Malaria Risk Drivers in the Brazilian Amazon: Land Use—Land Cover Interactions and Biological Diversity
Abstract
1. Introduction
2. Materials and Methods
2.1. Dataset
2.2. Malaria Cases and Population Data
2.3. Land Use–Land Cover (LULC)
2.4. Environmental Variables
2.5. Biological Diversity Variables
2.6. Model Building
3. Results
3.1. Malaria Cases
3.2. Spatial Clusters
3.3. LULC Change
3.4. Diversity Variables
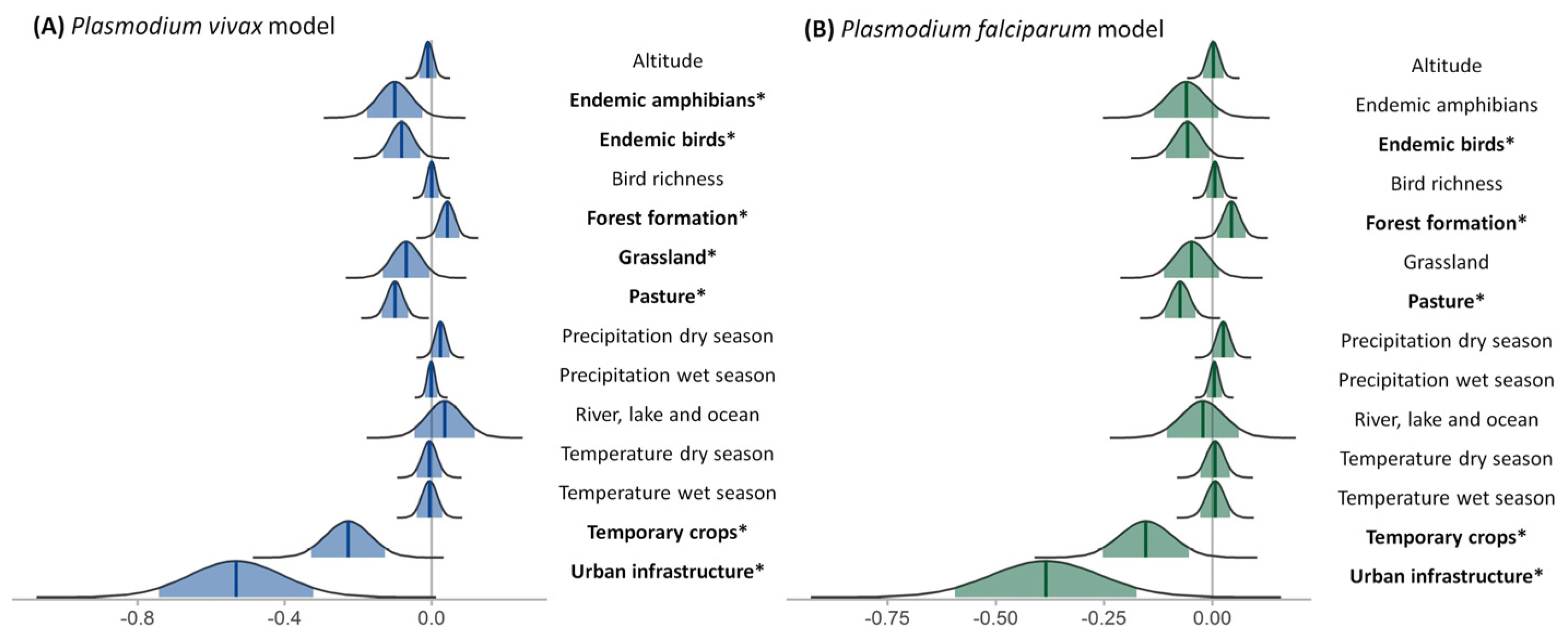
3.5. Covariate Significance
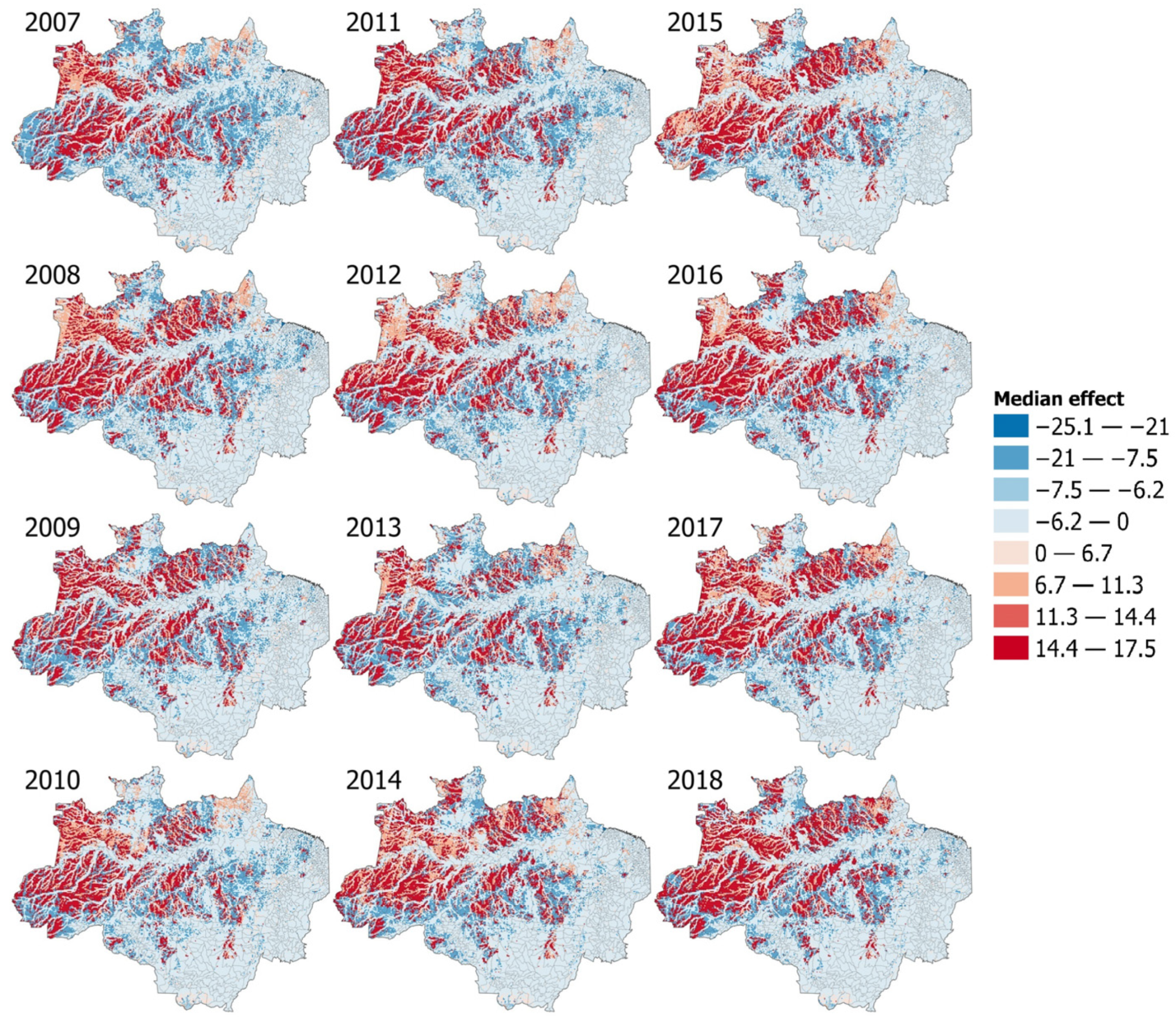
3.6. Interactions Models and Effect Maps
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Ayala, M.J.C.; Bastos, L.S.; Villela, D.A.M. On Multifactorial Drivers for Malaria Rebound in Brazil: A Spatio-Temporal Analysis. Malar. J. 2022, 21, 52. [Google Scholar] [CrossRef] [PubMed]
- Hiwat, H.; Bretas, G. Ecology of Anopheles Darlingi Root with Respect to Vector Importance: A Review. Parasites Vectors 2011, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Carlos, B.C.; Rona, L.D.P.; Christophides, G.K.; Souza-Neto, J.A. A Comprehensive Analysis of Malaria Transmission in Brazil. Pathog. Glob. Health 2019, 113, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.U.; Castro, M.C. Challenges for Malaria Elimination in Brazil. Malar. J. 2016, 15, 284. [Google Scholar] [CrossRef]
- Pimenta, P.F.; Orfano, A.S.; Bahia, A.C.; Duarte, A.P.; Ríos-Velásquez, C.M.; Melo, F.F.; Pessoa, F.A.; Oliveira, G.A.; Campos, K.M.; Villegas, L.M.; et al. An Overview of Malaria Transmission from the Perspective of Amazon Anopheles Vectors. Mem. Inst. Oswaldo Cruz. 2015, 110, 23–47. [Google Scholar] [CrossRef] [PubMed]
- Bauhoff, S.; Busch, J. Does Deforestation Increase Malaria Prevalence? Evidence from Satellite Data and Health Surveys. World Dev. 2020, 127, 104734. [Google Scholar] [CrossRef]
- Hahn, M.B.; Gangnon, R.E.; Barcellos, C.; Asner, G.P.; Patz, J.A. Influence of Deforestation, Logging, and Fire on Malaria in the Brazilian Amazon. PLoS ONE 2014, 9, e85725. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.J.; Mordecai, E.A. Amazon Deforestation Drives Malaria Transmission, and Malaria Burden Reduces Forest Clearing. Proc. Natl. Acad. Sci. USA 2019, 116, 22212–22218. [Google Scholar] [CrossRef]
- Barros, F.S.M.; Honório, N.A. Deforestation and Malaria on the Amazon Frontier: Larval Clustering of Anopheles Darlingi (Diptera: Culicidae) Determines Focal Distribution of Malaria. Am. J. Trop. Med. Hyg. 2015, 93, 939–953. [Google Scholar] [CrossRef]
- Vittor, A.Y.; Pan, W.; Gilman, R.H.; Tielsch, J.; Glass, G.; Shields, T.; Sánchez-Lozano, W.; Pinedo, V.V.; Salas-Cobos, E.; Flores, S.; et al. Linking deforestation to malaria in the Amazon: Characterization of the breeding habitat of the principal malaria vector, Anopheles darlingi. Am. J. Trop. Med. Hyg. 2009, 81, 5. [Google Scholar]
- Barbosa, L.M.C.; Scarpassa, V.M. Blood-Feeding Behavior of Anopheles Species (Diptera: Culicidae) in the District of Ilha de Santana, State of Amapá, Eastern Brazilian Amazon. Rev. Bras. Entomol. 2021, 65, e20200048. [Google Scholar] [CrossRef]
- Conn, J.E.; Segura, M.N.O.; Wilkerson, R.C.; Schlichting, C.D.; Póvoa, M.M.; Wirtz, R.A.; de Souza, R.T.L. Emergence of a New Neotropical Malaria Vector Facilitated by Human Migration and Changes in Land Use. Am. J. Trop. Med. Hyg. 2002, 66, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Rejmánková, E.; Grieco, J.; Achee, N.; Roberts, D.R. Ecology of Larval Habitats. In Anopheles Mosquitoes—New Insights into Malaria Vectors; Manguin, S., Ed.; InTech: Houston, TX, USA, 2013. [Google Scholar] [CrossRef]
- Roux, O.; Robert, V. Larval Predation in Malaria Vectors and Its Potential Implication in Malaria Transmission: An Overlooked Ecosystem Service? Parasites Vectors 2019, 12, 217. [Google Scholar] [CrossRef] [PubMed]
- Burkett-Cadena, N.D.; Vittor, A.Y. Deforestation and Vector-Borne Disease: Forest Conversion Favors Important Mosquito Vectors of Human Pathogens. Basic Appl. Ecol. 2018, 26, 101–110. [Google Scholar] [CrossRef]
- Laurance, S.G.W.; Meyer Steiger, D.B.; Ritchie, S.A. Land Use Influences Mosquito Communities and Disease Risk on Remote Tropical Islands: A Case Study Using a Novel Sampling Technique. Am. J. Trop. Med. Hyg. 2016, 94, 314–321. [Google Scholar] [CrossRef]
- Vanwambeke, S.O.; Lambin, E.F.; Eichhorn, M.P.; Flasse, S.P.; Harbach, R.E.; Oskam, L.; Somboon, P.; van Beers, S.; van Benthem, B.H.B.; Walton, C.; et al. Impact of Land-Use Change on Dengue and Malaria in Northern Thailand. EcoHealth 2007, 4, 37–51. [Google Scholar] [CrossRef]
- Ostfeld, R.; Glass, G.; Keesing, F. Spatial Epidemiology: An Emerging (or Re-Emerging) Discipline. Trends Ecol. Evol. 2005, 20, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Laporta, G.Z.; de Prado, P.I.K.L.; Kraenkel, R.A.; Coutinho, R.M.; Sallum, M.A.M. Biodiversity Can Help Prevent Malaria Outbreaks in Tropical Forests. PLoS Negl. Trop. Dis. 2013, 7, e2139. [Google Scholar] [CrossRef] [PubMed]
- Muylaert, R.; Sabino-Santos, G.; Prist, P.; Oshima, J.; Niebuhr, B.; Sobral-Souza, T.; Oliveira, S.; Bovendorp, R.; Marshall, J.; Hayman, D.; et al. Spatiotemporal Dynamics of Hantavirus Cardiopulmonary Syndrome Transmission Risk in Brazil. Viruses 2019, 11, 1008. [Google Scholar] [CrossRef]
- Suzán, G.; Marcé, E.; Giermakowski, J.T.; Mills, J.N.; Ceballos, G.; Ostfeld, R.S.; Armién, B.; Pascale, J.M.; Yates, T.L. Experimental Evidence for Reduced Rodent Diversity Causing Increased Hantavirus Prevalence. PLoS ONE 2009, 4, e5461. [Google Scholar] [CrossRef]
- Keesing, F.; Brunner, J.; Duerr, S.; Killilea, M.; LoGiudice, K.; Schmidt, K.; Vuong, H.; Ostfeld, R.S. Hosts as Ecological Traps for the Vector of Lyme Disease. Proc. R. Soc. B. 2009, 276, 3911–3919. [Google Scholar] [CrossRef]
- Johnson, P.T.J.; Lund, P.J.; Hartson, R.B.; Yoshino, T.P. Community Diversity Reduces Schistosoma mansoni Transmission, Host Pathology and Human Infection Risk. Proc. R. Soc. B. 2009, 276, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- Civitello, D.J.; Cohen, J.; Fatima, H.; Halstead, N.T.; Liriano, J.; McMahon, T.A.; Ortega, C.N.; Sauer, E.L.; Sehgal, T.; Young, S.; et al. Biodiversity Inhibits Parasites: Broad Evidence for the Dilution Effect. Proc. Natl. Acad. Sci. USA 2015, 112, 8667–8671. [Google Scholar] [CrossRef] [PubMed]
- Swaddle, J.P.; Calos, S.E. Increased Avian Diversity Is Associated with Lower Incidence of Human West Nile Infection: Observation of the Dilution Effect. PLoS ONE 2008, 3, e2488. [Google Scholar] [CrossRef] [PubMed]
- Louca, V.; Lucas, M.C.; Green, C.; Majambere, S.; Fillinger, U.; Lindsay, S.W. Role of Fish as Predators of Mosquito Larvae on the Floodplain of the Gambia River. J. Med. Entomol. 2009, 46, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.M.; Bonds, J.A.S.; Quinlan, M.M.; Mumford, J.D. Effects of the Removal or Reduction in Density of the Malaria Mosquito, Anopheles gambiae sl., on Interacting Predators and Competitors in Local Ecosystems. Med. Vet. Entomol. 2019, 33, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kweka, E.J.; Zhou, G.; Gilbreath, T.M.; Afrane, Y.; Nyindo, M.; Githeko, A.K.; Yan, G. Predation Efficiency of Anopheles Gambiae Larvae by Aquatic Predators in Western Kenya Highlands. Parasites Vectors 2011, 4, 128. [Google Scholar] [CrossRef]
- Russell, M.C.; Herzog, C.M.; Gajewski, Z.; Ramsay, C.; El Moustaid, F.; Evans, M.V.; Desai, T.; Gottdenker, N.L.; Hermann, S.L.; Power, A.G.; et al. Both Consumptive and Non-Consumptive Effects of Predators Impact Mosquito Populations and Have Implications for Disease Transmission. eLife 2022, 11, e71503. [Google Scholar] [CrossRef]
- Singh, S.P. Biological Control of Mosquitoes by Insectivorous Flycatcher Birds. J. Entomol. Res. 2013, 37, 359–364. [Google Scholar]
- Parham, P.E.; Michael, E. Modeling the Effects of Weather and Climate Change on Malaria Transmission. Environ. Health Perspect. 2010, 118, 620–626. [Google Scholar] [CrossRef]
- Achee, N.L.; Grieco, J.P.; Masuoka, P.; Andre, R.G.; Roberts, D.R.; Thomas, J.; Briceno, I.; King, R.; Rejmankova, E. Use of Remote Sensing and Geographic Information Systems to Predict Locations of Anopheles darlingi-Positive Breeding Sites Within the Sibun River in Belize, Central America. J. Med. Entomol. 2014, 43, 382–392. [Google Scholar] [CrossRef]
- Alimi, T.O.; Fuller, D.O.; Qualls, W.A.; Herrera, S.V.; Arevalo-Herrera, M.; Quinones, M.L.; Lacerda, M.V.G.; Beier, J.C. Predicting Potential Ranges of Primary Malaria Vectors and Malaria in Northern South America Based on Projected Changes in Climate, Land Cover and Human Population. Parasites Vectors 2015, 8, 431. [Google Scholar] [CrossRef]
- Baeza, A.; Santos-Vega, M.; Dobson, A.P.; Pascual, M. The Rise and Fall of Malaria under Land-Use Change in Frontier Regions. Nat. Ecol. Evol. 2017, 1, 108. [Google Scholar] [CrossRef]
- Chaves, L.S.M.; Bergo, E.S.; Conn, J.E.; Laporta, G.Z.; Prist, P.R.; Sallum, M.A.M. Anthropogenic Landscape Decreases Mosquito Biodiversity and Drives Malaria Vector Proliferation in the Amazon Rainforest. PLoS ONE 2021, 16, e0245087. [Google Scholar] [CrossRef] [PubMed]
- Kohara Melchior, L.A.; Chiaravalloti Neto, F. Spatial and Spatio-Temporal Analysis of Malaria in the State of Acre, Western Amazon, Brazil. Geospat. Health 2016, 11, 443. [Google Scholar] [CrossRef] [PubMed]
- Malha Municipal|IBGE. Available online: https://www.ibge.gov.br/geociencias/organizacao-do-territorio/malhas-territoriais/15774-malhas.html?=&t=acesso-ao-produto (accessed on 12 April 2023).
- Dados Para Cidadão a Partir da Fonte de Dados do Sivep-Malária e do Sinan, Para Notificações do Brasil de 2007 a 2023. Dados do Sivep-Malária Atualizados em 29/03/2023; Dados do Sinan Atualizados em: 14/03/2023. Available online: https://public.tableau.com/views/Dadosparacidado_201925_03_2020/Incio?%3Adisplay_static_image=y&%3AbootstrapWhenNotified=true&%3Aembed=true&%3Alanguage=en-US&:embed=y&:showVizHome=n&:apiID=host0#navType=0&navSrc=Parse (accessed on 12 April 2023).
- População|IBGE. Available online: https://www.ibge.gov.br/estatisticas/sociais/populacao.html (accessed on 12 April 2023).
- Souza, C.M.Z.; Shimbo, J.; Rosa, M.R.; Parente, L.L.A.; Alencar, A.; Rudorff, B.F.T.; Hasenack, H.; Matsumoto, M.G.; Ferreira, L.; Souza-Filho, P.W.M.; et al. Reconstructing Three Decades of Land Use and Land Cover Changes in Brazilian Biomes with Landsat Archive and Earth Engine. Remote Sens. 2020, 12, 2735. [Google Scholar] [CrossRef]
- Harris, I.; Jones, P.D.; Osborn, T.J.; Lister, D.H. Updated High-Resolution Grids of Monthly Climatic Observations—The CRU TS3.10 Dataset: Updated High-Resolution Grids of Monthly Climatic Observations. Int. J. Climatol. 2014, 34, 623–642. [Google Scholar] [CrossRef]
- Satyamurty, P.; de Castro, A.A.; Tota, J.; da Silva Gularte, L.E.; Manzi, A.O. Rainfall Trends in the Brazilian Amazon Basin in the Past Eight Decades. Theor. Appl. Climatol. 2010, 99, 139–148. [Google Scholar] [CrossRef]
- Amatulli, G.; Domisch, S.; Tuanmu, M.-N.; Parmentier, B.; Ranipeta, A.; Malczyk, J.; Jetz, W. A Suite of Global, Cross-Scale Topographic Variables for Environmental and Biodiversity Modeling. Sci. Data 2018, 5, 180040. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.P.; Leite, Y.L.R.; Mendes, S.L.; Ditchfield, A.D. Mammal Conservation in Brazil. Conserv. Biol. 2005, 19, 672–679. [Google Scholar] [CrossRef]
- Guerra, V.; Jardim, L.; Llusia, D.; Márquez, R.; Bastos, R.P. Knowledge Status and Trends in Description of Amphibian Species in Brazil. Ecol. Indic. 2020, 118, 106754. [Google Scholar] [CrossRef]
- Marini, M.A.; Garcia, F.I. Bird Conservation in Brazil. Conserv. Biol. 2005, 19, 665–671. [Google Scholar] [CrossRef]
- Jenkins, C.N.; Alves, M.A.S.; Uezu, A.; Vale, M.M. Patterns of Vertebrate Diversity and Protection in Brazil. PLoS ONE 2015, 10, e0145064. [Google Scholar] [CrossRef] [PubMed]
- Rue, H.; Martino, S.; Chopin, N. Approximate Bayesian Inference for Latent Gaussian Models by Using Integrated Nested Laplace Approximations. J. R. Stat. Soc. Ser. B Stat. Methodol. 2009, 71, 319–392. [Google Scholar] [CrossRef]
- Moraga, P.; Dean, C.; Inoue, J.; Morawiecki, P.; Noureen, S.R.; Wang, F. Bayesian Spatial Modelling of Geostatistical Data Using INLA and SPDE Methods: A Case Study Predicting Malaria Risk in Mozambique. Spat. Spatio-Temporal Epidemiol. 2021, 39, 100440. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Rubio, V. Bayesian Inference with INLA; Chapman & Hall/CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Besag, J.; York, J.; Molli, A. Bayesian Image Restoration, with Two Applications in Spatial Statistics. Ann. Inst. Stat. Math. 1991, 43, 1–20. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. vegan: Community Ecology Package. R Package Version 2.5-6. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 12 April 2023).
- Lindgren, F.; Rue, H. Bayesian Spatial Modelling with R-INLA. J. Stat. Soft. 2015, 63, 1–25. [Google Scholar] [CrossRef]
- Hess, C. coefINLA: Create Ggplot for INLA Fixed Effects Coefficients. R Package Version 0.02. 2020. Available online: https://github.com/hesscl/coefINLA (accessed on 12 April 2023).
- Langhi, D.M.; Orlando Bordin, J. Duffy Blood Group and Malaria. Hematology 2006, 11, 389–398. [Google Scholar] [CrossRef]
- Escalante, A.A.; Cepeda, A.S.; Pacheco, M.A. Why Plasmodium Vivax and Plasmodium Falciparum Are so Different? A Tale of Two Clades and Their Species Diversities. Malar. J. 2022, 21, 139. [Google Scholar] [CrossRef]
- White, M.T.; Karl, S.; Koepfli, C.; Longley, R.J.; Hofmann, N.E.; Wampfler, R.; Felger, I.; Smith, T.; Nguitragool, W.; Sattabongkot, J.; et al. Plasmodium Vivax and Plasmodium Falciparum Infection Dynamics: Re-Infections, Recrudescences and Relapses. Malar. J. 2018, 17, 170. [Google Scholar] [CrossRef]
- Programa Nacional de Prevenção e Controle da Malária. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/m/malaria/pncm/programa-nacional-de-prevencao-e-controle-da-malaria-pncm (accessed on 12 April 2023).
- Jackson, H.B.; Fahrig, L. Are Ecologists Conducting Research at the Optimal Scale?: Is Research Conducted at Optimal Scales? Glob. Ecol. Biogeogr. 2015, 24, 52–63. [Google Scholar] [CrossRef]
- Gonzalez-Daza, W.; Vivero-Gómez, R.J.; Altamiranda-Saavedra, M.; Muylaert, R.L.; Landeiro, V.L. Time-Lagged Response of Malaria Transmission to Climate and Land Use Change in a Colombian Amazonian Municipality: Implications for Early Warning Systems and Control Strategies. Research Square Platform LLC. 2023, 1–29. [Google Scholar] [CrossRef]
- Lucas, T.C.D.; Nandi, A.; Nguyen, M.; Rumisha, S.E.; Battle, K.E.; Howes, R.; Hendriks, C.; Python, A.; Hancock, P.; Cameron, E.; et al. Model Ensembles with Different Response Variables for Base and Meta Models: Malaria Disaggregation Regression Combining Prevalence and Incidence Data. bioRxiv 2019, 548719. [Google Scholar] [CrossRef]
- Eigenbrod, F.; Hecnar, S.J.; Fahrig, L. Sub-Optimal Study Design Has Major Impacts on Landscape-Scale Inference. Biol. Conserv. 2011, 144, 298–305. [Google Scholar] [CrossRef]
- Lambin, E.F.; Tran, A.; Vanwambeke, S.O.; Linard, C.; Soti, V. Pathogenic Landscapes: Interactions between Land, People, Disease Vectors, and Their Animal Hosts. Int. J. Health Geogr. 2010, 9, 54. [Google Scholar] [CrossRef]
- McGarigal, K.; Wan, H.Y.; Zeller, K.A.; Timm, B.C.; Cushman, S.A. Multi-Scale Habitat Selection Modeling: A Review and Outlook. Landsc. Ecol. 2016, 31, 1161–1175. [Google Scholar] [CrossRef]
- Oliveira, T.M.P.; Laporta, G.Z.; Bergo, E.S.; Chaves, L.S.M.; Antunes, J.L.F.; Bickersmith, S.A.; Conn, J.E.; Massad, E.; Sallum, M.A.M. Vector Role and Human Biting Activity of Anophelinae Mosquitoes in Different Landscapes in the Brazilian Amazon. Parasites Vectors 2021, 14, 236. [Google Scholar] [CrossRef] [PubMed]
- Tangena, J.-A.A.; Thammavong, P.; Wilson, A.L.; Brey, P.T.; Lindsay, S.W. Risk and Control of Mosquito-Borne Diseases in Southeast Asian Rubber Plantations. Trends Parasitol. 2016, 32, 402–415. [Google Scholar] [CrossRef]
- Fornace, K.M.; Diaz, A.V.; Lines, J.; Drakeley, C.J. Achieving Global Malaria Eradication in Changing Landscapes. Malar. J. 2021, 20, 69. [Google Scholar] [CrossRef]
- Loaiza, J.R.; Dutari, L.C.; Rovira, J.R.; Sanjur, O.I.; Laporta, G.Z.; Pecor, J.; Foley, D.H.; Eastwood, G.; Kramer, L.D.; Radtke, M.; et al. Disturbance and Mosquito Diversity in the Lowland Tropical Rainforest of Central Panama. Sci. Rep. 2017, 7, 7248. [Google Scholar] [CrossRef]
- Tadei, W.P.; Scarpassa, V.M.; Thatcher, B.D.; Santos, J.M.; Rafael, M.S.; Rodrigues, I.B. Ecologic Observations on Anopheline Vectors of Malaria in the Brazilian Amazon. Am. J. Trop. Med. Hyg. 1998, 59, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.M.T.; Vittor, A.; Rifai, S.; Valle, D. Does Deforestation Promote or Inhibit Malaria Transmission in the Amazon? A Systematic Literature Review and Critical Appraisal of Current Evidence. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160125. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Ostfeld, R.S.; Peterson, A.T.; Poulin, R.; de la Fuente, J. Effects of Environmental Change on Zoonotic Disease Risk: An Ecological Primer. Trends Parasitol. 2014, 30, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Schrama, M.; Hunting, E.R.; Beechler, B.R.; Guarido, M.M.; Govender, D.; Nijland, W.; van ‘t Zelfde, M.; Venter, M.; van Bodegom, P.M.; Gorsich, E.E. Human Practices Promote Presence and Abundance of Disease-Transmitting Mosquito Species. Sci. Rep. 2020, 10, 13543. [Google Scholar] [CrossRef] [PubMed]
- Svensson, J.R.; Lindegarth, M.; Jonsson, P.R.; Pavia, H. Disturbance–Diversity Models: What Do They Really Predict and How Are They Tested? Proc. R. Soc. B. 2012, 279, 2163–2170. [Google Scholar] [CrossRef] [PubMed]
- Springborn, M.R.; Weill, J.A.; Lips, K.R.; Ibáñez, R.; Ghosh, A. Amphibian Collapses Increased Malaria Incidence in Central America. Environ. Res. Lett. 2022, 17, 104012. [Google Scholar] [CrossRef]
- Ferraguti, M.; Martínez-de la Puente, J.; Jiménez–Clavero, M.Á.; Llorente, F.; Roiz, D.; Ruiz, S.; Soriguer, R.; Figuerola, J. A Field Test of the Dilution Effect Hypothesis in Four Avian Multi-Host Pathogens. PLoS Pathog. 2021, 17, e1009637. [Google Scholar] [CrossRef]
- Ferraguti, M.; Martínez-de la Puente, J.; Bensch, S.; Roiz, D.; Ruiz, S.; Viana, D.S.; Soriguer, R.C.; Figuerola, J. Ecological Determinants of Avian Malaria Infections: An Integrative Analysis at Landscape, Mosquito and Vertebrate Community Levels. J. Anim. Ecol. 2018, 87, 727–740. [Google Scholar] [CrossRef]
- Halliday, F.W.; Rohr, J.R. Measuring the Shape of the Biodiversity-Disease Relationship across Systems Reveals New Findings and Key Gaps. Nat. Commun. 2019, 10, 5032. [Google Scholar] [CrossRef]
- Mihaljevic, J.R.; Joseph, M.B.; Orlofske, S.A.; Paull, S.H. The Scaling of Host Density with Richness Affects the Direction, Shape, and Detectability of Diversity-Disease Relationships. PLoS ONE 2014, 9, e97812. [Google Scholar] [CrossRef]
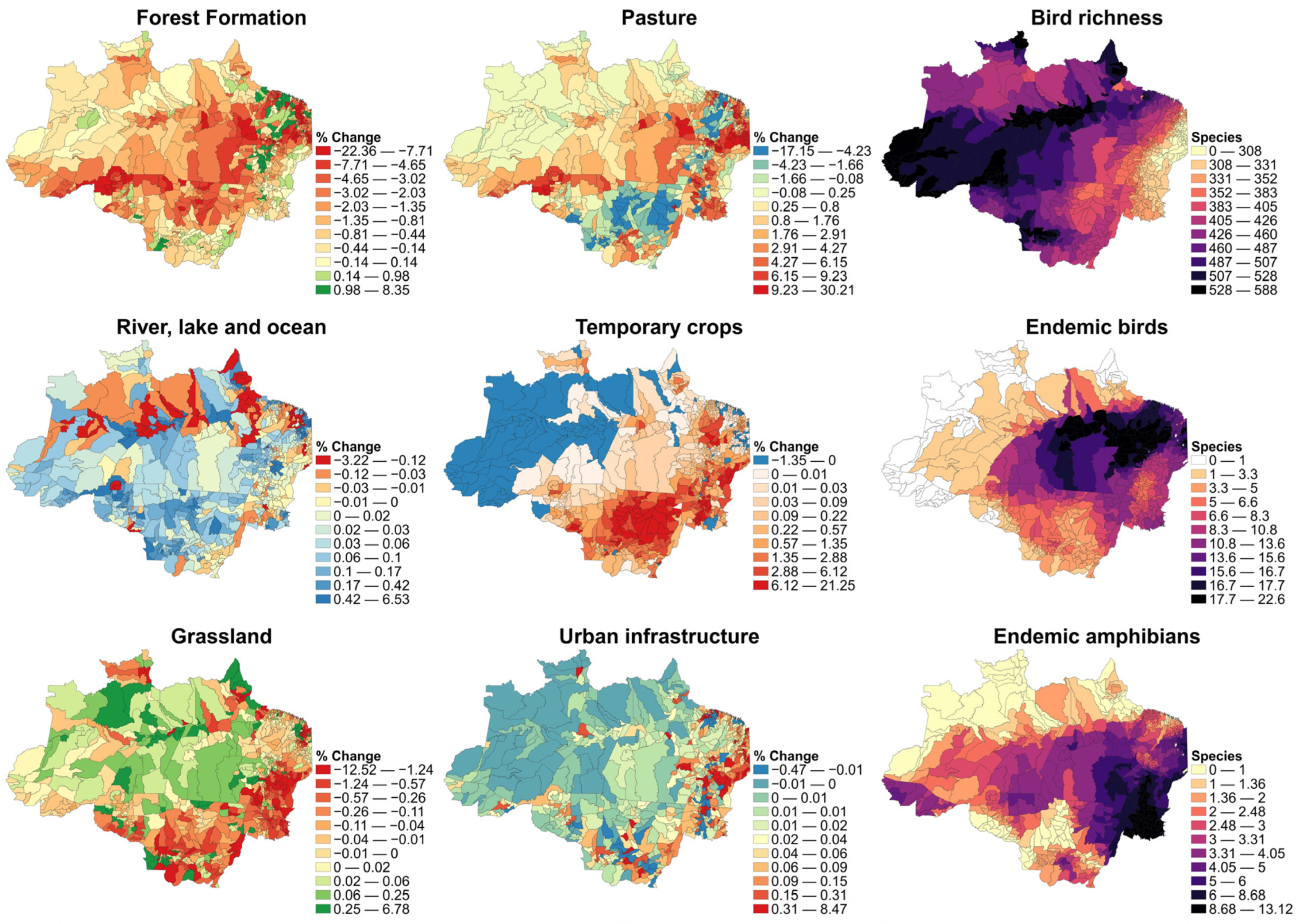
| Variable | Variable Type | Description |
|---|---|---|
| Altitude | Topographic | Municipality mean altitude, M.A.M.S.L. (static variable). |
| Precipitation wet season | Climatic | Total mean precipitation in the wet season (mm). |
| Precipitation dry season | Climatic | Total mean precipitation in the dry season (mm). |
| Temperature wet season | Climatic | Mean maximum temperature (°C) in the wet season. |
| Temperature dry season | Climatic | Mean maximum temperature (°C) in the dry season. |
| Forest Formation | Land use land cover | Dense rainforest, evergreen seasonal forest, open rainforest, semi-deciduous seasonal forest, deciduous seasonal forest, wooded savanna, and alluvial open rainforest (floodplain forests and Igapó forests) (% of municipality). |
| Grassland | Land use land cover | Regions within the Amazonia/Cerrado/Orinoco ecotone with a predominance of herbaceous strata (% of municipality). |
| Pasture | Land use land cover | Area of pasture, predominantly planted, linked to agricultural activity. Areas of natural pasture are predominantly classified as Grassland, which may or may not be grazed (% of municipality). |
| Temporary crops | Land use land cover | Areas occupied with agricultural crops of short or medium duration, generally with a vegetative cycle of less than one year, which after harvest require new planting to produce, composed mainly of cocoa, rubber, cashew nuts, palm oil, and açaí (% of municipality). |
| Urban Infrastructure | Land use land cover | Urbanized areas with a predominance of non-vegetated surfaces, including trails, roads, and buildings (% of municipality). |
| River, lakes and ocean | Land use land cover | As the name denotes, rivers, reservoirs, dams, ocean in the East coast zone in the Amazon region, lakes, and other water bodies (% of municipality). |
| Endemic amphibians * | Diversity | Mean endemic amphibians species number (static variable). |
| Endemic birds * | Diversity | Mean endemic bird number species (static variable). |
| Bird richness * | Diversity | Mean bird number of species (static variable). |
| P. vivax | P. falciparum | ||||||
|---|---|---|---|---|---|---|---|
| Cluster Time | Observed Cases | Expected Cases | Risk | Cluster Time | Observed Cases | Expected Cases | Risk |
| (1) 2013–2017 | 353,973 | 231,923.58 | 1.53 | (1) 2013–2018 | 83,331 | 47,915.42 | 1.74 |
| (2) 2010–2011 | 163,179 | 86,675.19 | 1.88 | (2) 2009–2012 | 65,919 | 38,388.91 | 1.72 |
| (3) 2007–2008 | 136,239 | 81,743.78 | 1.67 | (3) 2007–2008 | 21,794 | 9446.12 | 2.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez Daza, W.; Muylaert, R.L.; Sobral-Souza, T.; Lemes Landeiro, V. Malaria Risk Drivers in the Brazilian Amazon: Land Use—Land Cover Interactions and Biological Diversity. Int. J. Environ. Res. Public Health 2023, 20, 6497. https://doi.org/10.3390/ijerph20156497
Gonzalez Daza W, Muylaert RL, Sobral-Souza T, Lemes Landeiro V. Malaria Risk Drivers in the Brazilian Amazon: Land Use—Land Cover Interactions and Biological Diversity. International Journal of Environmental Research and Public Health. 2023; 20(15):6497. https://doi.org/10.3390/ijerph20156497
Chicago/Turabian StyleGonzalez Daza, William, Renata L. Muylaert, Thadeu Sobral-Souza, and Victor Lemes Landeiro. 2023. "Malaria Risk Drivers in the Brazilian Amazon: Land Use—Land Cover Interactions and Biological Diversity" International Journal of Environmental Research and Public Health 20, no. 15: 6497. https://doi.org/10.3390/ijerph20156497
APA StyleGonzalez Daza, W., Muylaert, R. L., Sobral-Souza, T., & Lemes Landeiro, V. (2023). Malaria Risk Drivers in the Brazilian Amazon: Land Use—Land Cover Interactions and Biological Diversity. International Journal of Environmental Research and Public Health, 20(15), 6497. https://doi.org/10.3390/ijerph20156497






