; Richter-Levin, G. From high anxiety trait to depression: A neurocognitive hypothesis. Trends Neurosci. 2009, 32, 312–320. [Google Scholar] [CrossRef]
- Hettema, J.M. What is the genetic relationship between anxiety and depression? Am. J. Med. Genet. Part C Semin. Med. Genet. 2008, 148C, 140–146. [Google Scholar] [CrossRef]
- Patten, S.B.; Marrie, R.A.; Carta, M.G. Depression in multiple sclerosis. Int. Rev. Psychiatry 2017, 29, 463–472. [Google Scholar] [CrossRef]
- Gay, M.-C.; Vrignaud, P.; Garitte, C.; Meunier, C. Predictors of depression in multiple sclerosis patients. Acta Neurol. Scand. 2010, 121, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Carandini, T.; Cercignani, M.; Galimberti, D.; Scarpini, E.; Bozzali, M. The distinct roles of monoamines in multiple sclerosis: A bridge between the immune and nervous systems? Brain Behav. Immun. 2021, 94, 381–391. [Google Scholar] [CrossRef]
- Koch-Henriksen, N.; Sørensen, P.S. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010, 9, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, B.A.; Chudzynski, J.; Storer, T.W.; Abrazado, M.; Penate, J.; Mooney, L.; Dickerson, D.; Rawson, R.A.; Cooper, C.B. Eight weeks of exercise training improves fitness measures in methamphetamine-dependent individuals in residential treatment. J. Addict. Med. 2013, 7, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Harbo, H.F.; Gold, R.; Tintore, M. Sex and gender issues in multiple sclerosis. Ther. Adv. Neurol. Disord. 2013, 6, 237–248. [Google Scholar] [CrossRef]
- Kalincik, T.; Vivek, V.; Jokubaitis, V.; Lechner-Scott, J.; Trojano, M.; Izquierdo, G.; Lugaresi, A.; Grand’Maison, F.; Hupperts, R.; Oreja-Guevara, C.; et al. Sex as a determinant of relapse incidence and progressive course of multiple sclerosis. Brain 2013, 136, 3609–3617. [Google Scholar] [CrossRef]
- Tomassini, V.; Pozzilli, C. Sex hormones, brain damage and clinical course of Multiple Sclerosis. J. Neurol. Sci. 2009, 286, 35–39. [Google Scholar] [CrossRef]
- Shirani, A.; Zhao, Y.; Karim, M.E.; Evans, C.; Kingwell, E.; van der Kop, M.L.; Oger, J.; Gustafson, P.; Petkau, J.; Tremlett, H. Association between use of interferon beta and progression of disability in patients with relapsing-remitting multiple sclerosis. JAMA 2012, 308, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Schoonheim, M.M.; Vigeveno, R.M.; Lopes, F.C.R.; Pouwels, P.J.; Polman, C.H.; Barkhof, F.; Geurts, J.J. Sex-specific extent and severity of white matter damage in multiple sclerosis: Implications for cognitive decline. Hum. Brain Mapp. 2013, 35, 2348–2358. [Google Scholar] [CrossRef] [PubMed]
- Marschallinger, R.; Mühlau, M.; Pongratz, V.; Kirschke, J.S.; Marschallinger, S.; Schmidt, P.; Sellner, J. Geostatistical Analysis of White Matter Lesions in Multiple Sclerosis Identifies Gender Differences in Lesion Evolution. Front. Mol. Neurosci. 2018, 11, 460. [Google Scholar] [CrossRef] [PubMed]
- Benedict, R.H.B.; Zivadinov, R. Risk factors for and management of cognitive dysfunction in multiple sclerosis. Nat. Rev. Neurol. 2011, 7, 332–342. [Google Scholar] [CrossRef]
- Golden, L.C.; Voskuhl, R. The importance of studying sex differences in disease: The example of multiple sclerosis. J. Neurosci. Res. 2016, 95, 633–643. [Google Scholar] [CrossRef]
- Kalron, A.; Aloni, R. Contrasting relationship between depression, quantitative gait characteristics and self-report walking difficulties in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 19, 1–5. [Google Scholar] [CrossRef]
- Haji, N.; Mandolesi, G.; Gentile, A.; Sacchetti, L.; Fresegna, D.; Rossi, S.; Musella, A.; Sepman, H.; Motta, C.; Studer, V.; et al. TNF-α-mediated anxiety in a mouse model of multiple sclerosis. Exp. Neurol. 2012, 237, 296–303. [Google Scholar] [CrossRef]
- Menculini, G.; Gentili, L.; Gaetani, L.; Mancini, A.; Sperandei, S.; Di Sabatino, E.; Chipi, E.; Salvadori, N.; Tortorella, A.; Parnetti, L.; et al. Clinical correlates of state and trait anxiety in multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 69, 104431. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Huang, S.; Wang, Y.; Lin, W.; Zhou, R.; Zou, H. Two-minute walk test: Reference equations for healthy adults in China. PLoS ONE 2018, 13, e0201988. [Google Scholar] [CrossRef]
- Langeskov-Christensen, D.; Feys, P.; Baert, I.; Riemenschneider, M.; Stenager, E.; Dalgas, U. Performed and perceived walking ability in relation to the Expanded Disability Status Scale in persons with multiple sclerosis. J. Neurol. Sci. 2017, 382, 131–136. [Google Scholar] [CrossRef]
- Kosak, M.; Smith, T. Comparison of the 2-, 6-, and 12-minute walk tests in patients with stroke. J. Rehabil. Res. Dev. 2004, 41, 103–107. [Google Scholar] [CrossRef]
- Downs, S. The Berg Balance Scale. J. Physiother. 2015, 61, 46. [Google Scholar] [CrossRef]
- Pérez, E.J.P.; De León, J.M.R.S.; Mota, G.R.; Alonso, S.M.; Aguilar, J.P.; Luque, I.L.; Sánchez, G. Inventario de síntomas prefrontales (ISP): Validez ecológica y convergencia con medidas neuropsicológicas. Rev. Neurol. 2016, 63, 241–251. [Google Scholar] [CrossRef]
- Sanz, J.; Vázquez, C. Fiabilidad, validez y datos normativos del Inventario para la Depresión de Beck. Psicothema 1998, 10, 303–318. [Google Scholar]
- Burgos Fonseca, P.; Gutiérrez Sepúlveda, A. Adaptación y Validación del Inventario Ansiedad Estado-Rasgo (STAI). 2013. Available online: http://repobib.ubiobio.cl/jspui/bitstream/123456789/265/3/BurgosFonseca_Pia.pdf (accessed on 14 April 2022).
- International Society for the Advancement of Kinanthropometry (ISAK). Topend Sports. 2008. Available online: https://www.topendsports.com/testing/isak.htm (accessed on 22 November 2022).
- Alvero Cruz RCaba-as Armesilla, M.; Herrero de Lucas, A.; Martínez Riaza, L.; Moreno Pascual, C.; Porta Manzanido, J.; Sillero Quintana, M.; Sirvent Belando, J. Protocolo de valoración de la composición corporal para el reconocimiento médico-deportivo. Documentos de consenso del grupo espaol de cineantropometría de la Federación Espa-ola de Medicina del Deportes. AMD 2009, 26, 166–179. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Wallis, O.; Bol, Y.; Köhler, S.; van Heugten, C. Anxiety in multiple sclerosis is related to depressive symptoms and cognitive complaints. Acta Neurol. Scand. 2019, 141, 212–218. [Google Scholar] [CrossRef]
- Leavitt, V.M.; Brandstadter, R.; Fabian, M.; Sand, I.K.; Klineova, S.; Krieger, S.; Lewis, C.; Lublin, F.; Miller, A.; Pelle, G.; et al. Dissociable cognitive patterns related to depression and anxiety in multiple sclerosis. Mult. Scler. J. 2019, 26, 1247–1255. [Google Scholar] [CrossRef]
- Magioncalda, P.; Martino, M.; Conio, B.; Lee, H.-C.; Ku, H.-L.; Chen, C.-J.; Inglese, M.; Amore, M.; Lane, T.J.; Northoff, G. Intrinsic brain activity of subcortical-cortical sensorimotor system and psychomotor alterations in schizophrenia and bipolar disorder: A preliminary study. Schizophr. Res. 2020, 218, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, S.N.; Dalley, S. The bipolar spectrum: Conceptions and misconceptions. Aust. N. Z. J. Psychiatry 2014, 48, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, Y.; Zhang, W.; Zhang, B.; Liu, X.; Mo, L.; Chen, Q. Multisensory Competition Is Modulated by Sensory Pathway Interactions with Fronto-Sensorimotor and Default-Mode Network Regions. J. Neurosci. 2015, 35, 9064–9077. [Google Scholar] [CrossRef]
- Conio, B.; Martino, M.; Magioncalda, P.; Escelsior, A.; Inglese, M.; Amore, M.; Northoff, G. Opposite effects of dopamine and serotonin on resting-state networks: Review and implications for psychiatric disorders. Mol. Psychiatry 2019, 25, 82–93. [Google Scholar] [CrossRef]
- Newland, P.; Salter, A.; Flach, A.; Flick, L.; Thomas, F.P.; Gulick, E.E.; Rantz, M.; Skubic, M. Associations Between Self-Reported Symptoms and Gait Parameters Using In-Home Sensors in Persons with Multiple Sclerosis. Rehabil. Nurs. 2019, 45, 80–87. [Google Scholar] [CrossRef]
- Ozsoy-Unubol, T.; Ata, E.; Cavlak, M.; Demir, S.; Candan, Z.; Yilmaz, F. Effects of Robot-Assisted Gait Training in Patients with Multiple Sclerosis. Am. J. Phys. Med. Rehabil. 2021, 101, 768–774. [Google Scholar] [CrossRef]
- Maggio, M.G.; Russo, M.; Cuzzola, M.F.; Destro, M.; La Rosa, G.; Molonia, F.; Bramanti, P.; Lombardo, G.; De Luca, R.; Calabrò, R.S. Virtual reality in multiple sclerosis rehabilitation: A review on cognitive and motor outcomes. J. Clin. Neurosci. 2019, 65, 106–111. [Google Scholar] [CrossRef]
- Barbarulo, A.M.; Lus, G.; Signoriello, E.; Trojano, L.; Grossi, D.; Esposito, M.; Costabile, T.; Lanzillo, R.; Saccà, F.; Morra, V.B.; et al. Integrated Cognitive and Neuromotor Rehabilitation in Multiple Sclerosis: A Pragmatic Study. Front. Behav. Neurosci. 2018, 12, 196. [Google Scholar] [CrossRef]
- Postigo-Alonso, B.; Galvao-Carmona, A.; Gavilan, C.C.; Jover, A.; Molina, S.; Peña-Toledo, M.A.; Valverde-Moyano, R.; Agüera, E. The effect of prioritization over cognitive-motor interference in people with relapsing-remitting multiple sclerosis and healthy controls. PLoS ONE 2019, 14, e02267755. [Google Scholar] [CrossRef]
- Voskuhl, R.R.; Gold, S. Sex-related factors in multiple sclerosis susceptibility and progression. Nat. Rev. Neurol. 2012, 8, 255–263. [Google Scholar] [CrossRef]
- Voskuhl, R.R.; Patel, K.; Paul, F.; Gold, S.M.; Scheel, M.; Kuchling, J.; Cooper, G.; Asseyer, S.; Chien, C.; Brandt, A.U.; et al. Sex differences in brain atrophy in multiple sclerosis. Biol. Sex Differ. 2020, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Zeydan, B.; Kantarci, O.H. Impact of Age on Multiple Sclerosis Disease Activity and Progression. Curr. Neurol. Neurosci. Rep. 2020, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Binzer, S.; Jiang, X.; Hillert, J.; Manouchehrinia, A. Depression and multiple sclerosis: A bidirectional Mendelian randomisation study. Mult. Scler. J. 2021, 27, 1799–1802. [Google Scholar] [CrossRef] [PubMed]
- Oreja-Guevara, C.; Ayuso Blanco, T.; Brieva Ruiz, L.; Hernández Pérez, M.Á.; Meca-Lallana, V.; Ramió-Torrentà, L. Cognitive Dysfunctions and Assessments in Multiple Sclerosis. Front. Neurol. 2019, 10, 581. [Google Scholar] [CrossRef]
- Meca-Lallana, V.; Gascón-Giménez, F.; Ginestal-López, R.C.; Higueras, Y.; Téllez-Lara, N.; Carreres-Polo, J.; Eichau-Madueño, S.; Romero-Imbroda, J.; Vidal-Jordana, Á.; Pérez-Miralles, F. Cognitive impairment in multiple sclerosis: Diagnosis and monitoring. Neurol. Sci. 2021, 42, 5183–5193. [Google Scholar] [CrossRef] [PubMed]
- Lansley, J.; Mataix-Cols, D.; Grau, M.; Radua, J.; Garriga, J.S. Localized grey matter atrophy in multiple sclerosis: A meta-analysis of voxel-based morphometry studies and associations with functional disability. Neurosci. Biobehav. Rev. 2013, 37, 819–830. [Google Scholar] [CrossRef]
- Zeqiraj, K.; Kruja, J.; Kabashi, S.; Mucaj, S. Epidemiological characteristics and functional disability of multiple sclerosis patients in Kosovo. Med. Arch. 2014, 68, 178–181. [Google Scholar] [CrossRef]
- Cameron, M.H.; Nilsagard, Y. Balance, gait, and falls in multiple sclerosis. Handb. Clin. Neurol. 2018, 159, 237–250. [Google Scholar] [CrossRef]
- Bollaert, R.E.; Jones, C.D.; Silic, P.; Motl, R.W. Depression, Anxiety, and Physical Activity in Older Adults with Multiple Sclerosis. J. Aging Phys. Act. 2022, 1, 1–7. [Google Scholar] [CrossRef]

| N (%) | ||
|---|---|---|
| MS type | Relapsing Remitting | 44 (65.7) |
| Secondary Progressive | 19 (28.4) | |
| Primary Progressive | 4 (5.9) | |
| Sex | Male | 20 (29.9) |
| Female | 47 (70.1) | |
| M ± SD | ||
| Age (y) | 44.6 ± 11.4 | |
| Time since diagnosis (y) | 12 ± 8.7 | |
| EDSS | 3.4 ± 2 | |
| Outcome | BERG | 2MWT | PSI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor coefficients | b | β | t | Sig. | B | β | t | Sig. | B | β | T | Sig. |
| STAI A/S | −0.27 | −0.27 | −1.98 | 0.055 | −0.82 | −0.25 | −2.10 | 0.042 | 1.44 | 0.58 | 4.75 | 0.000 |
| Age | −0.41 | −0.42 | −2.40 | 0.021 | −1.62 | −0.51 | −3.28 | 0.002 | 0.74 | 0.30 | 1.91 | 0.063 |
| Sex | 4.53 | 0.18 | 1.31 | 0.198 | 15.25 | 0.19 | 1.56 | 0.126 | −2.80 | −0.05 | −0.37 | 0.715 |
| Time from diagnosis | −0.23 | −0.19 | −1.06 | 0.295 | −0.77 | −0.20 | −1.22 | 0.230 | −0.89 | −0.30 | −1.81 | 0.079 |
| Model | F4, 38 = 5.07; p = 0.002 | F4, 39 = 8.76; p < 0.001 | F4, 40 = 7.79; p < 0.001 | |||||||||
| R2 = 0.59 | R2 = 0.69 | R2 = 0.66 | ||||||||||
| b | β | t | Sig. | B | β | t | Sig. | B | β | T | Sig. | |
| STAI A/T | −0.13 | −0.14 | −0.96 | 0.342 | −0.32 | −0.10 | −0.81 | 0.422 | 1.43 | 0.62 | 5.32 | 0.000 |
| Age | −0.40 | −0.41 | −2.22 | 0.033 | −1.64 | −0.52 | −3.10 | 0.004 | 0.48 | 0.20 | 1.28 | 0.209 |
| Sex | 3.30 | 0.13 | 0.94 | 0.354 | 12.43 | 0.15 | 1.23 | 0.225 | 1.04 | 0.02 | 0.14 | 0.886 |
| Time from diagnosis | −0.19 | −0.16 | −0.83 | 0.412 | −0.62 | −0.16 | −0.94 | 0.355 | −0.81 | −0.27 | −1.70 | 0.097 |
| Model | F4, 38 = 4.028; p = 0.008 | F4, 39 = 7.17; p < 0.001 | F4, 40 = 4.42; p < 0.001 | |||||||||
| R2 = 0.30 | R2 = 0.65 | R2 = 0.70 | ||||||||||
| b | β | t | Sig. | B | β | t | Sig. | B | β | T | Sig. | |
| BDI | −0.21 | −0.16 | −1.14 | 0.260 | −0.57 | −0.13 | −1.05 | 0.301 | 2.27 | 0.70 | 6.33 | 0.000 |
| Age | −0.38 | −0.39 | −2.11 | 0.041 | −1.59 | −0.50 | −3.00 | 0.005 | 0.36 | 0.15 | 1.02 | 0.314 |
| Sex | 2.67 | 0.11 | 0.76 | 0.454 | 10.92 | 0.13 | 1.08 | 0.287 | 7.79 | 0.13 | 1.16 | 0.251 |
| Time from diagnosis | −0.20 | −0.16 | −0.88 | 0.387 | −0.66 | −0.17 | −1.00 | 0.324 | −0.72 | −0.24 | −1.64 | 0.110 |
| Model | F4, 38 = 4.16; p = 0.007 | F4, 39 = 7.36; p < 0.001 | F4, 40 = 12.76; p < 0.001 | |||||||||
| R2 = 0.55 | R2 = 0.66 | R2 = 0.75 | ||||||||||
| b | β | t | Sig. | B | β | t | Sig. | |||||
| PSI | −0.05 | −0.11 | −0.77 | 0.444 | −0.08 | −0.06 | −0.48 | 0.635 | ||||
| Age | −0.40 | −0.41 | −2.17 | 0.037 | −1.66 | −0.52 | −3.06 | 0.004 | ||||
| Sex | 3.40 | 0.14 | 0.96 | 0.343 | 12.34 | 0.15 | 1.22 | 0.231 | ||||
| Time from diagnosis | −0.20 | −0.17 | −0.86 | 0.396 | −0.61 | −0.16 | −0.89 | 0.382 | ||||
| Model | F4, 38 = 3.91; p = 0.009 | F4, 39 = 6.98; p < 0.001 | ||||||||||
| R2 = 0.54 | R2 = 0.65 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuerda-Ballester, M.; Martínez-Rubio, D.; García-Pardo, M.P.; Proaño, B.; Cubero, L.; Calvo-Capilla, A.; Sancho-Cantus, D.; de la Rubia Ortí, J.E. Relationship of Motor Impairment with Cognitive and Emotional Alterations in Patients with Multiple Sclerosis. Int. J. Environ. Res. Public Health 2023, 20, 1387. https://doi.org/10.3390/ijerph20021387
Cuerda-Ballester M, Martínez-Rubio D, García-Pardo MP, Proaño B, Cubero L, Calvo-Capilla A, Sancho-Cantus D, de la Rubia Ortí JE. Relationship of Motor Impairment with Cognitive and Emotional Alterations in Patients with Multiple Sclerosis. International Journal of Environmental Research and Public Health. 2023; 20(2):1387. https://doi.org/10.3390/ijerph20021387
Chicago/Turabian StyleCuerda-Ballester, María, David Martínez-Rubio, María Pilar García-Pardo, Belén Proaño, Laura Cubero, Antonio Calvo-Capilla, David Sancho-Cantus, and Jose Enrique de la Rubia Ortí. 2023. "Relationship of Motor Impairment with Cognitive and Emotional Alterations in Patients with Multiple Sclerosis" International Journal of Environmental Research and Public Health 20, no. 2: 1387. https://doi.org/10.3390/ijerph20021387
APA StyleCuerda-Ballester, M., Martínez-Rubio, D., García-Pardo, M. P., Proaño, B., Cubero, L., Calvo-Capilla, A., Sancho-Cantus, D., & de la Rubia Ortí, J. E. (2023). Relationship of Motor Impairment with Cognitive and Emotional Alterations in Patients with Multiple Sclerosis. International Journal of Environmental Research and Public Health, 20(2), 1387. https://doi.org/10.3390/ijerph20021387










