High Adsorption of Hazardous Cr(VI) from Water Using a Biofilter Composed of Native Pseudomonas koreensis on Alginate Beads
Abstract
1. Introduction
2. Materials and Methods
2.1. Biofilter Preparation
2.2. Bacteria Immobilization
2.3. Kinetics Study
2.4. Determination of Dry Weight of Bacteria Attached to Alginate Beads
3. Results
3.1. Alginate Beads
3.2. Biosorption System Implementation
3.3. Cr(VI) Removal Tests for Optimization
3.4. Biosorption Effect on the Biofilter Surface
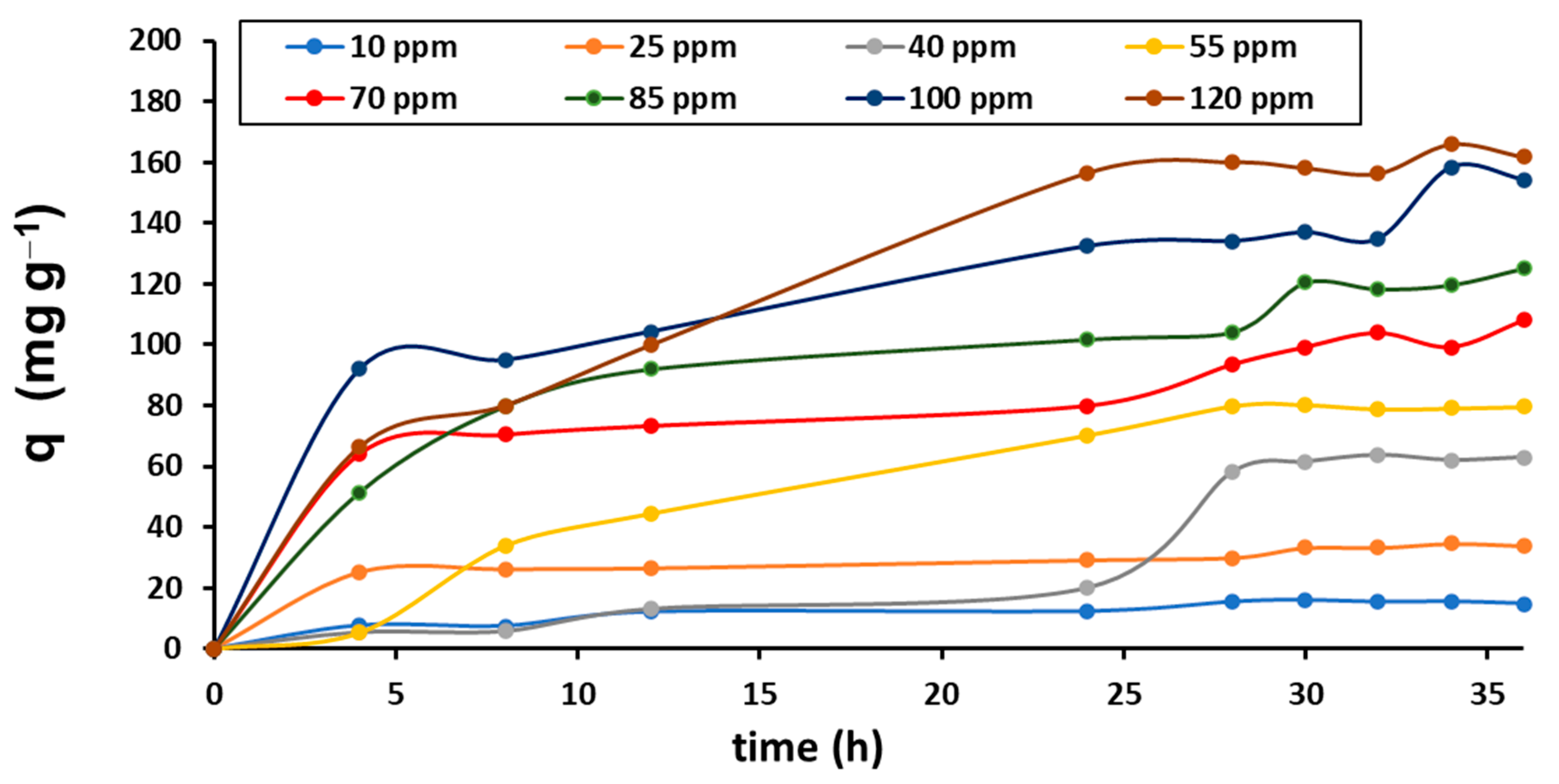
3.5. Kinetic Adsorption Experiments
4. Discussion
5. Conclusions
6. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gururajan, K.; Belur, P.D. Screening and selection of indigenous metal tolerant fungal isolates for heavy metal removal. Environ. Technol. Innov. 2018, 9, 91–99. [Google Scholar] [CrossRef]
- Sharma, S.K.; Petrusevski, B.; Amy, G. Review Paper Chromium removal from water: A review. J. Water Supply Res. Technol. 2008, 57, 541–553. [Google Scholar] [CrossRef]
- De Flora, S.; Bagnasco, M.; Serra, D.; Zanacchi, P. Genotoxicity of chromium compounds. A review. Mutat. Res. Genet. Toxicol. 1990, 238, 99–172. [Google Scholar] [CrossRef] [PubMed]
- Nickens, K.P.; Patierno, S.R.; Ceryak, S. Chromium genotoxicity: A double-edged sword. Chem. Biol. Interact. 2010, 188, 276–288. [Google Scholar] [CrossRef]
- DesMarias, T.L.; Costa, M. Mechanisms of chromium-induced toxicity. Curr. Opin. Toxicol. 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Kakavandi, B.; Kalantary, R.R.; Farzadkia, M.; Mahvi, A.H.; Esrafili, A.; Azari, A.; Yari, A.R.; Javid, A.B. Enhanced chromium (VI) removal using activated carbon modified by zero valent iron and silver bimetallic nanoparticles. J. Environ. Health Sci. Eng. 2014, 12, 115. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, N.; Li, Y.; Ren, B.; Ding, X.; Bian, H.; Yao, X. Total concentrations and sources of heavy metal pollution in global river and lake water bodies from 1972 to 2017. Glob. Ecol. Conserv. 2020, 22, e00925. [Google Scholar] [CrossRef]
- Yin, W.; Guo, Z.; Zhao, C.; Xu, J. Removal of Cr(VI) from aqueous media by biochar derived from mixture biomass precursors of Acorus calamus Linn. and feather waste. J. Anal. Appl. Pyrolysis 2019, 140, 86–92. [Google Scholar] [CrossRef]
- Covarrubias, S.A.; Peña Cabriales, J.J. Contaminación ambiental por metales pesados en México: Problemática y estrategias de fitorremediación. Rev. Int. Contam. Ambient. 2017, 33, 7–21. [Google Scholar] [CrossRef]
- Rangel Cordova, A.A.; Isarain Chávez, E.; Maldonado Vega, M. Caracterización y recuperación de sales de cromo hexavalente de un pasivo ambiental. Rev. Int. Conntam. Ambient. 2015, 31, 427–437. [Google Scholar]
- Paredes-Carrera, S.P.; Valencia-Martínez, R.F.; Valenzuela-Zapata, M.A.; Sànchez-Ochoa, J.C.; Castro-Sotelo, L.V. Study of hexavalent chromium sorption by hydrotalcites synthesized using ultrasound vs microwave irradation. Rev. Mex. Ing. Quím. 2015, 14, 429–436. [Google Scholar]
- Rangel Córdova, A.A.; Maldonado-Vega, M. Cromo hexavalente en la industria: Repercusiones a la salud y medidas de control. Rev. Mex. Salud Trab. 2014, 6, 77–84. [Google Scholar]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Joseph, L.; Jun, B.M.; Flora, J.R.V.; Park, C.M.; Yoon, Y. Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Application of three dimensional porous aerogels as adsorbent for removal of heavy metal ions from water/wastewater: A review study. Adv. Colloid Interface Sci. 2020, 284, 102247. [Google Scholar] [CrossRef] [PubMed]
- Aigbe, U.O.; Osibote, O.A. A review of hexavalent chromium removal from aqueous solutions by sorption technique using nanomaterials. J. Environ. Chem. Eng. 2020, 8, 104503. [Google Scholar] [CrossRef]
- Nur-E-Alam, M.; Mia, M.A.S.; Ahmad, F.; Rahman, M.M. An overview of chromium removal techniques from tannery effluent. Appl. Water Sci. 2020, 10, 205. [Google Scholar] [CrossRef]
- Almeida, J.C.; Cardoso, C.E.D.; Tavares, D.S.; Freitas, R.; Trindade, T.; Vale, C.; Pereira, E. Chromium removal from contaminated waters using nanomaterials—A review. Trends Anal. Chem. 2019, 118, 277–291. [Google Scholar] [CrossRef]
- Beni, A.A.; Esmaeili, A. Biosorption, an efficient method for removing heavy metals from industrial effluents: A Review. Environ. Technol. Innov. 2020, 17, 100503. [Google Scholar] [CrossRef]
- Paul, A.; Bhakta, J.N. Biosorption-driven green technology for the treatment of heavy metal(loids)-contaminated effluents. In Intelligent Environmental Data Monitoring for Pollution Management; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 71–91. ISBN 9780128196717. [Google Scholar]
- Papirio, S.; Frunzo, L.; Mattei, M.R.; Ferraro, A.; Race, M.; D’Acunto, B.; Pirozzi, F.; Esposito, G. Heavy metal removal from wastewaters by biosorption: Mechanisms and Modeling. In Sustainable Heavy Metal Remediation, Environmental Chemistry for a Sustainable World; Rene, E.R., Sahinkaya, E., Lewis, A., Lens, P.N.L., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 8, pp. 25–63. ISBN 978-3-319-58621-2. [Google Scholar]
- Nanda, M.; Kumar, V.; Sharma, D.K. Multimetal tolerance mechanisms in bacteria: The resistance strategies acquired by bacteria that can be exploited to ‘clean-up’ heavy metal contaminants from water. Aquat. Toxicol. 2019, 212, 1–10. [Google Scholar] [CrossRef]
- Priyadarshanee, M.; Das, S. Biosorption and removal of toxic heavy metals by metal tolerating bacteria for bioremediation of metal contamination: A comprehensive review. J. Environ. Chem. Eng. 2021, 9, 104686. [Google Scholar] [CrossRef]
- Gillan, D.C.; Roosa, S.; Kunath, B.; Billon, G.; Wattiez, R. The long-term adaptation of bacterial communities in metal-contaminated sediments: A metaproteogenomic study. Environ. Microbiol. 2015, 17, 1991–2005. [Google Scholar] [CrossRef] [PubMed]
- Fernández, P.M.; Viñarta, S.C.; Bernal, A.R.; Cruz, E.L.; Figueroa, L.I.C. Bioremediation strategies for chromium removal: Current research, scale-up approach and future perspectives. Chemosphere 2018, 208, 139–148. [Google Scholar] [CrossRef]
- Singh, P.; Itankar, N.; Patil, Y. Biomanagement of hexavalent chromium: Current trends and promising perspectives. J. Environ. Manag. 2021, 279, 111547. [Google Scholar] [CrossRef] [PubMed]
- Girijan, S.; Kumar, M. Immobilized biomass systems: An approach for trace organics removal from wastewater and environmental remediation. Curr. Opin. Environ. Sci. Health 2019, 12, 18–29. [Google Scholar] [CrossRef]
- Dzionek, A.; Wojcieszyńska, D.; Guzik, U. Natural carriers in bioremediation: A review. Electron. J. Biotechnol. 2016, 23, 28–36. [Google Scholar] [CrossRef]
- Silveira Martins, S.C.; Miranda Martins, C.; Guedes Fiuacute, L.M.; Tédde Santaella, S. Immobilization of microbial cells: A promising tool for treatment of toxic pollutants in industrial wastewater. Afr. J. Biotechnol. 2013, 12, 4412–4418. [Google Scholar]
- Calero, J.; Sánchez, Y.F.; Tórrez, R.; Hernann, E.; López, K. Elaboración y caracterización de microcápsulas gastrorresistentes de diclofenac obtenidas por gelificación iónica. Universitas 2008, 1, 27–30. [Google Scholar] [CrossRef]
- Hall, K.R.; Eagleton, L.C.; Acrivos, A.; Vermeulen, T. Pore- and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind. Eng. Chem. Fundam. 1966, 5, 212–223. [Google Scholar] [CrossRef]
- Quiton, K.G.; Doma, B.; Futalan, C.M.; Wan, M.W. Removal of chromium(VI) and zinc(II) from aqueous solution using kaolin-supported bacterial biofilms of Gram-negative E. coli and Gram-positive Staphylococcus epidermidis. Sustain. Environ. Res. 2018, 28, 206–213. [Google Scholar] [CrossRef]
- Meroufel, B.; Benali, O.; Benyahia, M.; Benmoussa, Y.; Zenasni, M.A. Adsorptive removal of anionic dye from aqueous solutions by Algerian kaolin: Characteristics, isotherm, kinetic and thermodynamic studies. J. Mater. Environ. Sci. 2013, 4, 482–491. [Google Scholar]
- Hernández Covarrubias, S.A. Inmovilización de Microorganismos en Esferas de Alginato como Protección contra Condiciones Adversas en un Tratamiento de agua Residual; Centro de Investigaciones Biológicas del Noroeste, S.C.: La Paz, Mexico, 2011. [Google Scholar]
- Ohemeng-Boahen, G.; Sewu, D.D.; Woo, S.H. Preparation and characterization of alginate-kelp biochar composite hydrogel bead for dye removal. Environ. Sci. Pollut. Res. 2019, 26, 33030–33042. [Google Scholar] [CrossRef]
- Guerini, M.; Perugini, P.; Grisoli, P. Evaluation of the effectiveness of N-Acetylcysteine (NAC) and N-acetylcysteine-cyclodextrins multi-composite in Pseudomonas aeruginosa biofilm formation. Appl. Sci. 2020, 10, 3466. [Google Scholar] [CrossRef]
- El-Naggar, N.E.A.; El-khateeb, A.Y.; Ghoniem, A.A.; El-Hersh, M.S.; Saber, W.E.I.A. Innovative low-cost biosorption process of Cr6+ by Pseudomonas alcaliphila NEWG-2. Sci. Rep. 2020, 10, 14043. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.I.E. Analysis of Variance. I. One-Way. In Basic Biostatistics for Medical and Biomedical Practitioners; Hoffman, J.I.E., Ed.; Elsevier Inc.: London, UK, 2019; pp. 391–417. ISBN 9780128170847. [Google Scholar]
- Guevara González, J.J.; Castañeda Carrión, I.N.; Juárez Alcántara, J.; Mendoza Fernández, A. Efecto de la temperatura y del pH sobre el crecimiento de Pseudomonas aeruginosa MBLA-04 en solución mínima de sales con detergente Ace. Rev. Rebiol 2013, 33, 1–8. [Google Scholar]
- Mollinedo Patzi, M.A.; González Villalobos, C. Bacterias Gram Negativas. Rev. Act. Clín. Med. 2014, 49, 2609–2613. [Google Scholar]
- Hlihor, R.M.; Figueiredo, H.; Tavares, T.; Gavrilescu, M. Biosorption potential of dead and living Arthrobacter viscosus biomass in the removal of Cr(VI): Batch and column studies. Process Saf. Environ. Prot. 2017, 108, 44–56. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; KIemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons: New York, NY, USA, 2005; ISBN 0471393622. [Google Scholar]
- Yaashikaa, P.R.; Senthil Kumar, P.; Mohan Babu, V.P.; Kanaka Durga, R.; Manivasagan, V.; Saranya, K.; Saravanan, A. Modelling on the removal of Cr(VI) ions from aquatic system using mixed biosorbent (Pseudomonas stutzeri and acid treated Banyan tree bark). J. Mol. Liq. 2019, 276, 362–370. [Google Scholar] [CrossRef]
- Gupta, A.; Balomajumder, C. Biosorptive performance of Escherichia coli supported on Waste tea biomass (WTB) for removal of Cr(VI) to avoid the contamination of ground water: A comparative study between biosorption and SBB system. Groundw. Sustain. Dev. 2015, 1, 12–22. [Google Scholar] [CrossRef]
- Fan, J.; Onal Okyay, T.; Frigi Rodrigues, D. The synergism of temperature, pH and growth phases on heavy metal biosorption by two environmental isolates. J. Hazard. Mater. 2014, 279, 236–243. [Google Scholar] [CrossRef]
- Aryal, M.; Liakopoulou-Kyriakides, M. Characterization of Mycobacterium sp. strain Spyr1 biomass and its biosorption behavior towards Cr(III) and Cr(VI) in single, binary and multi-ion aqueous systems. J. Chem. Technol. Biotechnol. 2014, 89, 559–568. [Google Scholar] [CrossRef]
- Saranya, K.; Sundaramanickam, A.; Shekhar, S.; Meena, M.; Sathishkumar, R.S.; Balasubramanian, T. Biosorption of multi-heavy metals by coral associated phosphate solubilising bacteria Cronobacter muytjensii KSCAS2. J. Environ. Manag. 2018, 222, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Ziagova, M.; Dimitriadis, G.; Aslanidou, D.; Papaioannou, X.; Litopoulou Tzannetaki, E.; Liakopoulou-Kyriakides, M. Comparative study of Cd(II) and Cr(VI) biosorption on Staphylococcus xylosus and Pseudomonas sp. in single and binary mixtures. Bioresour. Technol. 2007, 98, 2859–2865. [Google Scholar] [CrossRef]
- Simonescu, C.M.; Mason, T.J.; Călinescu, I.; Lavric, V.; Vînătoru, M.; Melinescu, A.; Culiţă, D.C. Ultrasound assisted preparation of calcium alginate beads to improve absorption of Pb+2 from water. Ultrason. Sonochem. 2020, 68, 105191. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Chakravarty, A.; Ikram, S. A comprehensive review on incredible renewable carriers as promising platforms for enzyme immobilization & thereof strategies. Int. J. Biol. Macromol. 2021, 167, 962–986. [Google Scholar] [PubMed]
- Omarova, E.O.; Lobakova, E.S.; Dolnikova, G.A.; Nekrasova, V.V.; Idiatulov, R.K.; Kashcheeva, P.B.; Perevertailo, N.G.; Dedov, A.G. Immobilization of bacteria on polymer matrices for degradation of crude oil and oil products. Moscow Univ. Biol. Sci. Bull. 2012, 67, 24–30. [Google Scholar] [CrossRef]
- Mangwani, N.; Shukla, S.K.; Rao, T.S.; Das, S. Calcium-mediated modulation of Pseudomonas mendocina NR802 biofilm influences the phenanthrene degradation. Colloids Surf. B Biointerfaces 2014, 114, 301–309. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Garcia Torres, S.G.; Ilyina, A.; Ramos-Gonzalez, R.; Carlos Hernandez, S.; Diaz-Jimenez, L. Interaction between Cobalt Ferrite Nanoparticles and Aspergillus Niger Spores. IEEE Trans. Nanobiosci. 2019, 18, 542–548. [Google Scholar] [CrossRef]
- Nakano, Y.; Takeshita, K.; Tsutsumi, T. Adsorption mechanism of hexavalent chromium by redox within condensed-tannin gel. Water Res. 2001, 35, 496–500. [Google Scholar] [CrossRef]

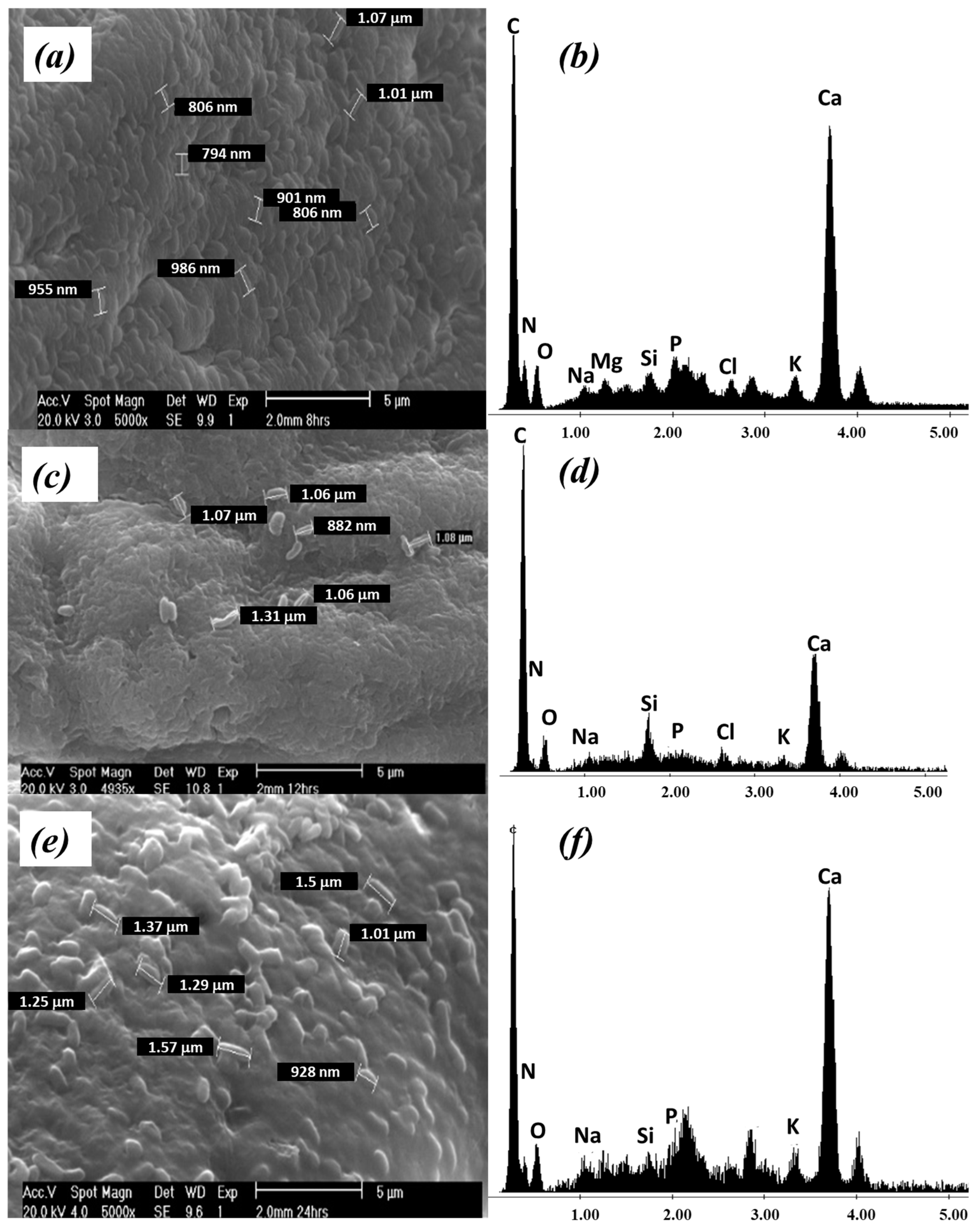
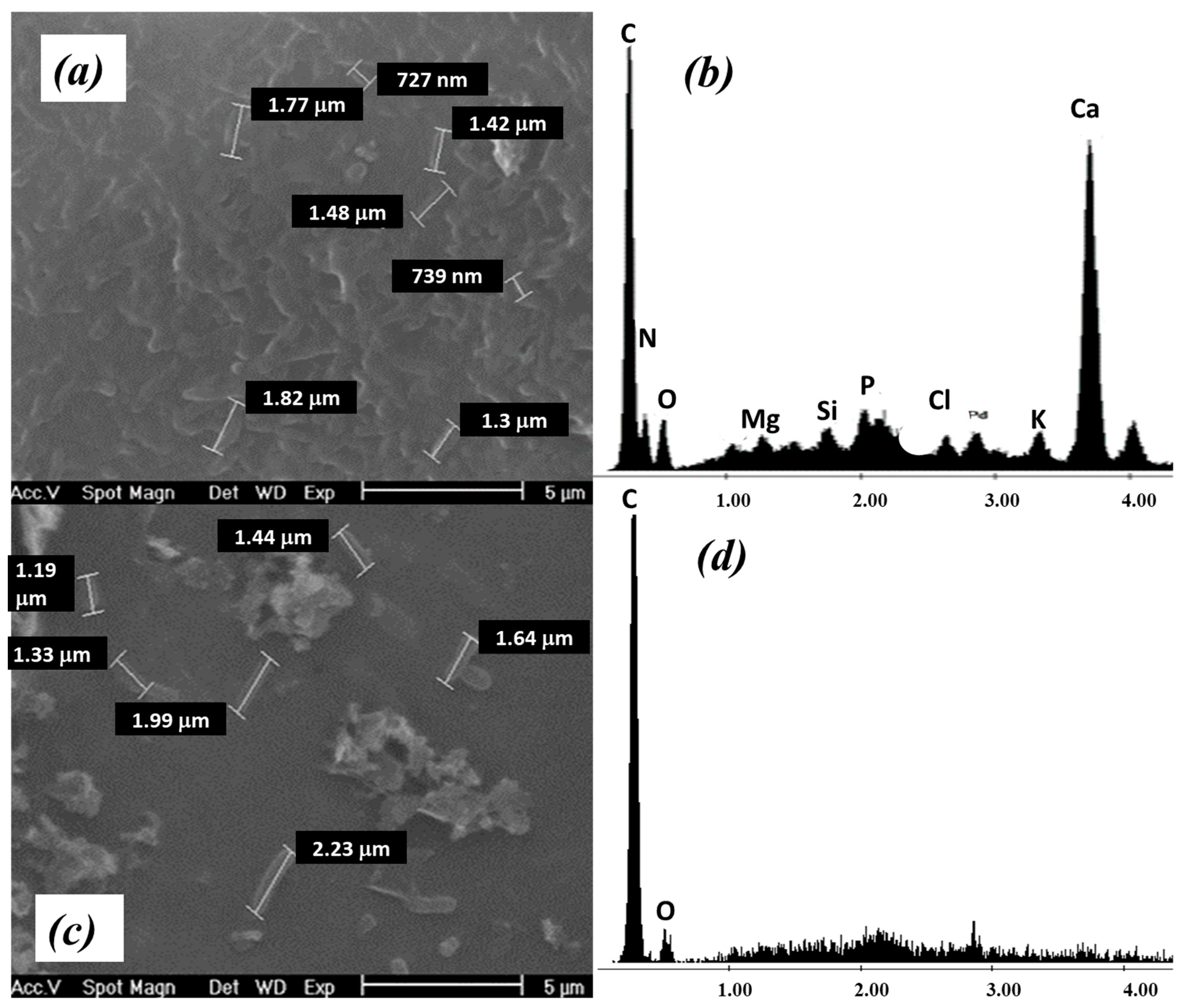

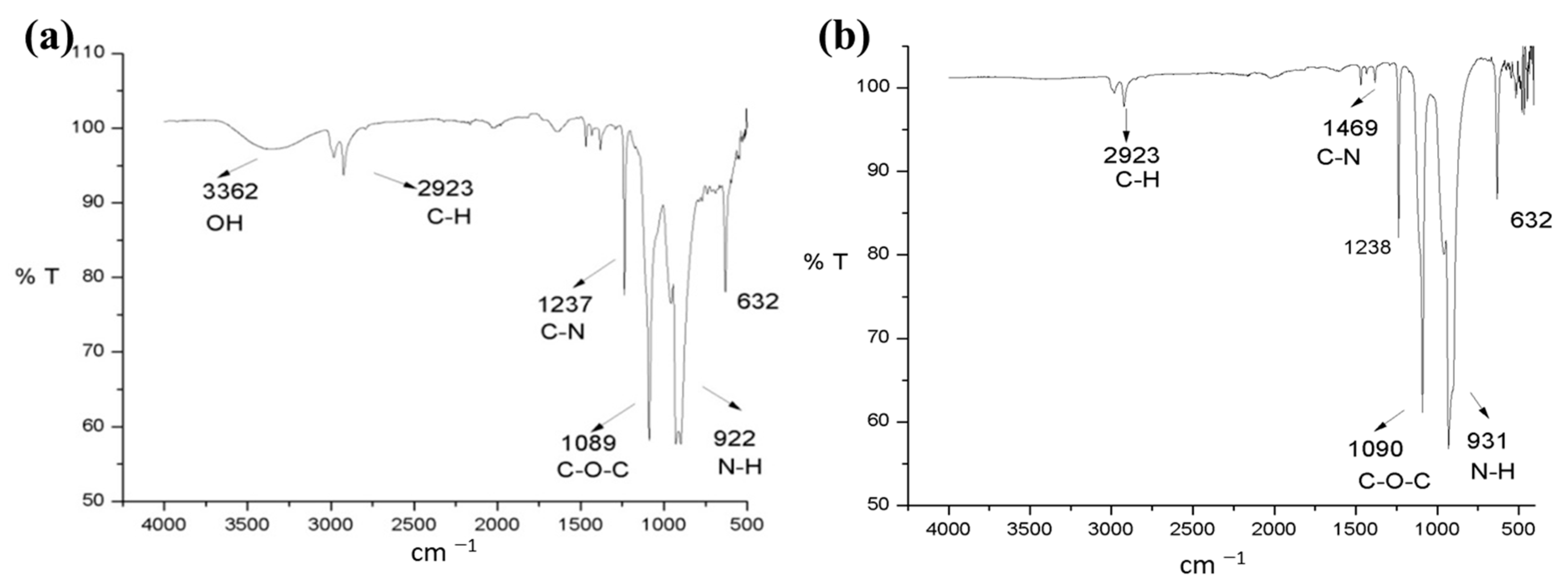
| Tip | Diameter nm | Items Beads/g | Set Weight g | Total Items Beads/Set | Surface mm2/Bead | Surface mm2/g |
|---|---|---|---|---|---|---|
| blue | 3.0 | 35.33 | 65 | 2296 | 28.27 | 998.9 |
| yellow | 2.4 | 84.30 | 56 | 4721 | 18.09 | 1525.6 |
| white | 2.0 | 130.66 | 60 | 7839 | 12.56 | 1641.2 |
| Sum of Squares | Degree of Freedom | Mean Square | F | p | FCV | |
|---|---|---|---|---|---|---|
| Between groups | 13,643.56 | 2 | 6821.78 | 2558.17 | 1.6 × 10−9 | 5.14 |
| Within groups | 16 | 6 | 2.67 | |||
| Total | 13,659.56 | 8 |
| Effect | Sum of Squares | Degree of Freedom | Sum of Means | F | p |
|---|---|---|---|---|---|
| Interception | 3332.79 | 1 | 3332.79 | 85,506.38 | 0.000 |
| Temperature | 0.05 | 1 | 0.05 | 1.35 | 0.254 |
| pH | 9.08 | 1 | 9.08 | 232.97 | 0.000 |
| [Adsorbent] | 66.16 | 3 | 22.05 | 565.77 | 0.000 |
| Temperature × pH | 0.59 | 1 | 0.59 | 15.12 | 0.001 |
| Temperature × [Adsorbent] | 0.13 | 3 | 0.04 | 1.14 | 0.348 |
| pH × [Adsorbent] | 6.22 | 3 | 2.07 | 53.19 | 0.000 |
| Temperature × pH × [Adsorbent] | 038 | 3 | 0.13 | 3.24 | 0.035 |
| Error | 1.25 | 32 | 0.04 |
| T (°C) | pH | Beads (gr) | Cr(VI) (mg L−1) | Treatment | |||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||
| 30 | 6.6 | 0.027 | 6.41 | * | |||
| 20 | 6.6 | 0.027 | 6.95 | * | |||
| 30 | 6.6 | 0.024 | 7.06 | * | * | ||
| 20 | 6.6 | 0.024 | 7.17 | * | * | ||
| 30 | 6.6 | 0.021 | 7.37 | * | * | ||
| 20 | 6.6 | 0.021 | 7.52 | * | |||
| Bacteria | Support | Model | R2 | qmax (mg g−1) | References |
|---|---|---|---|---|---|
| A. viscosus | None | Langmuir | 0.96 | 1161 | [41] |
| Staphylococcus epidermidis | Kaolin | Langmuir Freundlich Temkin Dubinin-Radushkevich | 0.99 0.99 0.80 0.82 | 56 | [32] |
| Escherichia coli | Kaolin | Langmuir Freundlich Temkin Dubinin-Radushkevich | 0.99 0.99 0.82 0.79 | 91 | [32] |
| Escherichia coli | Waste tea biomass | Langmuir Freundlich Temkin | 0.99 0.91 0.92 | 16.75 | [44] |
| Ochrobactrum intermedium | None | Langmuir | 0.96 | 51.96 | [45] |
| Cupriavidus metallidurans | None | Langmuir | 0.90 | 32.63 | [45] |
| Mycobacterium sp. | None | Langmuir Freundlich Temkin Hill-der Boer D-R | 0.99 0.92 0.97 0.98 0.97 | 61.51 | [46] |
| Cronobacter muytjensii | None | Langmuir | - | 73.8 | [47] |
| P. alcaliphila | Alginate beads | Langmuir Freundlich | 0.99 0.92 | 10 | [37] |
| P. koreensis | Alginate beads | Langmuir Freundlich | 0.98 0.97 | 625 | This study |
| P. stutzeri | None | Langmuir Freundlich Temkin Dubinin-Radushkevich | 0.89 0.99 0.98 0.71 | 27.47 | [43] |
| Pseudomonas sp. | None | Langmuir Freundlich | 0.95 0.99 | 95 | [48] |
| Model | KL | qmax (mg g−1) | RL | KF | 1/n |
|---|---|---|---|---|---|
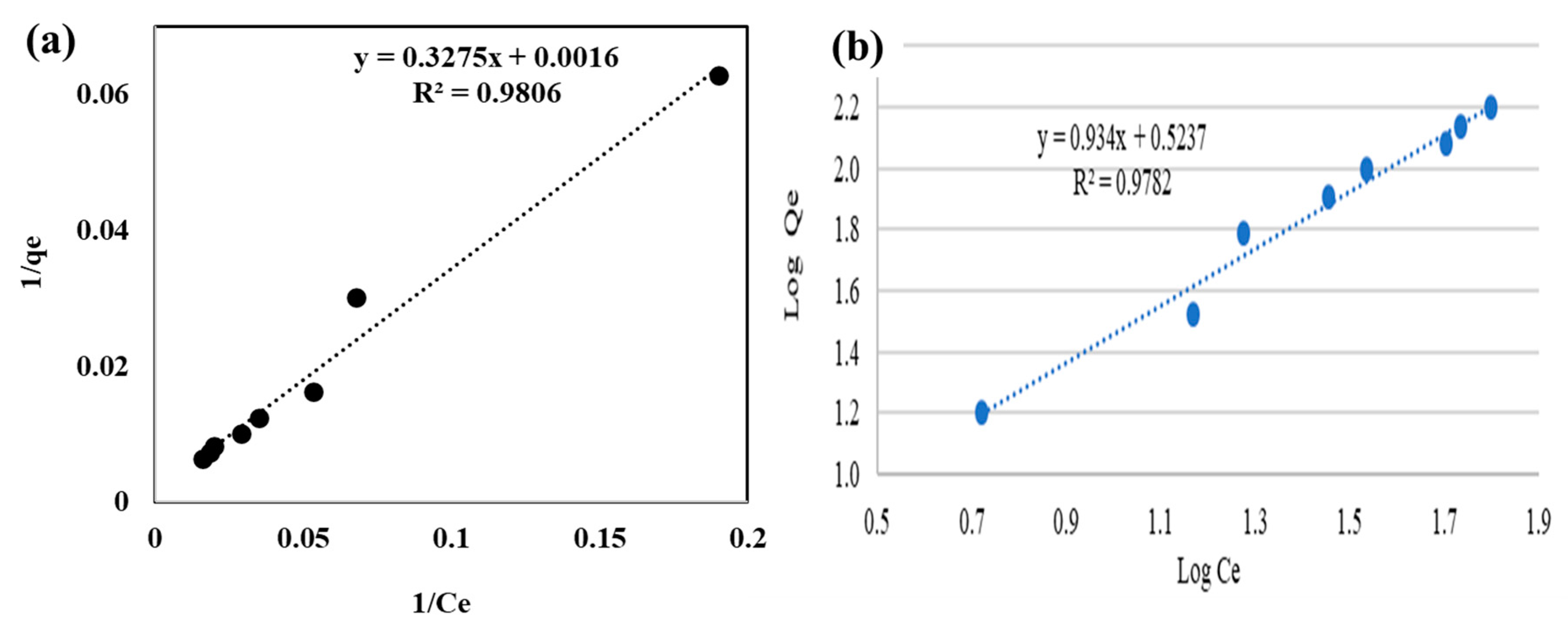
| Langmuir | 4.88 × 10−3 | 625 | 0.6304 | - | - |
| Freundlich | - | - | - | 3.33 | 0.9345 |
| Model | Parameters | 10 ppm | 70 ppm | 120 ppm |
|---|---|---|---|---|
| Pseudo-first-order | K1 | 0.101 | 0.075 | 0.179 |
| R2 | 0.671 | 0.818 | 0.934 | |
| Pseudo-second-order | K2 | 0.002 | 0.002 | 0.0003 |
| R2 | 0.777 | 0.966 | 0.961 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz-Jimenez, L.; Garcia-Torres, S.; Carlos-Hernandez, S. High Adsorption of Hazardous Cr(VI) from Water Using a Biofilter Composed of Native Pseudomonas koreensis on Alginate Beads. Int. J. Environ. Res. Public Health 2023, 20, 1385. https://doi.org/10.3390/ijerph20021385
Diaz-Jimenez L, Garcia-Torres S, Carlos-Hernandez S. High Adsorption of Hazardous Cr(VI) from Water Using a Biofilter Composed of Native Pseudomonas koreensis on Alginate Beads. International Journal of Environmental Research and Public Health. 2023; 20(2):1385. https://doi.org/10.3390/ijerph20021385
Chicago/Turabian StyleDiaz-Jimenez, Lourdes, Sandy Garcia-Torres, and Salvador Carlos-Hernandez. 2023. "High Adsorption of Hazardous Cr(VI) from Water Using a Biofilter Composed of Native Pseudomonas koreensis on Alginate Beads" International Journal of Environmental Research and Public Health 20, no. 2: 1385. https://doi.org/10.3390/ijerph20021385
APA StyleDiaz-Jimenez, L., Garcia-Torres, S., & Carlos-Hernandez, S. (2023). High Adsorption of Hazardous Cr(VI) from Water Using a Biofilter Composed of Native Pseudomonas koreensis on Alginate Beads. International Journal of Environmental Research and Public Health, 20(2), 1385. https://doi.org/10.3390/ijerph20021385










