Impact of Metformin on Periodontal and Peri-Implant Soft and Hard Tissue
Abstract
1. Introduction
2. Materials and Methods
2.1. PICO Questions
2.2. Selection Criteria
2.3. Search Methodology
2.4. Clinical Significance
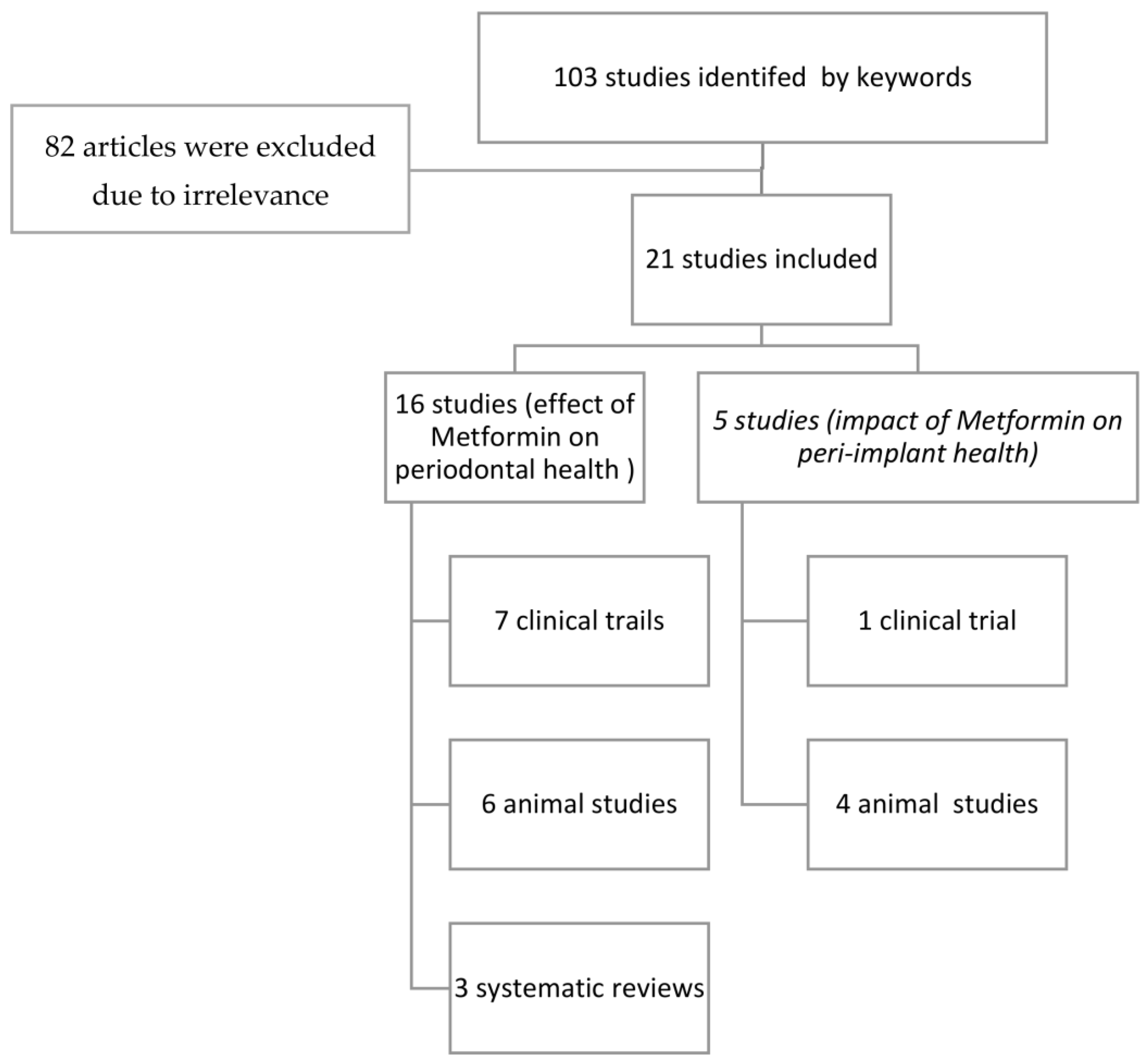
3. Results
3.1. Impact of Metformin on Periodontal Health
3.1.1. Animal Studies
3.1.2. Clinical Studies
3.1.3. Systematic Reviews
3.2. Impact of Metformin on Peri-Implant Health
3.2.1. Animal Studies
3.2.2. Clinical Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harada, S.; Rodan, G.A. Control of Osteoblast Function and Regulation of Bone Mass. Nature 2003, 423, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, M.E.; Gimble, J.M. Controlling the Balance between Osteoblastogenesis and Adipogenesis and the Consequent Therapeutic Implications. Curr. Opin. Pharmacol. 2004, 4, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M.; Bissett, S.M. Periodontitis: Oral Complication of Diabetes. Endocrinol. Metab. Clin. 2013, 42, 849–867. [Google Scholar] [CrossRef] [PubMed]
- Mealey, B.L. Periodontal Disease and Diabetes: A Two-Way Street. J. Am. Dent. Assoc. 2006, 137, S26–S31. [Google Scholar] [CrossRef]
- Mealey, B.L.; Oates, T.W. Diabetes Mellitus and Periodontal Diseases. J. Periodontol. 2006, 77, 1289–1303. [Google Scholar] [CrossRef]
- Bailey, C.J. Metformin: Historical Overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef]
- Cortizo, A.M.; Sedlinsky, C.; McCarthy, A.D.; Blanco, A.; Schurman, L. Osteogenic Actions of the Anti-Diabetic Drug Metformin on Osteoblasts in Culture. Eur. J. Pharmacol. 2006, 536, 38–46. [Google Scholar] [CrossRef]
- Bak, E.J.; Park, H.G.; Kim, M.; Kim, S.W.; Kim, S.; Choi, S.-H.; Cha, J.-H.; Yoo, Y.-J. The Effect of Metformin on Alveolar Bone in Ligature-induced Periodontitis in Rats: A Pilot Study. J. Periodontol. 2010, 81, 412–419. [Google Scholar] [CrossRef]
- Vestergaard, P.; Rejnmark, L.; Mosekilde, L. Relative Fracture Risk in Patients with Diabetes Mellitus, and the Impact of Insulin and Oral Antidiabetic Medication on Relative Fracture Risk. Diabetologia 2005, 48, 1292–1299. [Google Scholar] [CrossRef]
- Blümel, J.E.; Arteaga, E.; Aedo, S.; Arriola-Montenegro, J.; López, M.; Martino, M.; Miranda, C.; Miranda, O.; Mostajo, D.; Ñañez, M. Metformin Use Is Associated with a Lower Risk of Osteoporosis in Adult Women Independent of Type 2 Diabetes Mellitus and Obesity. REDLINC IX Study. Gynecol. Endocrinol. 2020, 36, 421–425. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, C.; Hu, Y.; Peng, B. Protective Effect of Metformin on Periapical Lesions in Rats by Decreasing the Ratio of Receptor Activator of Nuclear Factor Kappa B Ligand/Osteoprotegerin. J. Endod. 2012, 38, 943–947. [Google Scholar] [CrossRef]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Madathil, S.A.; Mali, M.; Almas, K. Efficacy of Metformin in the Management of Periodontitis: A Systematic Review and Meta-Analysis. Saudi Pharm. J. 2018, 26, 634–642. [Google Scholar] [CrossRef]
- De Araújo, A.A.; de Pereira, A.S.B.F.; de Medeiros, C.A.C.X.; de Brito, G.A.C.; de Leitão, R.F.C.; de Araújo, L.S.; Guedes, P.M.M.; Hiyari, S.; Pirih, F.Q.; de Araújo Júnior, R.F. Effects of Metformin on Inflammation, Oxidative Stress, and Bone Loss in a Rat Model of Periodontitis. PLoS ONE 2017, 12, e0183506. [Google Scholar] [CrossRef]
- Pradeep, A.R.; Rao, N.S.; Naik, S.B.; Kumari, M. Efficacy of Varying Concentrations of Subgingivally Delivered Metformin in the Treatment of Chronic Periodontitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2013, 84, 212–220. [Google Scholar] [CrossRef]
- Pradeep, A.R.; Nagpal, K.; Karvekar, S.; Patnaik, K.; Naik, S.B.; Guruprasad, C.N. Platelet-rich Fibrin with 1% Metformin for the Treatment of Intrabony Defects in Chronic Periodontitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2015, 86, 729–737. [Google Scholar] [CrossRef]
- Schurman, L.; McCarthy, A.D.; Sedlinsky, C.; Gangoiti, M.V.; Arnol, V.; Bruzzone, L.; Cortizo, A.M. Metformin Reverts Deleterious Effects of Advanced Glycation End-Products (AGEs) on Osteoblastic Cells. Exp. Clin. Endocrinol. Diabetes 2008, 116, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, A.R.; Patnaik, K.; Nagpal, K.; Karvekar, S.; Ramamurthy, B.L.; Naik, S.B.; Suke, D.; Singh, P.; Raju, A. Efficacy of Locally-delivered 1% Metformin Gel in the Treatment of Intrabony Defects in Patients with Chronic Periodontitis: A Randomized, Controlled Clinical Trial. J. Investig. Clin. Dent. 2016, 7, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.S.; Pradeep, A.R.; Kumari, M.; Naik, S.B. Locally Delivered 1% Metformin Gel in the Treatment of Smokers with Chronic Periodontitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2013, 84, 1165–1171. [Google Scholar] [CrossRef]
- Inouye, K.A.S.; Bisch, F.C.; Elsalanty, M.E.; Zakhary, I.; Khashaba, R.M.; Borke, J.L. Effect of Metformin on Periimplant Wound Healing in a Rat Model of Type 2 Diabetes. Implant. Dent. 2014, 23, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wu, X.; Ling-Ling, E.; Wang, D.-S.; Liu, H.-C. The Transmembrane Transport of Metformin by Osteoblasts from Rat Mandible. Arch. Oral Biol. 2009, 54, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Zhen, D.; Chen, Y.; Tang, X. Metformin Reverses the Deleterious Effects of High Glucose on Osteoblast Function. J. Diabetes Its Complicat. 2010, 24, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Serrão, C.; Ferreira Bastos, M.; Ferreira Cruz, D.; de Souza Malta, F.; Camacho Vallim, P.; Mendes Duarte, P. Role of Metformin in Reversing the Negative Impact of Hyperglycemia on Bone Healing Around Implants Inserted in Type 2 Diabetic Rats. Int. J. Oral Maxillofac. Implant. 2017, 32, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, T.T.; Dündar, S.; Bozoğlan, A.; Karaman, T.; Kahraman, O.E.; Özcan, E.C. The Effects of Metformin on the Bone Filling Ration around of TiAl6Va4 Implants in Non Diabetic Rats. J. Oral. Biol. Craniofac. Res. 2020, 10, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Gadicherla, S.; Smriti, K.; Roy, S.; Pentapati, K.-C.; Rajan, J.; Walia, A. Comparison of Extraction Socket Healing in Non-Diabetic, Prediabetic, and Type 2 Diabetic Patients. Clin. Cosmet. Investig. Dent. 2020, 12, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Ding, F.; Liu, X.; Wang, L.; Wang, X.; Zhang, S.; Zhao, G.; Song, Y. Clinical and Radiographic Variables Related to Implants with Simultaneous Grafts among Type 2 Diabetic Patients Treated with Different Hypoglycemic Medications: A Retrospective Study. BMC Oral Health 2021, 21, 214. [Google Scholar] [CrossRef]
- Malta, F.S.; Garcia, R.P.; Azarias, J.S.; Ribeiro, G.K.D.R.; Miranda, T.S.; Shibli, J.A.; Bastos, M.F. Impact of Hyperglycemia and Treatment with Metformin on Ligature-Induced Bone Loss, Bone Repair and Expression of Bone Metabolism Transcription Factors. PLoS ONE 2020, 15, e0237660. [Google Scholar] [CrossRef]
- Pereira, A.; Brito, G.; Lima, M.; Silva Júnior, A.; Silva, E.; de Rezende, A.; Bortolin, R.; Galvan, M.; Pirih, F.; Araújo Júnior, R.; et al. Metformin Hydrochloride-Loaded PLGA Nanoparticle in Periodontal Disease Experimental Model Using Diabetic Rats. Int. J. Mol. Sci. 2018, 19, 3488. [Google Scholar] [CrossRef]
- Xu, W.; Tan, W.; Li, C.; Wu, K.; Zeng, X.; Xiao, L. Metformin-Loaded β-TCP/CTS/SBA-15 Composite Scaffolds Promote Alveolar Bone Regeneration in a Rat Model of Periodontitis. J. Mater. Sci. Mater. Med. 2021, 32, 145. [Google Scholar] [CrossRef]
- Kominato, H.; Takeda, K.; Mizutani, K.; Mikami, R.; Kido, D.; Buranasin, P.; Saito, N.; Takemura, S.; Nakagawa, K.; Nagasawa, T.; et al. Metformin Accelerates Wound Healing by Akt Phosphorylation of Gingival Fibroblasts in Insulin-resistant Prediabetes Mice. J. Periodontol. 2022, 93, 258–270. [Google Scholar] [CrossRef]
- Kurian, I.G.; Dileep, P.; Ipshita, S.; Pradeep, A.R. Comparative Evaluation of Subgingivally-delivered 1% Metformin and Aloe Vera Gel in the Treatment of Intrabony Defects in Chronic Periodontitis Patients: A Randomized, Controlled Clinical Trial. J. Investig. Clin. Dent. 2018, 9, e12324. [Google Scholar] [CrossRef] [PubMed]
- Pankaj, D.; Sahu, I.; Kurian, I.G.; Pradeep, A.R. Comparative Evaluation of Subgingivally Delivered 1.2% Rosuvastatin and 1% Metformin Gel in Treatment of Intrabony Defects in Chronic Periodontitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2018, 89, 1318–1325. [Google Scholar] [CrossRef]
- Khalifehzadeh, S.; Haghanifar, S.; Jenabian, N.; Kazemi, S.; Hajiahmadi, M. Clinical and Radiographic Evaluation of Applying 1% Metformin Biofilm with Plasma Rich in Growth Factor (PRGF) for Treatment of Two-Wall Intrabony Periodontal Defects: A Randomized Clinical Trial. J. Dent. Res. Dent. Clin. Dent. Prospects. 2019, 13, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Akram, Z.; Vohra, F.; Javed, F. Locally Delivered Metformin as Adjunct to Scaling and Root Planing in the Treatment of Periodontal Defects: A Systematic Review and Meta-Analysis. J. Periodont. Res. 2018, 53, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, A.C.; Grisa, T.A.; Muniz, F.W.M.G.; Rösing, C.K.; Cavagni, J. Effect of Adjuvant Use of Metformin on Periodontal Treatment: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2019, 23, 2659–2666. [Google Scholar] [CrossRef] [PubMed]
- Bastos, M.F.; Serrão, C.R.; Miranda, T.S.; Cruz, D.F.; de Souza Malta, F.; Duarte, P.M. Effects of Metformin on Bone Healing around Titanium Implants Inserted in Non-Diabetic Rats. Clin. Oral Implant. Res. 2017, 28, e146–e150. [Google Scholar] [CrossRef]
- Araújo, M.G.; Lindhe, J. Dimensional Ridge Alterations Following Tooth Extraction. An Experimental Study in the Dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef]
- Devlin, H.; Garland, H.; Sloan, P. Healing of Tooth Extraction Sockets in Experimental Diabetes Mellitus. J. Oral Maxillofac. Surg. 1996, 54, 1087–1091. [Google Scholar] [CrossRef]
- Nevins, M.L.; Karimbux, N.Y.; Weber, H.P.; Giannobile, W.V.; Fiorellini, J.P. Wound Healing around Endosseous Implants in Experimental Diabetes. Int. J. Oral Maxillofac. Implant. 1998, 13, 620–629. [Google Scholar] [CrossRef]
- Jang, W.G.; Kim, E.J.; Bae, I.-H.; Lee, K.-N.; Kim, Y.D.; Kim, D.-K.; Kim, S.-H.; Lee, C.-H.; Franceschi, R.T.; Choi, H.-S.; et al. Metformin Induces Osteoblast Differentiation via Orphan Nuclear Receptor SHP-Mediated Transactivation of Runx2. Bone 2011, 48, 885–893. [Google Scholar] [CrossRef]
- Bosi, E. Metformin—The Gold Standard in Type 2 Diabetes: What Does the Evidence Tell Us? Diabetes Obes. Metab. 2009, 11, 3–8. [Google Scholar] [CrossRef]
- Al Jofi, F.E.; Ma, T.; Guo, D.; Schneider, M.P.; Shu, Y.; Xu, H.H.K.; Schneider, A. Functional Organic Cation Transporters Mediate Osteogenic Response to Metformin in Human Umbilical Cord Mesenchymal Stromal Cells. Cytotherapy 2018, 20, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Hui, A.L.; Back, L.; Ludwig, S.; Gardiner, P.; Sevenhuysen, G.; Dean, H.J.; Sellers, E.; McGavock, J.; Morris, M.; Jiang, D.; et al. Effects of Lifestyle Intervention on Dietary Intake, Physical Activity Level, and Gestational Weight Gain in Pregnant Women with Different Pre-Pregnancy Body Mass Index in a Randomized Control Trial. BMC Pregnancy Childbirth 2014, 14, 331. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G.; Lindhe, J. Ridge Alterations Following Tooth Extraction with and without Flap Elevation: An Experimental Study in the Dog. Clin. Oral Implant. Res. 2009, 20, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-X.; Wang, G.-Y.; Su, N.; Ma, J.; Li, Y.-K. Effects of Different Doses of Metformin on Bone Mineral Density and Bone Metabolism in Elderly Male Patients with Type 2 Diabetes Mellitus. World J. Clin. Cases 2020, 8, 4010–4016. [Google Scholar] [CrossRef]
- McDonough, A.K.; Rosenthal, R.S.; Cao, X.; Saag, K.G. The Effect of Thiazolidinediones on BMD and Osteoporosis. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 507–513. [Google Scholar] [CrossRef]
- Wang, P.; Ma, T.; Guo, D.; Hu, K.; Shu, Y.; Xu, H.H.K.; Schneider, A. Metformin Induces Osteoblastic Differentiation of Human Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells. J. Tissue Eng. Regen. Med. 2018, 12, 437–446. [Google Scholar] [CrossRef]
- Pernicova, I.; Korbonits, M. Metformin—Mode of Action and Clinical Implications for Diabetes and Cancer. Nat. Rev. Endocrinol. 2014, 10, 143–156. [Google Scholar] [CrossRef]
| Study (Author and Year) | Study Design | Types of Periodontal Lesions | Methods | Intervention | No. of Subjects | Follow -Up | Outcomes |
|---|---|---|---|---|---|---|---|
| Pereira et al., 2018 [28] | In vivo prospective | Ligature-induced periodontitis | Histopathological, immunohistochemical analysis and Micro-CT analysis | MET-loaded PLGA | Control 80 Test: 80 | 10 days | MET-loaded PLGA reduced inflammation and periodontitis-associated bone in diabetic rats. |
| Xu et al., 2021 [29] | In vivo prospective | Ligature-induced periodontitis | Micro-CT and histological analysis | Composite scaffold loading metformin (MET) implanted in the alveolar bone defects | Not reported | 7 and 14 days | MET has a positive impact on alveolar bone regeneration in periodontitis. |
| Araújo et al., 2017 [14] | In vivo prospective | Ligature-induced periodontists | Histopathological, immunohistochemical analysis and micro-CT analysis | MET-loaded PLGA | Five groups composed of twenty-one rats each: (1) Without ligature and water. (2) With Ligature and water. (3) Ligature with 50 mg/kg MF. (4) Ligature + 100 mg/kg MF. (5) Ligature + 200 mg/kg MF. | 11 days | Metformin reduces the inflammation, oxidative stress, as well as bone loss. |
| Kominato et al., 2022 [30] | In vivo prospective | ligature-induced periodontist | Histological and histomorphometry analysis, in vitro wound healing, and cell proliferation assay | Metformin effects and the underlying mechanism in the acceleration of gingival wound healing under minimal influence of glycemic control | 20 mice | 7 days | Metformin enhanced delayed gingival wound healing in insulin- resistant prediabetes. |
| Malta et al., 2020 [27] | In vivo prospective | ligature-induced periodontitis | Histometric and immunohistochemical analyses | Oral metformin | 80 rats | 84 days | Metformin and hyperglycemia have negative influence on bone repair. |
| Bak et al., 2010 [9] | In vivo | Ligature-induced periodontitis | Histologic examination and microcomputed tomography | Oral metformin | Control: 5 Test: 5 | 10 days | Metformin increases the osteoblastic differentiation in periodontitis, which may exert a favorable impact on alveolar bone. |
| Study (Author and Year) | Study Design | Types of Periodontal Lesions | Methods | Intervention | No. of Subjects | Follow -Up | Outcomes |
|---|---|---|---|---|---|---|---|
| Rao et al., 2013 [19] | RCT | Vertical bony defects in smokers with generalized chronic periodontitis | Clinical and radiographic parameters | SRP plus 1% MF (local delivery) (35 sites) | SRP plus placebo (36 sites) | 6 months | Pocket reduction and mean values of CAL gain > in the MF group at all of the follow-up visits. MF group shows significantly higher mean percentage of bone fill (26.17–6.66%) vs. placebo sites (3.75–8.06%) |
| Pradeep et al., 2013 [15] | RCT | Intrabody defects (IBDs) in patients with chronic periodontitis. | Clinical and radiographic parameters | (30 sites) SRP and placebo groups | (3 groups of 30 sites each) SRP with 0.5%, 1%, and 1.5% local MF gel. | 6 months | At 3 and 6 months, mean values of PD reduction and CAL gain were more in MF groups than the placebo. Significantly greater reduction in the depth of IBD in the MF groups in comparison to the placebo group, with greatest decrease of 1% MF. |
| Pradeep et al., 2015 [16] | RCT | Intrabody defects (IBDs) in patients with chronic periodontitis (CP). | Clinical and radiographic parameters | OFD alone (32 sites) | 32 sites were treated with OFD and PRF, 31 sites treated with OFD with 1% MF, and 31 sites with OFD and PRF plus 1% MF | 9 months | Significantly greater reduction in PD and RAL gain in the groups of PRF, 1% MF, and PRF + 1% MF than the OFD-only group. PRF + 1% MF group had greater reduction in PD and mean RAL gain and significantly higher percentage of radiographic defect depth reduction compared to PRF or MF alone at 9 months. |
| Kurian, et al., 2018 [31] | RCT | Intrabody defects in chronic periodontitis patients. | Clinical and radiographic parameters | SRP + placebo gel (30 sites). | 30 sites: SRP + 1% MtF gel) 30 sites: SRP + Aloe veragel). | 12 months | Significant PPD reduction and CAL gain at 6 and 12 months of using 1% MtF group compared to the AV and placebo groups. |
| Pradeep et al., 2016 [18] | RCT | Moderate and severe chronic periodontitis (CP). | Clinical and radiographic parameters | SRP plus placebo (34 sites) | SRP plus 1% MF (36 sites) | 9 months | In CP patient sites treated with SPR and locally delivered, MF had greater decrease in PD and more CAL gain with significant intrabony defect depth reduction |
| Pankaj et al., 2018 [32] | RCT | Intrabody defects in chronic periodontitis | Clinical and radiographic parameters | (30 sites) received SRP plus placebo gel | 30 sites: SRP plus 1.2% RSV gel 30 sites: SRP plus 1% MF gel | 12 months | Significant PD reduction and CAL gains, as well as improved bone fill reported with locally delivered 1.2% RSV and 1% MF gel in comparison with placebo gel. |
| Khalifehzedeh et al., 2019 [33] | RCT | Two-wall intrabody periodontal defects. | Clinical and radiographic parameters | OFD (6 sites) | 6 sites each: 1% MF, plasma rich in growth factor (PRGF), and PRGF and MF. | 6 months | The group of 1% MF with PRGF showed significant radiographic changes when compared with other groups. |
| Author | Intervention and Defect Studied | Number and Types of Included Studies | Outcome |
|---|---|---|---|
| Akram et al., 2018 [34] | Adjunctive use of locally delivered MF to SRP in treatment of periodontal defects | 5 RCT and 3 in meta-analysis. | All the involved experiments revealed significant BD fill, PD reduction, and CAL gain with adjunctive use of locally delivered MF as opposed to SRP alone. Meta-analysis: a statistically significant PD reduction (WMD = −3.11, 95% CI = −3.63 to −2.59, p < 0.001) and CAL gain (WMD = −2.83, 95% CI = −3.32 to −2.34, p < 0.001), BD fill (WMD = −2.96, 95% CI = −3.99 to −1.93, p < 0.001) for SRP + MF treatment compared to SRP. |
| Nicolini et al., 2019 [35] | Adjuvant effects of MF on the outcomes of mechanical periodontal treatment | 4 RCT | For PD and CAL, the findings demonstrated a weighted mean difference of 2.12 mm (95% CI 1.83–2.42) and 2.29 mm (95% CI 1.72–2.86), respectively, favoring the group subjected to 1% adjunct MF. |
| Najeeb et al., 2018 [13] | MF in the treatment of periodontitis | 2 animal studies 4 RCT | The topical usage of MF led to histological, clinical, and radiographic outcome improvements. Meta-analysis showed that application of MF improved the clinical and radiographic outcomes of SRP; however, a heterogeneity of the results was evident. |
| Study (Author and Year) | Study Design | Methods | Intervention | No. of Subjects | Follow-Up | Outcomes |
|---|---|---|---|---|---|---|
| Bastos et al., 2017 [36] | In vivo prospective implant placement | Histometric measurements of bone-to-implant contact and bone area, in addition to immunohistochemical analysis | Metformin (40 mg/kg/day by gavage). | Control: 10 Test: 10 | 30 days | MF negatively affected osseointegration by decreasing both the bone area (BA) and bone-to-implant contact (BIC) percentages and increasing the expression of RANKL around titanium implants in nondiabetic rats. |
| Inouye et al., 2014 [20] | In vivo prospective implant placement | Microcomputed tomography and blood analysis | 1 × 3 mm titanium in healed sites. Metformin (40 mg/kg). | Nondiabetics: 12 Diabetic: 12 Diabetic on metformin: 12 | 4 weeks | After metformin use, hyperglycemic type 2 diabetic rats presented improvements in blood glucose as well as wound healing around implants. |
| Yıldırım et al., 2020 [24] | In vivo (bone filling around implants in rats) | Histopathologic analysis | TiAl6Va4 implants inserted in the metaphyseal part of the tibial bone. Metformin (40 mg/kg). | Controls: 10 Metformin 10 | 28 days | The ratios of bone filling among the rats in the metformin group were 55.50 ± 14.034, and in the CNT group were 37.78 ± 13.017 (statistically higher). |
| Serrão et al., 2017 [23] | In vivo (reversing the negative effects of hyperglycemia in rats) | Histometric measurements: bone-to-implant contact (BIC), bone area (BA), κB ligand RANKL- and OPG-stained cells | Titanium implants were placed in tibiae. Metformin (40 mg/kg). | DM II DM II on metformin (40 mg/kg/day), starting on the 15th day after implant placement). Control group: nondiabetic rats without MF treatment. | 30 days | The percentages of BIC and BA in the cortical bone were decreased in the DM and MDM groups opposing the control group (p < 0.05) in the DM group compared with the control group. The percentage of BA in the medullary region was decreased (p < 0.05). The highest number of OPG-stained cells was in the MDM group; however, the highest ratio of RANKL/OPG in the medullary area was in the DM group (p < 0.05). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljofi, F.E.; Alesawy, A.; Alzaben, B.; Alshaikh, M.; Alotaibi, N.; Aldulaijan, H.A.; Alshehri, S.; Aljoghaiman, E.; Al-Dulaijan, Y.A.; AlSharief, M. Impact of Metformin on Periodontal and Peri-Implant Soft and Hard Tissue. Int. J. Environ. Res. Public Health 2023, 20, 1095. https://doi.org/10.3390/ijerph20021095
Aljofi FE, Alesawy A, Alzaben B, Alshaikh M, Alotaibi N, Aldulaijan HA, Alshehri S, Aljoghaiman E, Al-Dulaijan YA, AlSharief M. Impact of Metformin on Periodontal and Peri-Implant Soft and Hard Tissue. International Journal of Environmental Research and Public Health. 2023; 20(2):1095. https://doi.org/10.3390/ijerph20021095
Chicago/Turabian StyleAljofi, Faisal E., Aminah Alesawy, Bader Alzaben, Marwa Alshaikh, Norah Alotaibi, Hajer A. Aldulaijan, Sami Alshehri, Eman Aljoghaiman, Yousif A. Al-Dulaijan, and Mishali AlSharief. 2023. "Impact of Metformin on Periodontal and Peri-Implant Soft and Hard Tissue" International Journal of Environmental Research and Public Health 20, no. 2: 1095. https://doi.org/10.3390/ijerph20021095
APA StyleAljofi, F. E., Alesawy, A., Alzaben, B., Alshaikh, M., Alotaibi, N., Aldulaijan, H. A., Alshehri, S., Aljoghaiman, E., Al-Dulaijan, Y. A., & AlSharief, M. (2023). Impact of Metformin on Periodontal and Peri-Implant Soft and Hard Tissue. International Journal of Environmental Research and Public Health, 20(2), 1095. https://doi.org/10.3390/ijerph20021095








