The Potential Environmental and Social Influence of the Inorganic Salt Hydrates Used as a Phase Change Material for Thermal Energy Storage in Solar Installations
Abstract
1. Introduction
2. Thermophysical Parameters of the Salt and the Costs of Thermal Energy Storage
3. Assessment of Chemical Hazards of Inorganic Salt Hydrates for the Environment and Humans
4. Analysis of the Defects of Salt Hydrates in Terms of Environmental Hazards with an Indication of the Possibility of Minimizing Them
4.1. Phase Separation and Supercooling
4.1.1. MgCl2·6H2O
4.1.2. Mg(NO3)2·6H2O (MNH)
4.1.3. Na2SO4·10H2O (SSD)
4.1.4. CH3COONa ·3H2O (SAT)
4.1.5. Na2CO3·10H2O (SCD)
4.1.6. Na2HPO4·12H2O (SD)
4.1.7. Ba(OH)2·8H2O
4.1.8. CaCl2·6H2O
4.2. Corrosiveness
5. Evaluation of the Possibility of Utilizing Used Salts
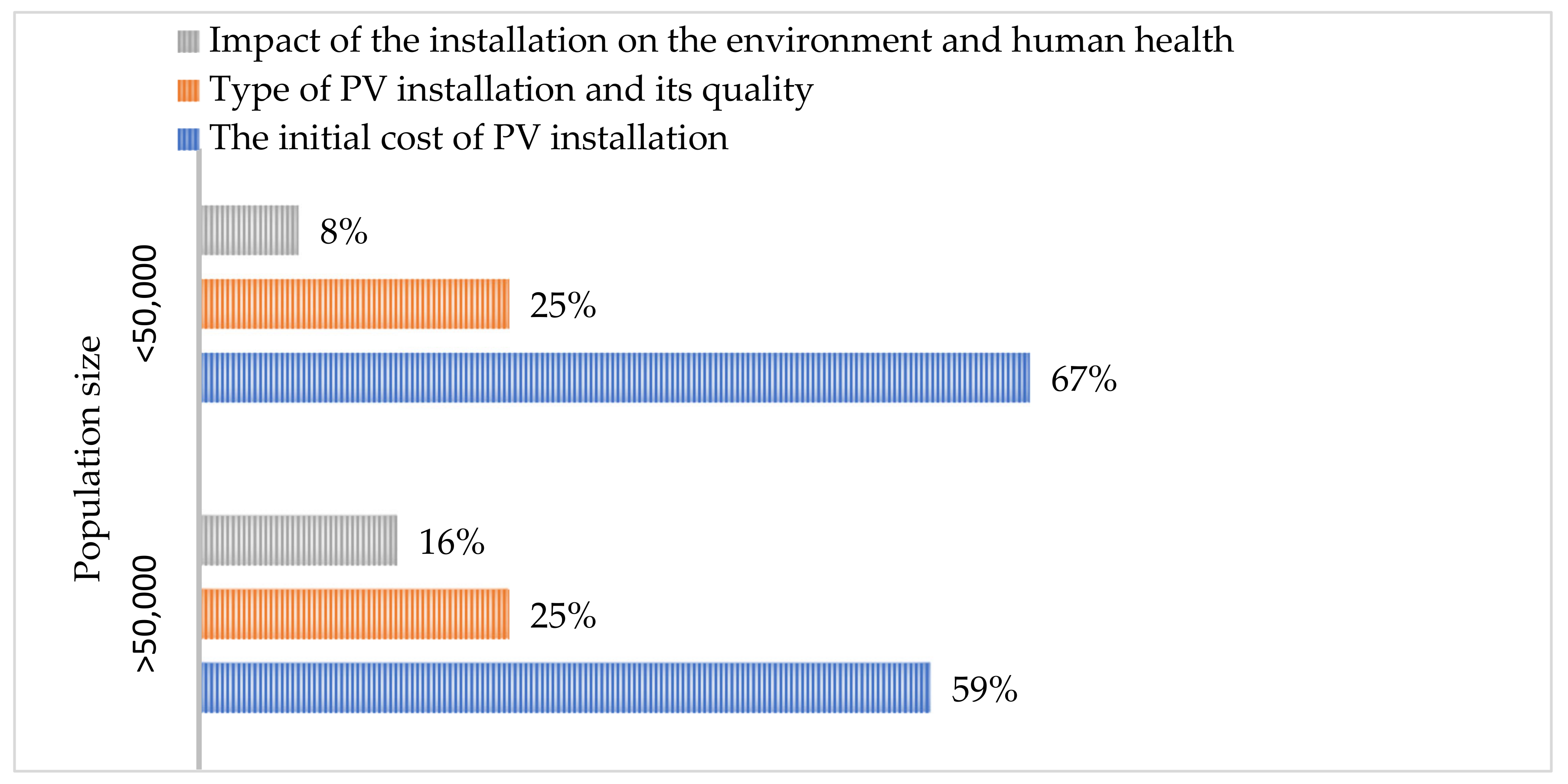
6. Social Approach to PV Technology with PCM Modules
7. Conclusions
- Higher values of energy storage density translate into a smaller volume of material necessary to accumulate a given amount of heat, which can minimize the amount of waste. The most advantageous from an economic point of view, due to the relation of price and density, are: calcium chloride hexahydrate, in the first place, and disodium hydrogen phosphate dodecahydrate, in the second place. On the other hand, magnesium chloride hexahydrate and sodium acetate trihydrate seem to be the most unfavorable and can generate the largest volume of waste.
- Based on the risk level of the substance on the GHS definitions, it can be indicated that magnesium chloride hexahydrate, magnesium nitrate hexahydrate, sodium sulfate decahydrate, sodium acetate trihydrate, and disodium hydrogen phosphate dodecahydrate are substances practically harmless to human health. On the other hand, sodium carbonate decahydrate, calcium chloride hexahydrate and barium hydroxide octahydrate are medium-harmful salts.
- Disodium hydrogen phosphate dodecahydrate turned out to be the most promising salt in terms of the thermophysical, economic, and environmental properties for use in solar installations. The waste of this substance is not hazardous waste, and the unfavorable defects (supercooling, phase separation, and corrosiveness) can be easily eliminated, e.g., by encapsulation in expanded graphite. As we believe that it is the most pro-environmental substance, it is advisable to work on the creation of eutectic substances based on disodium hydrogen phosphate dodecahydrate to model its thermophysical properties for various applications.
- Waste code 16 10—hydrated liquid waste is proposed for off-site recovery or disposal for used salt hydrates; 16 10 01—hydrated liquid waste containing hazardous wastes (for magnesium chloride hexahydrate, sodium sulfate decahydrate, sodium acetate trihydrate, sodium carbonate decahydrate, calcium chloride hexahydrate, and barium hydroxide octahydrate); or 16 10 02—hydrated liquid waste other than those mentioned in 16 10 01 (for magnesium nitrate hexahydrate, disodium hydrogen phosphate dodecahydrate.) Waste with the assumed waste code 16 10 02 can be collected in septic tanks and accepted to liquid waste collection points operating in water and wastewater companies.
- The basis for sustainable development is the documentation of salt in terms of all aspects at the same time: technical, economic, environmental, and social. Such activities should be taken into account when designing new technologies in the field of solar installations using inorganic salt hydrates. Further environmental assessments of substances for other uses could be carried out in the future.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marrero, R.J.; Hernández-Cabrera, J.A.; Fumero, A.; Hernández, B. Social Acceptance of Gas, Wind, and Solar Energies in the Canary Islands. Int. J. Environ. Res. Public Health 2021, 18, 9672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sun, J.; Ma, J.; Xu, F.; Qiu, L. Environmental Assessment of a Hybrid Solar-Biomass Energy Supplying System: A Case Study. Int. J. Environ. Res. Public Health 2019, 16, 2222. [Google Scholar] [CrossRef] [PubMed]
- Nartowska, E.; Styś-Maniara, M.; Porowski, R.; Emersleben, A. Management of salt hydrates in photovoltaic installations in light of existing environmental legislation. Struct. Environ. 2022, 14, 11–17. [Google Scholar] [CrossRef]
- Elias, C.N.; Stathopoulos, V.N. A comprehensive review of recent advances in materials aspects of phase change materials in thermal energy storage. Energy Procedia 2019, 161, 385–394. [Google Scholar] [CrossRef]
- Shukla, A.; Kant, K.; Sharma, A.; Biwole, P.H. Cooling methodologies of photovoltaic module for enhancing electrical efficiency: A review. Sol. Energy Mater. Sol. Cells 2017, 160, 275–286. [Google Scholar] [CrossRef]
- El Hammoumi, A.; Chtita, S.; Motahhir, S.; El Ghzizal, A. Solar PV energy: From material to use, and the most commonly used techniques to maximize the power output of PV systems: A focus on solar trackers and floating solar panels. Energy Rep. 2022, 8, 11992–12010. [Google Scholar] [CrossRef]
- Sharma, N.K.; Gaur, M.K.; Malvi, C.S. Application of phase change materials for cooling of solar photovoltaic panels: A review. Mater. Proc. 2021, 47, 6759–6765. [Google Scholar] [CrossRef]
- Choo, Y.M.; Wei, W. Salt hydates as phase change materials for photovoltaics thermal management. Energy Sci. Eng. 2022, 10, 1630–1642. [Google Scholar] [CrossRef]
- Li, J.; Chen, H.; Jia, L.; Zhu, X.; Qin, G.; Chen, Y. Preparation and characterization of Na2HPO4·12H2O polymethyl methacrylate nanocapsule for efficient thermal energy storage. J. Energy Storage 2022, 53, 105133. [Google Scholar] [CrossRef]
- Zhang, Z.; Lian, Y.; Xu, X.; Xu, X.; Fang, G.; Gu, M. Synthesis and characterization of microencapsulated sodium sulfate decahydrate as phase change energy storage materials. Appl. Energy 2019, 255, 113830. [Google Scholar] [CrossRef]
- Trausel, F.; de Jong, A.J.; Cuypers, R. A Review on the Properties of Salt Hydrates for Thermochemical Storage. Energy Procedia 2014, 48, 447–452. [Google Scholar] [CrossRef]
- Song, X.; Zhang, G.; Tan, H.; Chang, L.; Cai, L.; Xu, G.; Deng, Z.; Han, Z. Review on Thermophysical Properties and Corrosion Perfomrance of Molten Salt in High Temperature Thermal Energy Storage. Earth Environ. Sci. 2020, 474, 052071. [Google Scholar] [CrossRef]
- Donkers, P.A.J.; Sögütoglu, L.C.; Huinink, H.P.; Fischer, H.R.; Adan, O.C.G. A review of salt hydrates for seasonal heat storage in domestic applications. Appl. Energy 2017, 199, 45–68. [Google Scholar] [CrossRef]
- Nkuissi, H.J.T.; Konan, F.K.; Bouchaib Hartiti, B.; Ndjaka, J.M. Toxic Materials Used in Thin Film Photovoltaics and Their Impacts on Environment. In Reliability and Ecological Aspects of Photovoltaic Modules; Gok, A., Ed.; IntechOpen: London, UK, 2020; pp. 121–138. [Google Scholar] [CrossRef]
- Jastrzębski, P.; Saługa, P.W. Innowacyjne metody magazynowania ciepła. Zesz. Nauk. Inst. Gospod. Surowcami Miner. Energią Pol. Akad. Nauk. 2018, 105, 225–232. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Zong, J.; Ma, J.; Fang, Y. Experimental Research of the Heat Storage Performance of a Magnesium Nitrate Hexahydrate-Based Phase Change Material for Building Heating. Energies 2021, 14, 7108. [Google Scholar] [CrossRef]
- Dutil, Y.; Rousse, D.; Lassue, S.; Zalewski, L.; Joulin, A.; Virgone, J.; Kuznik, F.; Johannes, K.; Dumas, J.-P.; Bédécarrats, J.-P.; et al. Modeling phase change materials behavior in building applications: Comments on material characterization and model validation. Renew. Energy 2014, 61, 132–135. [Google Scholar] [CrossRef]
- Material Safety Data Sheet, MSDS. Available online: https://www.sigmaaldrich.com/PL/en/sds/sial/m2670 (accessed on 27 June 2022).
- Höhlein, S.; König-Haagen, A.; Brüggemann, D. Thermophysical Characterization of MgCl₂·6H₂O, Xylitol and Erythritol as Phase Change Materials (PCM) for Latent Heat Thermal Energy Storage (LHTES). Materials 2017, 10, 444. [Google Scholar] [CrossRef]
- Prices for high purity salts. Available online: https://www.sigmaaldrich.com/PL/en/products/chemistry-and-biochemicals/lab-chemicals/salts#High-Purity-Salts-for-Instrumental-Analysis (accessed on 27 June 2022).
- Material Safety Data Sheet, MSDS. Available online: https://www.sigmaaldrich.com/PL/en/sds/sigald/237175 (accessed on 27 June 2022).
- Material Safety Data Sheet, MSDS. Available online: https://www.sigmaaldrich.com/PL/en/sds/sigald/403008 (accessed on 27 June 2022).
- Material Safety Data Sheet, MSDS. Available online: https://www.sigmaaldrich.com/PL/en/sds/sigald/236500 (accessed on 27 June 2022).
- Oliver, D.E.; Bissell, A.J.; Liu, X.; Tang, C.C.; Pulham, C.R. Crystallisation studies of sodium acetate trihydrate-suppression of incongruent melting and sub-cooling to produce a reliable, high-performance phase-change material. CrystEngComm 2021, 23, 700–706. [Google Scholar] [CrossRef]
- Yuan, S. Study on stability of carbon residue/Sodium acetate trihydrate composite phase change material from sludge pyrolysis. J. Phys. Conf. Ser. 2022, 2174, 012032. [Google Scholar] [CrossRef]
- Kumar, R.; Vyas, S.; Kumar, R.; Dixit, A. Development of sodium acetate trihydrate-ethylene glycol composite phase change materials with enhanced thermophysical properties for thermal comfort and therapeutic applications. Sci. Rep. 2017, 7, 5203. [Google Scholar] [CrossRef]
- Material Safety Data Sheet, MSDS. Available online: https://www.sigmaaldrich.com/PL/en/sds/sigald/71360 (accessed on 27 June 2022).
- Zui, Z.; Kai, L.; Weiliang, Y.E.; Hua, F.; Yan, W.; Jingtao, L. Research progress of disodium hydrogen phosphate dodecahydrate phase change material. Chem. Ind. Eng. Prog. 2022, 41, 827–836. [Google Scholar] [CrossRef]
- Ren, S.; Li, J.; Huang, K.; Bai, Y.; Wang, C. Effect of composite orders of graphene oxide on thermal properties of Na2HPO4•12H2O/expanded vermiculite composite phase change materials. J. Energy Storage 2021, 41, 102980. [Google Scholar] [CrossRef]
- Material Safety Data Sheet, MSDS. Available online: https://www.sigmaaldrich.com/PL/en/sds/sigald/04273 (accessed on 27 June 2022).
- Material Safety Data Sheet, MSDS. Available online: https://www.sigmaaldrich.com/PL/en/sds/sigald/b2507 (accessed on 27 June 2022).
- Wang, Q.; Wang, J.; Chen, Y.; Zhao, C.Y. Experimental investigation of barium hydroxide octahydrate as latent heat storage materials. Solar Energy 2019, 177, 99–107. [Google Scholar] [CrossRef]
- Material Safety Data Sheet, MSDS. Available online: https://www.sigmaaldrich.com/PL/en/sds/aldrich/442909 (accessed on 27 June 2022).
- Cao, S.; Luo, X.; Han, X.; Lu, X.; Zou, C. Development of a New Modified CaCl2 ·6H2O Composite Phase Change Material. Energies 2022, 15, 824. [Google Scholar] [CrossRef]
- Singh, H.K.; Buddhi, D. Experimental investigationon CaCl2.6H2O for subcooling behavior and its correction for low temperature thermal energy storage. Int. J. Appl. Eng. Res. 2018, 13, 9858–9867. [Google Scholar]
- Saikrishnan, V.; Karthikeyan, A.; Beemkumar, N.; Ganesan, S.; Yuvarajan, D. The thermal performance analyses of the solar energy-powered thermal energy storage system with MgCl2·6H2O as PCM. J. Braz. Soc. Mech. Sci. Eng. 2020, 42, 31. [Google Scholar] [CrossRef]
- Gupta, N.; Kumar, A.; Dhasmana, H.; Kumar, A.; Verma, A.; Shukla, P.; Jain, C.K. Effect of shape and size of carbon materials on the thermophysical properties of magnesium nitrate hexahydrate for solar thermal energy storage applications. J. Energy Storage 2021, 41, 102899. [Google Scholar] [CrossRef]
- Ling, Z.; Li, S.; Zhang, Z.; Fang, X.; Gao, X.; Xu, T. A shape-stabilized MgCl2·6H2O–Mg(NO3)2·6H2O/expanded graphite composite phase change material with high thermal conductivity and stability. J. Appl. Electrochem. 2018, 48, 1131–1138. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B.; Xie, B.; Zhao, X.; Chen, J. Tailored phase change behavior of Na2SO4·10H2O/expanded graphite composite for thermal energy storage. Energy Convers. Manag. 2020, 208, 112586. [Google Scholar] [CrossRef]
- Shen, Y.; Li, X.; Zhang, S.; Sun, Y.; Zeng, J.; Hai, C.; Ren, X.; Zhu, S.; Zhou, Y. Surface evolution of eutectic MgCl2·6H2O-Mg(NO3)2·6H2O phase change materials for thermal energy storage monitored by scanning probe microscopy. Appl. Surf. Sci. 2021, 565, 150549. [Google Scholar] [CrossRef]
- Xiao, Q.; Fan, J.; Li, L.; Xu, T.; Yuan, W. Solar thermal energy storage based on sodium acetate trihydrate phase change hydrogels with excellent light-to-thermal conversion performance. Energy 2018, 165, 1240–1247. [Google Scholar] [CrossRef]
- Wang, G.; Xu, C.; Kong, W.; Englmair, G.; Fan, J.; Wei, G.; Furbo, S. Review on sodium acetate trihydrate in flexible thermal energy storages: Properties, challenges and applications. J. Energy Storage 2021, 40, 102780. [Google Scholar] [CrossRef]
- Rathod, A.C.; Bandela, C.V.; Rehman, A.R. Experimental Study on Phase Change Material based Thermal Energy Storage System. Int. Res. J. Eng. Technol. 2017, 4, 56–72. Available online: https://www.irjet.net/archives/V4/i11/IRJET-V4I11331.pdf (accessed on 7 July 2022).
- Pichandi, R.; Kulandaivelu, K.M.; Alagar, K.; Dhevaguru, H.K.; Ganesamoorthy, S. Performance enhancement of photovoltaic module by integrating eutectic inorganic phase change material. Energy Sources Part A Recovery Util. Environ. Eff. 2020. [Google Scholar] [CrossRef]
- Lan, X.Z.; Tan, Z.C.; Yue, D.T.; Shi, Q.; Yang, C.G. Heat Storage Performance of Disodium Hydrogen Phosphate Dodecahydrate: Prevention of Phase Separation by Thickening and Gelling Method. Chin. J. Chem. 2007, 25, 921–925. [Google Scholar] [CrossRef]
- Xie, N.; Huang, Z.; Luo, Z.; Gao, X.; Fang, Y.; Zhang, Z. Inorganic Salt Hydrate for Thermal Energy Storage. Appl. Sci. 2017, 7, 1317. [Google Scholar] [CrossRef]
- Zhao, S.; Yan, S.; Ming, T.; Ao, C.; Zhao, L.; Zhao, X. Experimental study of the preparation and modification of Ba(OH)2·8H2O high-performance composite phase change materials. J. Therm. Anal. Calorim. 2022, 147, 13239–13252. [Google Scholar] [CrossRef]
- Han, X.; Zhang, X.; Hua, W.; Yuan, W.; Jia, X.; Wang, Z.F. Preparation and application of composite EG/Ba(OH)2·8H2O form-stable phase change material for solar thermal storage. Energy Res. 2019, 43, 2227–2240. [Google Scholar] [CrossRef]
- Pan, C.; Charles, J.; Vermaak, N.; Romero, C.; Neti, S.; Zheng, Y.; Chen, C.H.; Bonner, R. Experimental, numerical and analytic study of unconstrained melting in a vertical cylinder with a focus on mushy region effects. Int. J. Heat Mass Transf. 2018, 124, 1015–1024. [Google Scholar] [CrossRef]
- Rezvanpour, M.; Borooghani, D.; Torabi, F.; Pazoki, M. Using CaCl2·6H2O as a phase change material for thermo-regulation and enhancing photovoltaic panels’ conversion efficiency: Experimental study and TRNSYS validation. Renew. Energy 2020, 146, 1907–1921. [Google Scholar] [CrossRef]
- Lizana, J.; Chacartegui, R.; Barrios-Padura, A.; Valverde, J.M.; Ortiz, C. Identification of best available thermal energy storage compounds for low—to- moderate temperature storage applications in buildings. Mater. Construcción 2018, 68, 331. [Google Scholar] [CrossRef]
- Journal of Laws 2019 Pos. 1311. Regulation on substances particularly harmful to the aquatic environment and conditions to be met when discharging sewage into waters or into the ground, as well as when discharging rainwater or snowmelt into water or into water facilities (Poland). Date of text 12 Jul 2019. Available online: https://faolex.fao.org/docs/pdf/pol192320.pdf (accessed on 1 May 2022).
- Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC) No 1907/2006 (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/eli/reg/2008/1272/oj (accessed on 1 May 2022).
- Council Directive 91/689/EEC of 12 December 1991 on hazardous waste. Official Journal of the European Communities. L377, vol.34, ISSN 0378-6978. Available online: https://eurlex.europa.eu/legalcontent/EN/TXT/PDF/?uri=OJ:L:1991:377:FULL&from=EN (accessed on 1 May 2022).
- United Nations. Globally Harmonized System of Classification and Labeling of Chemicals. ST/SG/AC.10/30/Rev.4 2011. [Online]. Available online: https://www.unece.org/fileadmin/DAM/trans/danger/publi/ghs/ghs_rev04/English/ST-SG-AC10-30-Rev4e.pdf (accessed on 27 June 2022).
- Melcer, A.; Klugmann-Radziemska, E.; Lewandowski, W.M. Materiały zmiennofazowe. Właściwości, klasyfikacja, zalety i wady. Przem. Chem. 2012, 7, 1000–1011. [Google Scholar]
- Pilar, R.; Svoboda, L.; Honcova, P.; Oravova, L. Study of magnesium chloride hexahydrate as heat storage material. Thermochim. Acta 2012, 546, 81–86. [Google Scholar] [CrossRef]
- Ci, E.; Wang, H.; Xiaoqing Li, X.; Zhang, Y.; Zhang, Z.; Li, J. Preparation and property enhancement of magnesium nitrate hexahydrate-lithium nitrate eutectic/expanded graphite composite phase change materials. Energy Storage Sci. Technol. 2022, 11, 30–37. [Google Scholar] [CrossRef]
- Honcova, P.; Pilar, R.; Danielik, V.; Soska, P.; Sadovska, G.; Honc, D. Suppressing supercooling in magnesium nitrate hexahydrate and evaluating corrosion of aluminium alloy container for latent heat storage application. J. Therm. Anal. Calorim. 2017, 129, 1573–1581. [Google Scholar] [CrossRef]
- Honcova, P.; Sádovská, G.; Pastvová, J.; Kotál, P.; Seidel, J.; Sazama, P.; Pila, R. Improvement of thermal energy accumulation by incorporation of carbon nanomaterial into magnesium chloride hexahydrate and magnesium nitrate hexahydrate. Renew. Energy 2021, 168, 1015–1026. [Google Scholar] [CrossRef]
- Tie, J.; Liu, X.; Tie, S.; Zhang, I.; Jiang, S.; Tao, R. Packing and properties of composite phase change energy storage materials based on SiC nanowires and Na2SO4·10H2O. J. Therm. Anal. Calorim. 2020, 139, 855–862. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Svensson, G.; Hiebler, S.; Mehling, H. Thermal performance of sodium acetate trihydrate thickened with different materials as phase change energy storage material. Appl. Therm. Eng. 2003, 23, 1697. [Google Scholar] [CrossRef]
- Takahiro, W.; Fumiko, K.; Yoshihiro, M. Studies on Salt Hydrates for Latent Heat Storage. IV. Crystallization in the Binary System CH3CO2Na-H2O. Bull. Chem. Soc. Jpn. 1983, 56, 3827. [Google Scholar] [CrossRef]
- Zheng, M.; Li, Y.; Zhang, S.; Peng, X.; Jin, H. Sodium acetate-based hydrated salt for solar thermal storage. Nanomater. Energy 2021, 10, 128–136. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.; Lai, A.; Li, G.; Wang, L. Study on Modification of Phase Change Energy Storage Materials Suitable for Biogas Fermentation. In Proceedings of the 2nd International Conference on Power and Energy Engineering (ICPEE 2018), Xiamen, China, 3–5 September 2018; Volume 192, p. 012055. [Google Scholar] [CrossRef]
- Xiao, Q.; Yuan, W.; Li, L.; Xu, T. Fabrication and characteristics of composite phase change material based on Ba(OH)2·8H2O for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 179, 339–345. [Google Scholar] [CrossRef]
- Hua, W.; Zhang, X.; Liu, F.; Han, X.; Yuan, W.; Wang, X. Preparation and thermal properties of composite barium hydroxide octahydrate for energy storage. Chem. Ind. Eng. Prog. 2018, 37, 4384–4389. [Google Scholar] [CrossRef]
- Xu, X.; Dong, Z.; Memon, S.A.; Bao, X.; Cui, H. Preparation and Supercooling Modification of Salt Hydrate Phase Change Materials Based on CaCl2·2H2O/CaCl2. Materials 2017, 10, 691. [Google Scholar] [CrossRef]
- Xu, X.; Cui, H.; Memon, S.A.; Yang, H.; Tang, W. Development of novel composite PCM for thermal energy storage using CaCl2·6H2O with graphene oxide and SrCl2·6H2O. Energy Build. 2017, 156, 163–172. [Google Scholar] [CrossRef]
- Cui, K.; Liu, L.; Sun, M. Study on improving the heat storage property of Ba(OH)2·8H2O with paraffin. Mater. Res. Express 2017, 4, 125502. [Google Scholar] [CrossRef]
- Mehralia, M.; ten Elshofb, J.E.; Shahia, M.; Mahmoudia, A. Simultaneous solar-thermal energy harvesting and storage via shape stabilized salt hydrate phase change material. Chem. Eng. J. 2021, 405, 126624. [Google Scholar] [CrossRef]
- Shah, K.W.; Huseien, G.F. Chapter 17—Nanostructures encapsulated phase-change materials for sustained thermal energy storage in concrete. In Micro and Nano Technologies, Green Nanomaterials for Industrial Applications; Shanker, U., Hussain, C.M., Rani, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 477–507. ISBN 9780128232965. [Google Scholar] [CrossRef]
- Ushak, S.; Marín, P.; Galazutdinova, Y.; Cabeza, L.F.; Farid, M.M.; Grágeda, M. Compatibility of materials for macroencapsulation of inorganic phase change materials: Experimental corrosion study. Appl. Therm. Eng. 2016, 107, 410–419. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, G.; Muthumani, A. Corrosion of metals exposed to 25% magnesium chloride solution and tensile stress: Field and laboratory studies. Case Stud. Constr. Mater. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Xi, Y.; Xie, Z. Corrosion Effects of Magnesium Chloride and Sodium Chloride on Automobile Components by Principal Investigators; Report No. CDOT-DTD-R-2002-4; Colorado Department of Transportation Research Branch: Denver, CO, USA, 2002. [Google Scholar]
- Danielik, V.; Šoška, P.; Felgerová, K.; Zemanová, M. The corrosion of carbon steel in nitrate hydrates used as phase change materials. Mater. Corros. 2017, 68, 416–422. [Google Scholar] [CrossRef]
- Nagano, K.; Ogawa, K.; Mochida, T.; Hayashi, K.; Ogoshi, H. Performance of heat charge/discharge of magnesium nitrate hexahydrate and magnesium chloride hexahydrate mixture to a single vertical tube for latent heat storage system. Appl. Therm. Eng. 2004, 24, 209–220. [Google Scholar] [CrossRef]
- Farrell, A.J.; Norton, B.; Kennedy, D.M. Corrosive effects of salt hydrate phase change materials used with aluminium and copper. J. Mater. Process. Technol. 2006, 175, 198–205. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Roca, J.; Illa, J.; Badia, F.; Mehling, H.; Hiebler, S.; Ziegler, F. Middle term immersion corrosion tests on metal-salt hydrate pairs used for latent heat storage in the 32 to 36°C temperature range. Mater. Corros. 2001, 52, 748–754. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Roca, J.; Illa, K.; Badia, F.; Mehling, H.; Hiebler, S.; Ziegler, F. Corrosion Experiments on Salt Hydrates used as Phase Change Materials in Cold Storage. In IEA ECES IA Annex 17 Kick-Off Workshop; International Energy Agency: Lleida, Spain, 2001. [Google Scholar]
- Available online: https://www.corrosionpedia.com/definition/2782/sodium-carbonate (accessed on 10 May 2022).
- Purohit, B.K.; Sistla, V.S. Inorganic salt hydrate for thermal energy storage application: A review. Energy Storage 2020, 3, e212. [Google Scholar] [CrossRef]
- Sheng, Q.; Xing, Y. Preparation and heat transfer performance of Ba(OH)2·8H2O/copper foam phase change composites. Fuhe Cailiao Xuebao/Acta Mater. Compos. Sin. 2014, 31, 1566–1572. [Google Scholar]
- Mao, F.; Zhang, X.; Wang, Y.; Chen, W. Compatibility study of barium hydroxide octahydrate and phase change heat storage container. Taiyangneng Xuebao/Acta Energ. Sol. Sin. 2017, 38, 2292–2296. [Google Scholar]
- Available online: https://dixonvalve.com/sites/default/files/documents/corrosion-resistance%20chart.pdf (accessed on 1 September 2022).
- Kumar, N.; Chavez, R.; Banerjee, D. Experimental Measurement of Corrosion Involving Inorganics (Salt Hydrates) Phase Change Materials (PCM) for Thermal Energy Storage (TES) Applications. Intersoc. Conf. Therm. Phenom. Electron. Syst. (ITherm) 2018, 17, 117–125. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Roca, J.; Illa, J.; Badia, F.; Mehling, H.; Hiebler, S.; Ziegler, F. Immersion corrosion tests on metal-salt hydrate pairs used for latent heat storage in the 32 to 36 °C temperature range. Mater. Corros. 2001, 52, 140–146. [Google Scholar] [CrossRef]
- Waste Act. Legislation. Announcement of the Speaker of the Sejm of the Republic of Poland of 16 April 2020 on the Announcement of the Consolidated Text of the Act on Waste. J. Laws 2020, item 797. Available online: https://faolex.fao.org/docs/pdf/pol182641.pdf (accessed on 1 April 2022).
- Regulation of the Minister for the Environment as of the 9th of December 2014 Concerning the Catalogue of Waste (Journal of Laws 2014, item 1923). Available online: https://faolex.fao.org/docs/pdf/pol192338.pdf (accessed on 1 April 2022).
- Report Photovoltaics Market in Poland. Institute of Renewable Energy, iEO ec brec, Warsaw 2022. Available online: https://ieo.pl/pl/aktualnosci/1591-raport-rynek-fotowoltaiki-w-polsce-2022 (accessed on 17 July 2022).
- Szreder, M. Methods and Techniques of Opinion Polls; Polish Economic Publishing House: Warsaw, Poland, 2010; ISBN 978-83-208-1843-7. (In Polish) [Google Scholar]
- Segreto, M.; Principe, L.; Desormeaux, A.; Torre, M.; Tomassetti, L.; Tratzi, P.; Paolini, V.; Petracchini, F. Trends in Social Acceptance of Renewable Energy Across Europe—A Literature Review. Int. J. Environ. Res. Public Health 2020, 17, 9161. [Google Scholar] [CrossRef]

| CAS Number | Salt Hydrates | Melting Temperature °C | Heat of Fusion J/g | Density g/cm3 | Cost €/kg |
|---|---|---|---|---|---|
| 7791-18-6 | MgCl2·6H2O | 116.7 [18] 115 [19] | 166.9 [19] | 1.57 [18] | 164.9 [20] |
| Magnesium Chloride Hexahydrate | |||||
| 13446-18-9 | Mg(NO3)2·6H2O | 89 [15,21] | 162 [15] | 1.64 [21] | 115.6 [20] |
| Magnesium Nitrate Hexahydrate | |||||
| 7727-73-3 | Na2SO4·10H2O | 32.4 [10,22] | 251 [10] | 1.46 [22] | 104.7 [20] |
| Sodium Sulfate Decahydrate | |||||
| 6131-90-4 | CH3COONa·3H2O | 57.9 [23] 58 [24] | 260 [25] 250 [26] | 1.45 [23] | 112.6 [20] |
| Sodium Acetate Trihydrate | |||||
| 6132-02-1 | Na2CO3·10H2O | 30 [27] 32.4 [8] | 247 [8] | 1.44 [27] | 47.4 [20] |
| Sodium Carbonate Decahydrate | |||||
| 10039-32-4 | Na2HPO4·12H2O | 35 [28,29] | 256.6 [28] 278.8 [29] | 1.52 [28,30] | 40.2 [20] |
| Disodium Hydrogen Phosphate Dodecahydrate | |||||
| 12230-71-6 | Ba(OH)2·8H2O | 78 [31,32] | 233–332 [32] | 2.18 [31] | 127.1 [20] |
| Barium Hydroxide Octahydrate | |||||
| 7774-34-7 | CaCl2·6H2O | 30 [33] 28.6 [34] 29 [35] | 169.5 [34] 160 [35] | 1.71 [33] 1.83 [35] | 31.4 [20] |
| Calcium Chloride Hexahydrate | |||||
| Hydrated Salts | Environmental Hazards | Public Health Hazards |
|---|---|---|
| MgCl2·6H2O | Fire may release hazardous vapours—hydrogen chloride gas, magnesium oxide. Hygroscopic. Corrosive. Hazardous waste (HW). | Use with adequate ventilation. |
| Mg(NO3)2·6H2O | Avoid strong heating. Decomposition products: magnesium and nitrogen oxides. Hygroscopic. It can cause excessive eutrophication. Increases the flammability of other substances. | Use with adequate ventilation. Dusts may cause irritation of the respiratory and digestive systems. Absorption in the body leads to the formation of methemoglobin. |
| Na2SO4·10H2O | Fire may release hazardous vapours—sulphur and sodium oxides. Reacts exothermically with strong acids, aluminium and magnesium. Avoid moisture and heating. HW. | Use with adequate ventilation.At a concentration of >500 mg/L of water, it may cause irritation of the digestive tract. |
| CH3COONa·3H2O | Fire may release hazardous vapours. Possibility of explosion—reacts with nitrates. Exothermic reaction with fluorine. Hygroscopic. HW. | Use with adequate ventilation. Avoid inhalation of dusts. |
| Na2CO3·10H2O | Hazardous vapours—carbon and sodium oxides. Hygroscopic. High concentration in water can cause alkalization. Dangerous reactions with aluminium, alkaline earth metals in powder form, organic nitro compounds, fluorine, and non-metal oxides. Violent reactions with sulphuric acid, phosphorus pentoxide, fluorine, lithium, 2,4,6-trinitrotoluene, trichlorethylene, and aluminum. HW. | No special ventilation is required. May cause corneal damage. Irritating to eyes. Eye Irrit. 2, H319. |
| Na2HPO4·12H2O | Hazardous vapour—phosphorus oxides. Reacts exothermically with strong acids, antipyrine and acetates. | Use with adequate ventilation. |
| Ba(OH)2·8H2O | Fire may release hazardous vapours. Barium oxide. Sensitive to air. Reacts with carbon dioxide. Exothermic reaction with hydrogen sulphide and acids. HW. | It is extremely destructive to the respiratory system, eyes, and skin. Corrosive and poisonous to the body. Acute toxicity inhalation H332, dusty H302, corrosive to skin H314. |
| CaCl2·6H2O | Dangerous gases—chlorine, hydrogen chloride, chlorine oxides, and calcium oxides. Strongly hygroscopic. Dangerous reactions—boron and calcium oxides, bromine trifluoride; 2-furancarboxylic acid, reacts violently with zinc with evolution of gas; exothermic occurs vinyl ether polymerization reaction catalysis; reacts with water releasing heat. Calcium ions combine with sulphate or carbon ions in the soil to form stable, inorganic salts. Chlorine ions are mobile in the soil. HW. | Use with adequate ventilation. Irritating to eyes. Eye Irrit. 2, H319. Skin irritation with frequent contact. |
| Salt Hydrate | Preventive Measure | |
|---|---|---|
| Against Phase Separation | Against Supercooling | |
| MgCl2·6H2O | 85% by weight g-C3N4 + 2% by weight SrCl2·6H2O [38] | |
| 1% by weight SrCO3, 0.5% by weight Sr(OH)2, Mg(OH)2 [57] sufficiently large sample weight 100 g [19] | ||
| Mg(NO3)2·6H2O | carbon sphere [37]; Magnesium Nitrate Hexahydrate-Lithium Nitrate eutectic salt 85∶15 mass ratio [58] | |
| 0.5–2% by weight Mg(OH)2, BaO, MgO and Sr(OH)2 [59] 3% graphite + 3% graphene [60] | ||
| Na2SO4·10H2O | MPCM microcapsules with a silica shell [10]; microcapsules + 1.0 mL Triton X-100 + tetraethyl orthosilicate (TEOS) and 3-aminopropyltriethoxysilane (APTS) [10]; SSD-CBO synthesized by SSD, carboxymethyl cellulose (CMC), borax decahydrate, and OP-10 + 7% by weight EG [39] | |
| 1–5% by weight nanowires SIC [61] | ||
| CH3COONa·3H2O | methylhydroxyethylcellulose 30% by weight [62]; polyvinyl alcohol combined with paraffin oil (1%), acetone (0.5%) and additional water (3.5%) [63]; SAT adsorbed in expanded vermiculite [62]; poly(methacrylic acid-co-methyl methacrylate (PMMA-co-MMA) 0.8% sodium acetate 57.78%, water 41.42% [23]; sodium salt of poly(methacrylic acid) (Na-PMAA) 0.67%, Sodium Acetate 57.85%, water 41.48% [23]; Sodium Acetate Trihydrate/Disodium Hydrogen Phosphate Dodecahydrate 8.5:1.5 + 1.5% Sodium Metasilicate Nonahydrate [64] | silicon carbide, bentonite, expanded graphite, copper nanoparticles, Disodium Hydrogen Phosphate, Tetrasodium Pyrophosphate [23]; Na4P2O7·10H2O and thickening agent polyacrylamide [24]; 2–3% by weight EG in water SAT [25] |
| Na2CO3·10H2O | Sodium Carbonate Decahydrate (SCD)-Na2CO3.10H2O and Magnesium Sulphate Heptahydrate (MSH)—MgSO4·7H2O in proportions 70:30 [44]. | |
| Disodium Hydrogen Phosphate Dodecahydrate and Sodium Carbonate Decahydrate encapsulated into EG [42] | ||
| Na2HPO4·12H2O | 15% by weight EG [42], mixing Na2HPO4·12H2O, sodium carboxymethyl cellulose (CMC), aluminum oxide (Al2O3), and poly(vinylpyrrolidone) (PVP) in a mass ratio of 95.8:2:2:0.2 [65], | |
| sodium acrylate 3.0–5.0% (by weight), cross-linking agent N,N-methylene bisacrylamide 0.10–0.20% (by weight), K2S2O8 and Na2SO3 (mass ratio 1:1) 0.06–0.12% (w/w) [45]; borax, carboxymethyl cellulose (CMC) [45] | ||
| Ba(OH)2·8H2O | Ba(OH)2·8H2O/MEG—expanded graphite modified with polyoxyethylene octylphenol ether [66] | |
| 95.1%Ba(OH)2·8H2O + 2%Ba(OH)2·H2O + 2.9%H2O [67]; 3% by weight xanthan gum (XG) [47] | 1% copper powder, 1% calcium fluoride and 1% calgon (by weight) [31]; 3% calcium fluoride (by weight) [47] | |
| CaCl2·6H2O | BaI2·6H2O, SrCl2·6H2O i SrBr2·6H2O [50] 66.21% by weight CaCl2·2H2O [68] 0.5 wt % nano SiO2 particles [68] nanosheets GO, SrCl2·6H2O [69] | |
| Salt Hydrates | Corrosiveness of Materials Due to Salt Hydrates | |
|---|---|---|
| Corrosion-Prone Materials | Corrosion-Resistant Materials | |
| MgCl2·6H2O | copper, aluminium, and stainless steel [73] carbon steel (C 1010); aluminium steel (Al 1100) 1 [74]; metal sheets (aluminium, copper, stainless steel) [75] | carbon foam, diatomite, expanded graphite and expanded perlite [46] polyethylene, polyvinyl chloride, unplasticized, butyl rubber 2 [75] |
| Mg(NO3)2·6H2O | mild carbon steel [76]; carbon steel, copper, brass 3 [77] | aluminum, SUS316 steel 3 [77] mild carbon steel 4, aluminium alloy 5 [59,60] |
| Na2SO4·10H2O | Copper 6 [78] | aluminum [78] |
| CH3COONa·3H2O | brass [42]; copper [42,79,80]; aluminium [42,79] | steel, stainless steel, glass, polyethylene, polypropylene [42]; aluminium [79] |
| Na2CO3·10H2O | aluminium, copper [80]; plastics (polyacrylates and polysulfide), aluminium, lead, zinc, and zinc brasses [81] | stainless steel, carbon steel, nickel cast iron, nickel and nickel-base alloys, acrylonitrile-butadiene-styrene (ABS), chlorinated polyvinyl chloride (CPVC), nylon, polyethylene, polypropylene, polyvinyl chloride (PVC), Teflon, other fluorocarbons, and some elastomers [81] |
| Na2HPO4·12H2O | aluminium [79] | carbon foam, diatomaceous earth, expanded graphite, and expanded perlite [82] |
| Ba(OH)2·8H2O | copper foam [83]; 20# carbon steel, T2 red copper, H62 brass [84]; aluminium bronze [85] | 304 austenitic stainless steel [84]; monel, brass, hastelloy, stainless steel 304, nylon, polypropylene [85] |
| CaCl2·6H2O | aluminium (AL 1000 series) [86]; aluminium [87] | stainless steel (SS 347) [86]; carbon foam, diatomite, expanded graphite, and expanded perlite [46]; silver [87] |
| Inorganic Salt Hydrates | Criteria | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T a | C b,** | E c | H d | Ps e | S f | Cr g | Ʃ | |||
| Magnesium Chloride Hexahydrate | 1 | 1 | 1 | 3 | 2 | 2 | 1 | 11 | ||
| Magnesium Nitrate Hexahydrate | 1 | 2 | 3 | 3 | 2 | 2 | 2 | 15 | ||
| Sodium Sulphate Decahydrate | 2 | 2 | 2 | 3 | 1 | 2 | 2 | 14 | ||
| Sodium Acetate Trihydrate | 3 | 1 | 2 | 3 | 2 | 1 (2 *) | 2 | 15 | ||
| Sodium Carbonate Decahydrate | 2 | 3 | 1 | 2 | 2 | 2 | 2 | 14 | ||
| Disodium Hydrogen Phosphate Dodecahydrate | 3 | 3 | 3 | 3 | 3 | 1 | 2 | 18 | ||
| Barium Hydroxide Octahydrate | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 11 | ||
| Calcium Chloride Hexahydrate | 2 | 3 | 1 | 2 | 2 | 2 | 1 | 13 | ||
| Criteria | Scale | |||||||||
| 1 | 2 | 3 | ||||||||
| a T—Thermophysical parameters | medium | good | very good | |||||||
| b C—Cost **; | high | medium | low | |||||||
| c E—Adverse impact on the Environment; | ||||||||||
| d H—Adverse impact on the Health; | ||||||||||
| e Ps—Phase separation; | ||||||||||
| f S—Supercooling | ||||||||||
| g Cr—Corrosiveness | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nartowska, E.; Styś-Maniara, M.; Kozłowski, T. The Potential Environmental and Social Influence of the Inorganic Salt Hydrates Used as a Phase Change Material for Thermal Energy Storage in Solar Installations. Int. J. Environ. Res. Public Health 2023, 20, 1331. https://doi.org/10.3390/ijerph20021331
Nartowska E, Styś-Maniara M, Kozłowski T. The Potential Environmental and Social Influence of the Inorganic Salt Hydrates Used as a Phase Change Material for Thermal Energy Storage in Solar Installations. International Journal of Environmental Research and Public Health. 2023; 20(2):1331. https://doi.org/10.3390/ijerph20021331
Chicago/Turabian StyleNartowska, Edyta, Marta Styś-Maniara, and Tomasz Kozłowski. 2023. "The Potential Environmental and Social Influence of the Inorganic Salt Hydrates Used as a Phase Change Material for Thermal Energy Storage in Solar Installations" International Journal of Environmental Research and Public Health 20, no. 2: 1331. https://doi.org/10.3390/ijerph20021331
APA StyleNartowska, E., Styś-Maniara, M., & Kozłowski, T. (2023). The Potential Environmental and Social Influence of the Inorganic Salt Hydrates Used as a Phase Change Material for Thermal Energy Storage in Solar Installations. International Journal of Environmental Research and Public Health, 20(2), 1331. https://doi.org/10.3390/ijerph20021331






