Evaluation of the Effect of Perfluorohexane Sulfonate on the Proliferation of Human Liver Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
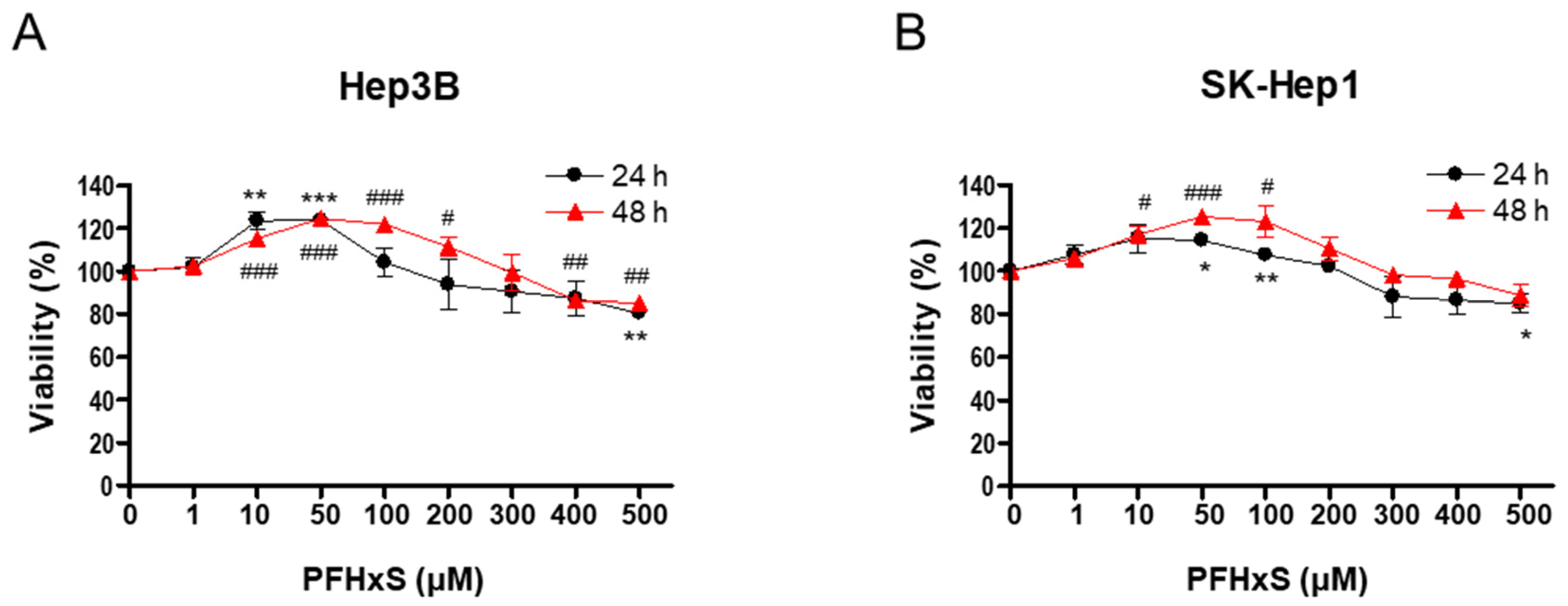
2.3. Cell Viability Assay
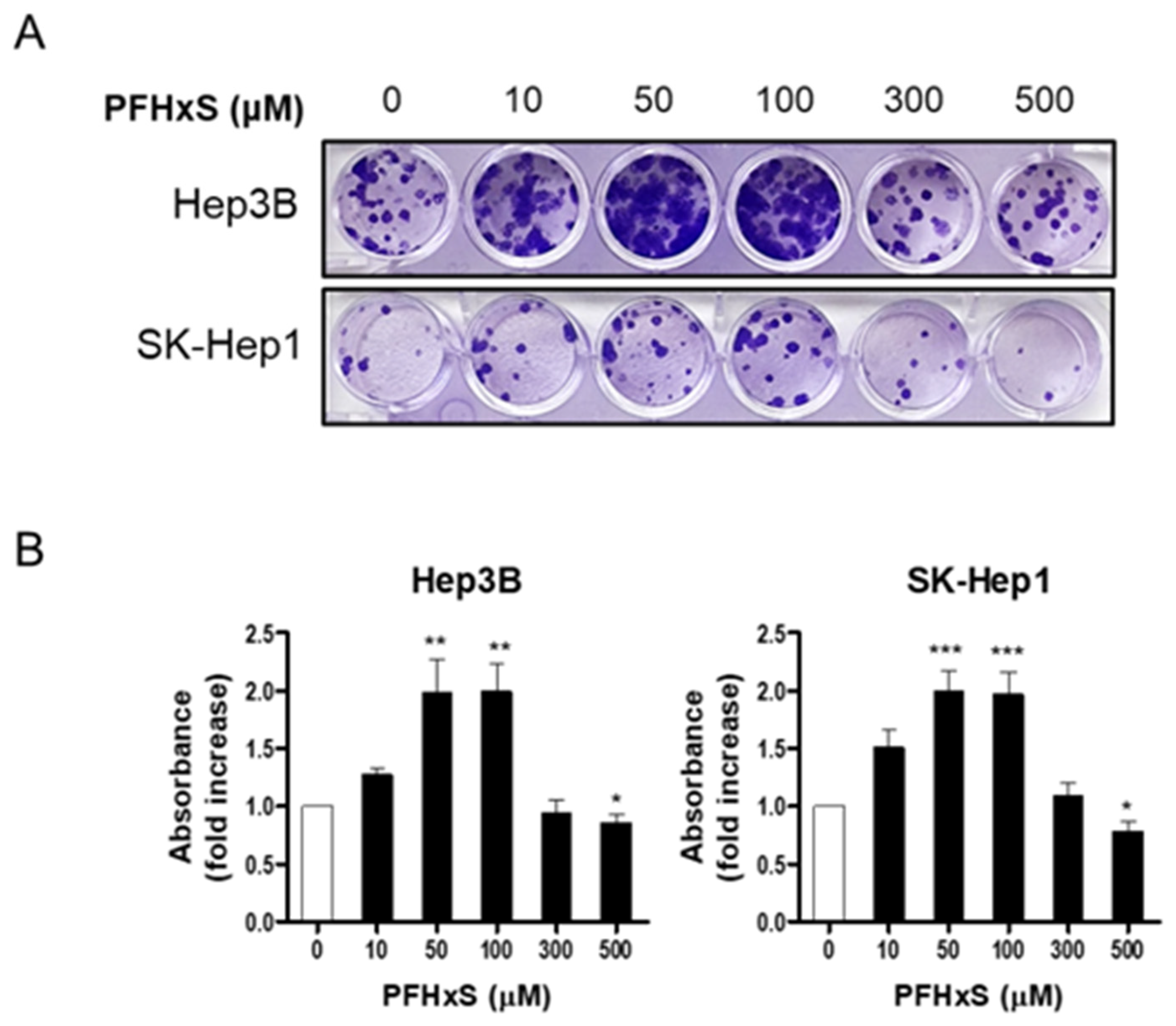
2.4. Colony Formation Assay
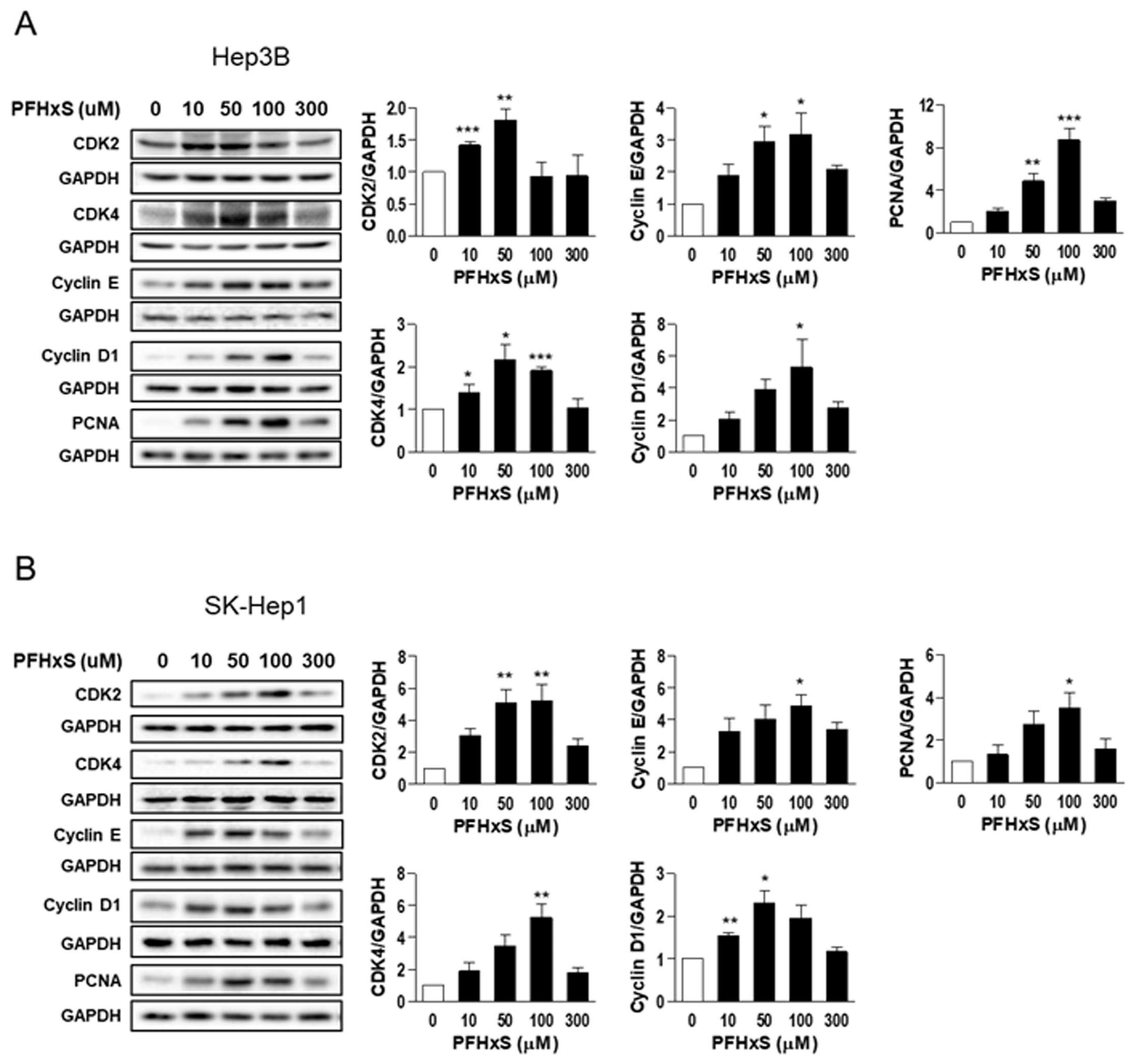
2.5. Western Blotting
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ebnesajjad, S. Introduction to Fluoropolymers: Materials, Technology and Applications. In PDL Handbook Series; William Andrew: Norwich, NY, USA, 2013. [Google Scholar]
- Brase, R.A.; Mullin, E.J.; Spink, D.C. Legacy and Emerging Per- and Polyfluoroalkyl Substances: Analytical Techniques, Environmental Fate, and Health Effects. Int. J. Mol. Sci. 2021, 22, 995. [Google Scholar] [CrossRef] [PubMed]
- Krafft, M.P.; Riess, J.G. Selected physicochemical aspects of poly- and perfluoroalkylated substances relevant to performance, environment and sustainability—Part one. Chemosphere 2015, 129, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Ruan, T.; Field, J.; Cousins, I.; Lohmann, R.; Jiang, G. Emerging Contaminants: Fluorinated Alternatives to Existing PFAS. Environ. Sci. Technol. 2022, 56, 6001–6003. [Google Scholar] [CrossRef] [PubMed]
- Caron-Beaudoin, E.; Ayotte, P.; Sidi, E.A.L.; McHugh, N.G.-L.; Lemire, M. Exposure to perfluoroalkyl substances (PFAS) and associations with thyroid parameters in First Nation children and youth from Quebec. Environ. Int. 2019, 128, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, L.; Norström, K.; Viktor, T.; Cousins, A.P.; Josefsson, S. Stockholm Arlanda Airport as a source of per- and polyfluoroalkyl substances to water, sediment and fish. Chemosphere 2015, 129, 33–38. [Google Scholar] [CrossRef]
- Giesy, J.P.; Kannan, K. Global Distribution of Perfluorooctane Sulfonate in Wildlife. Environ. Sci. Technol. 2001, 35, 1339–1342. [Google Scholar] [CrossRef]
- Hu, X.C.; Andrews, D.Q.; Lindstrom, A.B.; Bruton, T.A.; Schaider, L.A.; Grandjean, P.; Lohmann, R.; Carignan, C.C.; Blum, A.; Balan, S.A.; et al. Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ. Sci. Technol. Lett. 2016, 3, 344–350. [Google Scholar] [CrossRef]
- Roth, K.; Yang, Z.; Agarwal, M.; Liu, W.; Peng, Z.; Long, Z.; Birbeck, J.; Westrick, J.; Liu, W.; Petriello, M.C. Exposure to a mixture of legacy, alternative, and replacement per- and polyfluoroalkyl substances (PFAS) results in sex-dependent modulation of cholesterol metabolism and liver injury. Environ. Int. 2021, 157, 106843. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Liu, S.; Zhou, J.L.; Zheng, C.; Weifeng, J.; Chen, B.; Zhang, T.; Qiu, W. PFAS and their substitutes in groundwater: Occurrence, transformation and remediation. J. Hazard. Mater. 2021, 412, 125159. [Google Scholar] [CrossRef]
- Ateia, M.; Maroli, A.; Tharayil, N.; Karanfil, T. The overlooked short- and ultrashort-chain poly- and perfluorinated substances: A review. Chemosphere 2019, 220, 866–882. [Google Scholar] [CrossRef]
- Butenhoff, J.L.; Chang, S.-C.; Ehresman, D.J.; York, R.G. Evaluation of potential reproductive and developmental toxicity of potassium perfluorohexanesulfonate in Sprague Dawley rats. Reprod. Toxicol. 2009, 27, 331–341. [Google Scholar] [CrossRef]
- Das, K.P.; Wood, C.R.; Lin, M.T.; Starkov, A.A.; Lau, C.; Wallace, K.B.; Corton, J.C.; Abbott, B.D. Perfluoroalkyl acids-induced liver steatosis: Effects on genes controlling lipid homeostasis. Toxicology 2017, 378, 37–52. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, Z.; Magand, O.; Thollot, A.; Ebinghaus, R.; Mi, W.; Dommergue, A. Occurrence of legacy and emerging organic contaminants in snow at Dome C in the Antarctic. Sci. Total. Environ. 2020, 741, 140200. [Google Scholar] [CrossRef]
- Huang, M.; Dzierlenga, A.; Robinson, V.; Waidyanatha, S.; DeVito, M.; Eifrid, M.; Granville, C.; Gibbs, S.; Blystone, C. Toxicokinetics of perfluorobutane sulfonate (PFBS), perfluorohexane-1-sulphonic acid (PFHxS), and perfluorooctane sulfonic acid (PFOS) in male and female Hsd:Sprague Dawley SD rats after intravenous and gavage administration. Toxicol. Rep. 2019, 6, 645–655. [Google Scholar] [CrossRef]
- Kim, S.-J.; Shin, H.; Lee, Y.-B.; Cho, H.-Y. Sex-specific risk assessment of PFHxS using a physiologically based pharmacokinetic model. Arch. Toxicol. 2018, 92, 1113–1131. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Jiang, J.; Zhang, X.-X. Environmental exposure to low-dose perfluorohexanesulfonate promotes obesity and non-alcoholic fatty liver disease in mice fed a high-fat diet. Environ. Sci. Pollut. Res. Int. 2022, 29, 49279–49290. [Google Scholar] [CrossRef]
- Jain, R.B.; Ducatman, A. Selective Associations of Recent Low Concentrations of Perfluoroalkyl Substances With Liver Function Biomarkers: NHANES 2011 to 2014 Data on US Adults Aged ≥20 Years. J. Occup. Environ. Med. 2019, 61, 293–302. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Singal, A.G.; Pillai, A.; Tiro, J. Early Detection, Curative Treatment, and Survival Rates for Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis: A Meta-analysis. PLoS Med. 2014, 11, e1001624. [Google Scholar] [CrossRef] [PubMed]
- Poisson, J.; Lemoinne, S.; Boulanger, C.; Durand, F.; Moreau, R.; Valla, D.; Rautou, P.-E. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J. Hepatol. 2017, 66, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Choi, S.-Y.; Yang, J.-H. AMP-activated protein kinase is involved in perfluorohexanesulfonate -induced apoptosis of neuronal cells. Chemosphere 2016, 149, 1–7. [Google Scholar] [CrossRef]
- Foulds, C.E.; Treviño, L.S.; York, B.; Walker, C.L. Endocrine-disrupting chemicals and fatty liver disease. Nat. Rev. Endocrinol. 2017, 13, 445–457. [Google Scholar] [CrossRef]
- Ioannou, G.N. Epidemiology and risk-stratification of NAFLD-associated HCC. J. Hepatol. 2021, 75, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Behr, A.C.; Kwiatkowski, A.; Ståhlman, M.; Schmidt, F.F.; Luckert, C.; Braeuning, A.; Buhrke, T. Impairment of bile acid metabolism by perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in human HepaRG hepatoma cells. Arch. Toxicol. 2020, 94, 1673–1686. [Google Scholar] [CrossRef]
- Goodrich, J.A.; Walker, D.; Lin, X.; Wang, H.; Lim, T.; McConnell, R.; Conti, D.V.; Chatzi, L.; Setiawan, V.W. Exposure to perfluoroalkyl substances and risk of hepatocellular carcinoma in a multiethnic cohort. JHEP Rep. 2022, 4, 100550. [Google Scholar] [CrossRef]
- Cao, L.; Guo, Y.; Chen, Y.; Hong, J.; Wu, J.; Hangbiao, J. Per-/polyfluoroalkyl substance concentrations in human serum and their associations with liver cancer. Chemosphere 2022, 296, 134083. [Google Scholar] [CrossRef] [PubMed]
- Gomis, M.I.; Vestergren, R.; Borg, D.; Cousins, I.T. Comparing the toxic potency in vivo of long-chain perfluoroalkyl acids and fluorinated alternatives. Environ. Int. 2018, 113, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Naile, J.E.; Wiseman, S.; Bachtold, K.; Jones, P.D.; Giesy, J.P. Transcriptional effects of perfluorinated compounds in rat hepatoma cells. Chemosphere 2012, 86, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Olsen, G.W.; Burris, J.M.; Ehresman, D.J.; Froehlich, J.W.; Seacat, A.M.; Butenhoff, J.L.; Zobel, L.R. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 2007, 115, 1298–1305. [Google Scholar] [CrossRef]
- Bjork, J.A.; Dawson, D.A.; Krogstad, J.O.; Wallace, K.B. Transcriptional effects of binary combinations of PFAS in FaO cells. Toxicology 2021, 464, 152997. [Google Scholar] [CrossRef]
- Ojo, A.F.; Peng, C.; Ng, J.C. Genotoxicity assessment of per- and polyfluoroalkyl substances mixtures in human liver cells (HepG2). Toxicology 2022, 482, 153359. [Google Scholar] [CrossRef] [PubMed]
- Ojo, A.F.; Xia, Q.; Peng, C.; Ng, J.C. Evaluation of the individual and combined toxicity of perfluoroalkyl substances to human liver cells using biomarkers of oxidative stress. Chemosphere 2021, 281, 130808. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, K.H.; Oh, H.S.; Lee, C.; Eun, H.; Lee, Y.J. Evaluation of the Effect of Perfluorohexane Sulfonate on the Proliferation of Human Liver Cells. Int. J. Environ. Res. Public Health 2023, 20, 6868. https://doi.org/10.3390/ijerph20196868
Sim KH, Oh HS, Lee C, Eun H, Lee YJ. Evaluation of the Effect of Perfluorohexane Sulfonate on the Proliferation of Human Liver Cells. International Journal of Environmental Research and Public Health. 2023; 20(19):6868. https://doi.org/10.3390/ijerph20196868
Chicago/Turabian StyleSim, Kyeong Hwa, Hyeon Seo Oh, Chuhee Lee, Heesoo Eun, and Youn Ju Lee. 2023. "Evaluation of the Effect of Perfluorohexane Sulfonate on the Proliferation of Human Liver Cells" International Journal of Environmental Research and Public Health 20, no. 19: 6868. https://doi.org/10.3390/ijerph20196868
APA StyleSim, K. H., Oh, H. S., Lee, C., Eun, H., & Lee, Y. J. (2023). Evaluation of the Effect of Perfluorohexane Sulfonate on the Proliferation of Human Liver Cells. International Journal of Environmental Research and Public Health, 20(19), 6868. https://doi.org/10.3390/ijerph20196868






