Real-World Effectiveness in Hypertension and Hyperlipidemia Collaborative Management between Pharmacies and Primary Care in Portugal: A Multicenter Pragmatic Controlled Trial (USFarmácia®)
Abstract
1. Introduction
2. Materials and Methods
2.1. Objectives
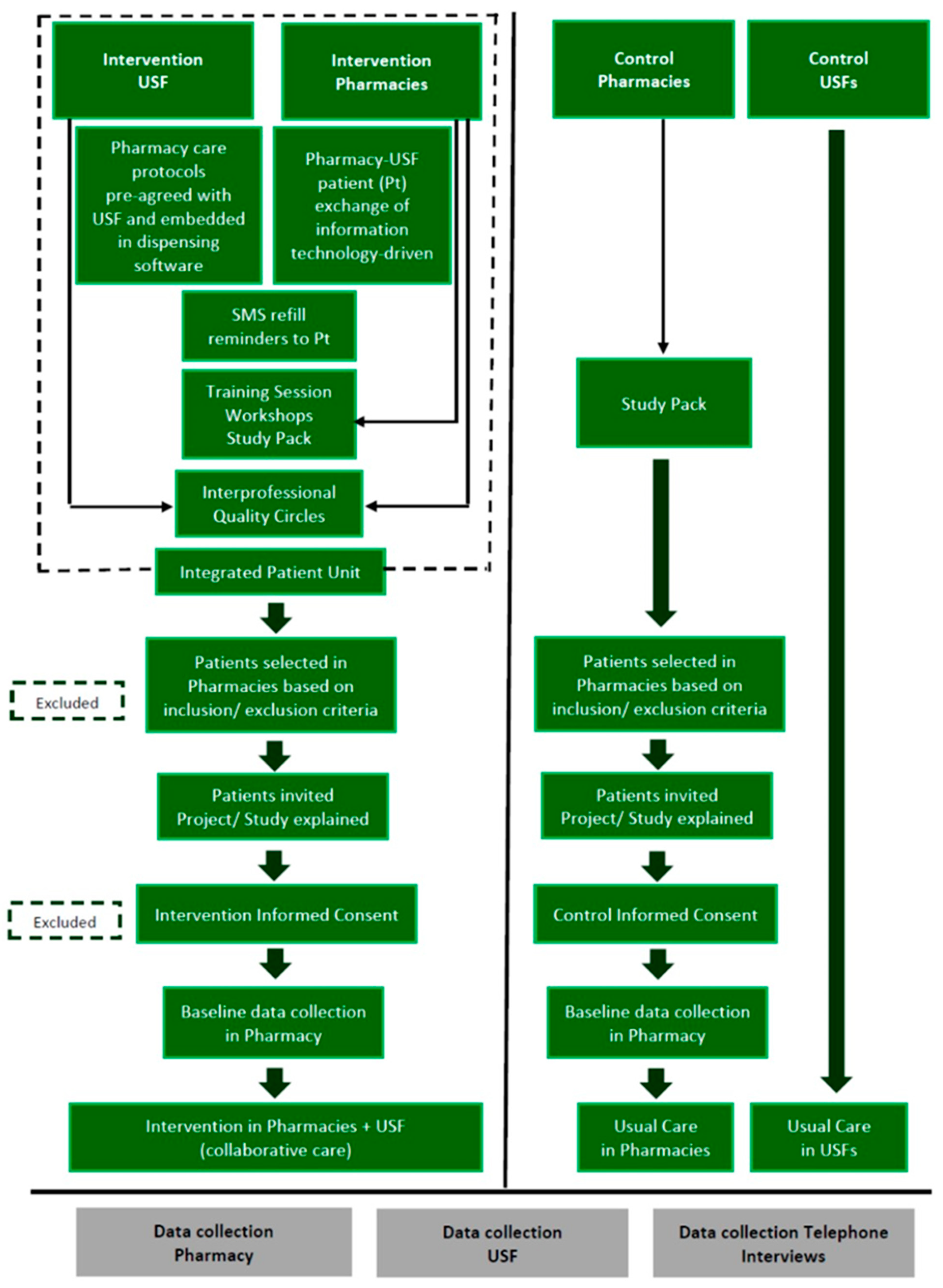
2.2. Trial Design
2.3. Participants
2.3.1. Eligibility Criteria for Intervention Primary Care Units and Pharmacies
2.3.2. Eligibility Criteria for Control Primary Care Units and Pharmacies
2.3.3. Patient Inclusion Criteria
- Mobile phone users (to receive Short Message Service (SMS) refill reminders);
- Registered at the EHR RSE® 2018 (SPMS EPE, Lisboa, Portugal) (to consent to data exchange between pharmacists and GPs);
- Members of the Pharmacy Customer Loyalty Program Saúda® (to enable reimbursement to intervention pharmacies);
- Holders of a Patient Record in the pharmacy dispensing claims software Sifarma® 2000 2.9.4.3 (Glintt, Sintra, Portugal) (to enable longitudinal tracking).
2.3.4. Method of Recruitment
2.3.5. Settings and Locations
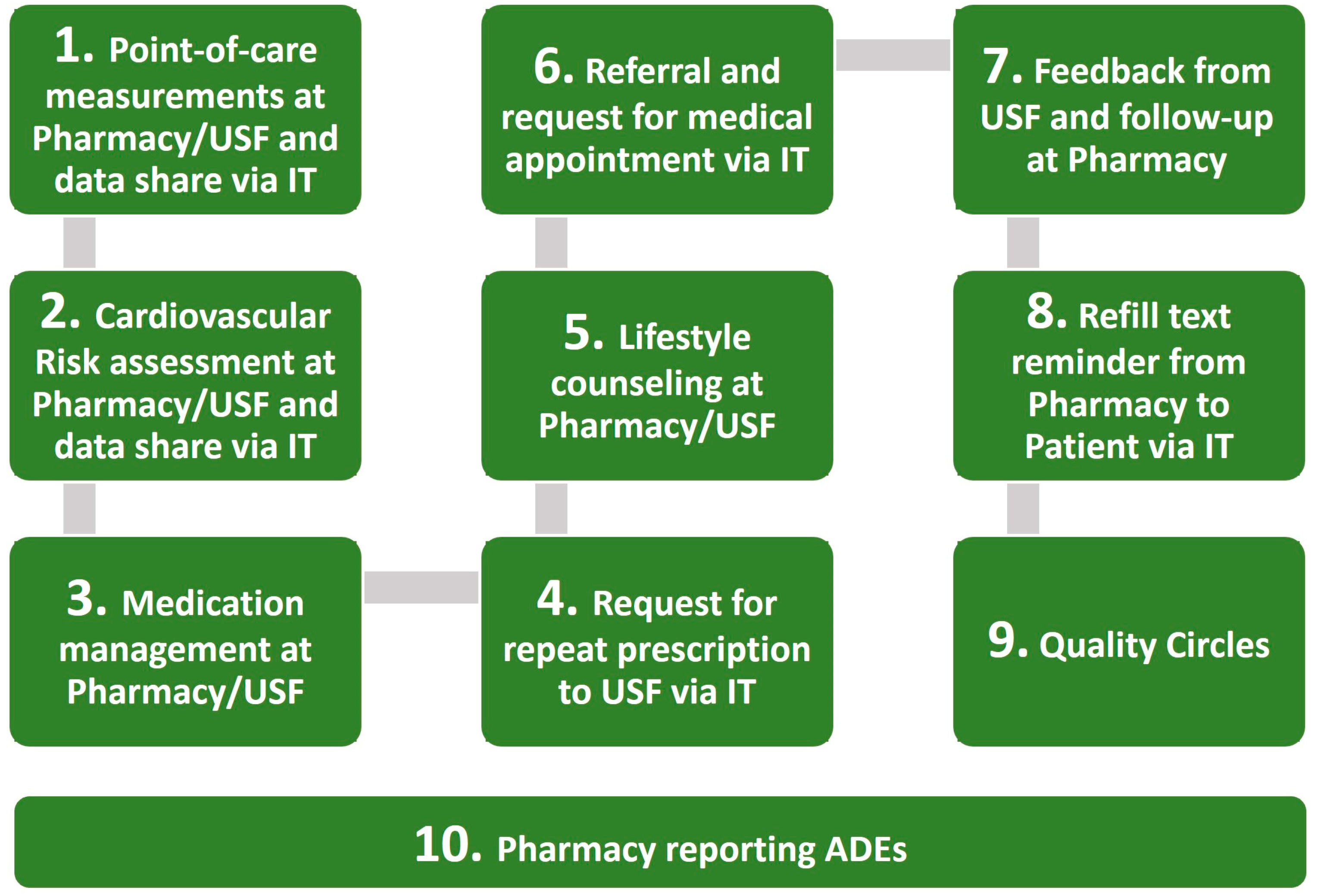
2.4. Interventions
2.4.1. Intervention
2.4.2. Usual Care
2.4.3. Time Span
2.4.4. Post-Trial Procedures and Ethical Considerations
2.5. Outcomes
2.5.1. Primary Outcomes
2.5.2. Data Collection
2.5.3. Data Management
2.6. Sample Size
2.6.1. Sample Size Calculation
2.6.2. Interim Analyses
2.6.3. Withdrawal Procedures
2.7. Other Indicators
2.8. Assignment Method
2.9. Blinding
2.10. Statistical Methods
2.10.1. Descriptive Statistics
2.10.2. Primary Outcomes
2.10.3. Subgroup Analyses
2.10.4. Missing Data
3. Results
3.1. Primary Care Units and Pharmacies
3.2. Participant Flow
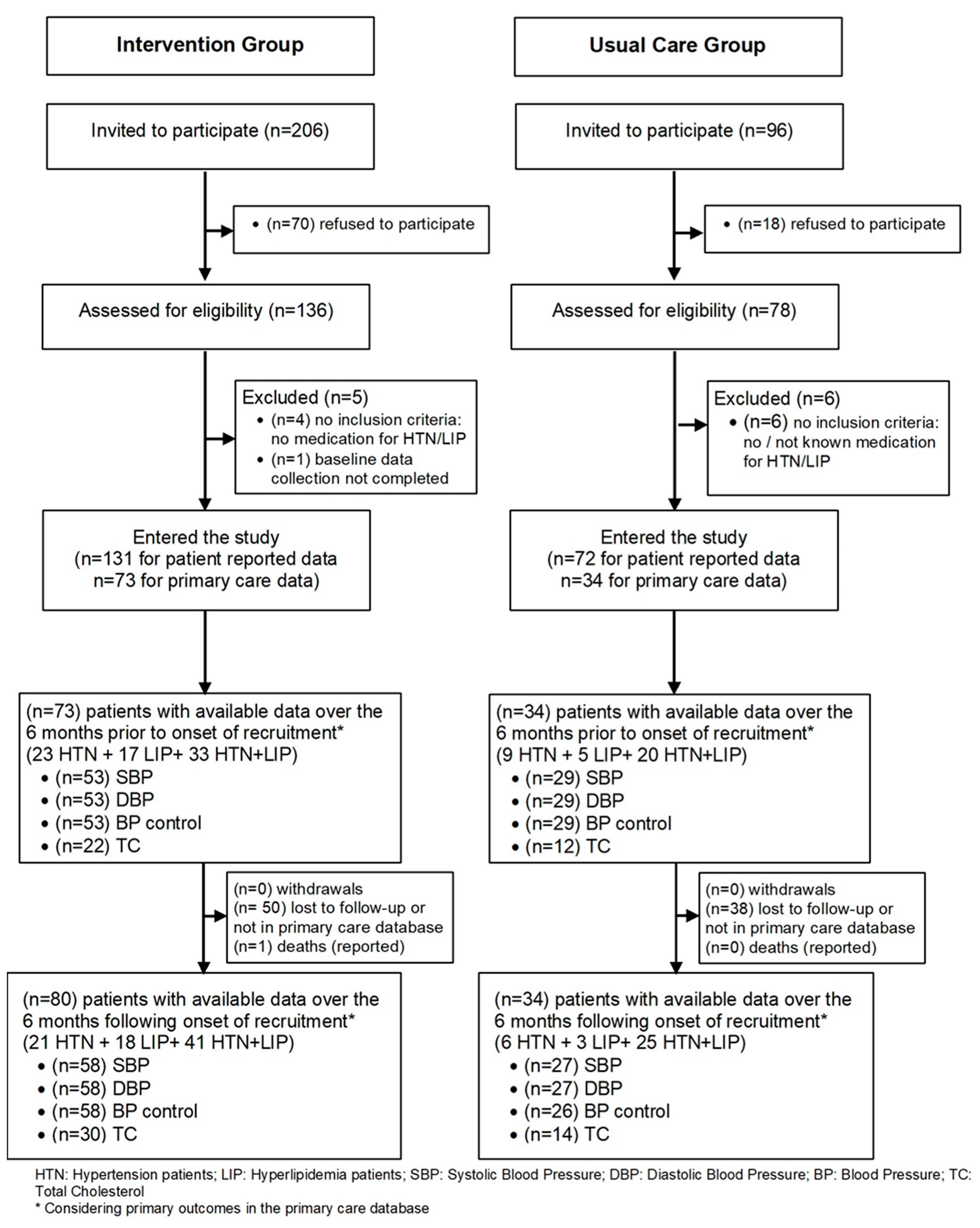
3.2.1. Patient Flow
3.2.2. Protocol Deviations
3.3. Recruitment
3.4. Baseline Data and Baseline Equivalence
3.5. Numbers Analyzed
3.6. Outcomes and Estimation
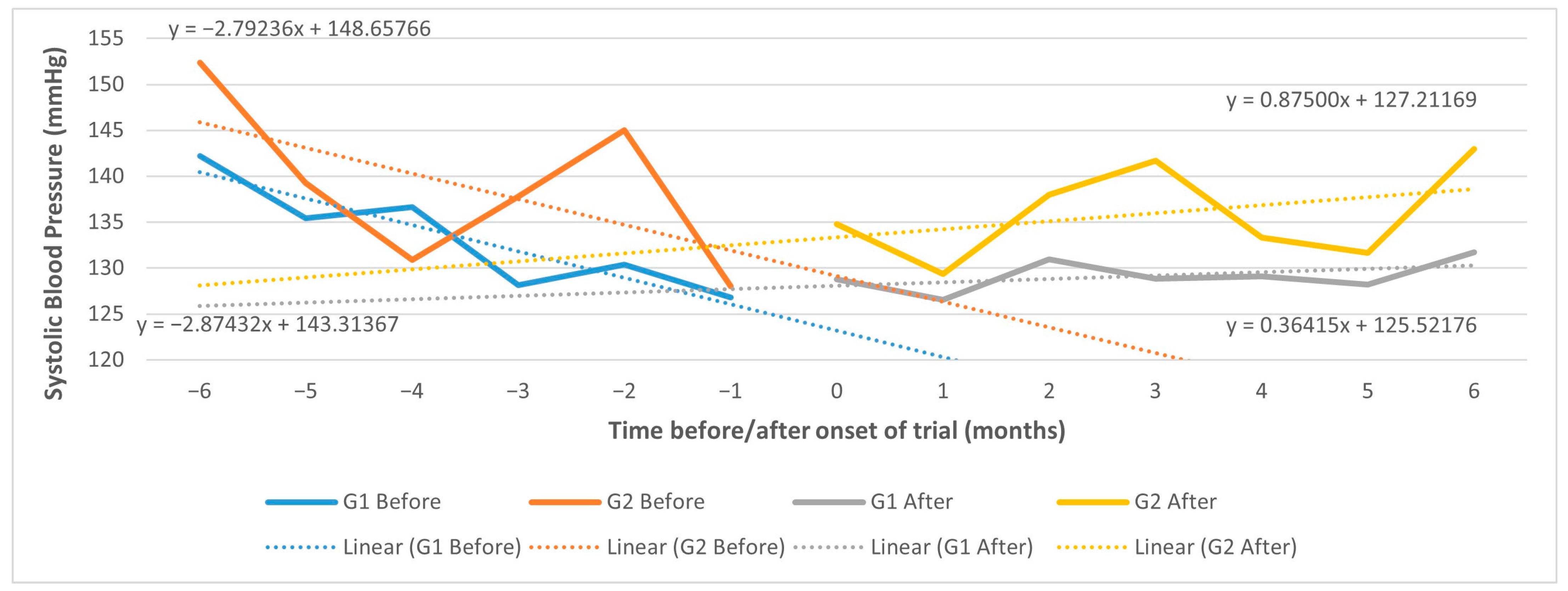
3.6.1. Using Difference-in-Differences in GLM
3.6.2. Using Controlled Interrupted Time Series (CITS)
3.7. Process Indicators
3.8. Adverse Events
3.9. Pharmacy Profiling
4. Discussion
4.1. Summary of Findings
4.1.1. Limitations
4.1.2. Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Espiga de Macedo, M.; Ferreira, R. A Hipertensão Arterial nos Cuidados de Saúde Primários, em Portugal: Contributo para o conhecimento epidemiológico da população em 2013. Hypertension in Primary Health Care in Portugal: Contribution to knowledge in population epidemiology in 2013. Rev. Factores Risco 2015, 36, 47–56. (In Portuguese) [Google Scholar]
- Mariano, C.; Antunes, M.; Rato, Q.; Bourbon, M. _e_LIPID: Caraterização do perfil lipídico da população portuguesa. e-LIPID: Lipid profile of Portuguese population. Bol. Epidemiol. 2015, 14, 7–10. (In Portuguese) [Google Scholar]
- Marques da Silva, P.; Lima, M.J.; Neves, P.M.; Espiga de Macedo, M. Prevalence of cardiovascular risk factors and other comorbidities in patients with hypertension in Portuguese primary health care populations: The PRECISE study. Rev. Port. Cardiol. 2019, 38, 427–437. [Google Scholar] [CrossRef]
- OECD/European Observatory on Health Systems and Policies. Portugal: Country Health Profile 2021, State of Health in the EU; OECD Publishing: Paris, France, 2021. [Google Scholar]
- World Health Organization Regional Office for Europe. Health Services Delivery Programme. Division of Health Systems and Public Health. Ambulatory Care Sensitive Conditions in Portugal—April 2016. Available online: https://www.euro.who.int/en/countries/portugal/publications/ambulatory-care-sensitive-conditions-in-portugal-2016 (accessed on 15 December 2022).
- Sarmento, J.; Rocha, J.V.M.; Santana, R. Defining ambulatory care sensitive conditions for adults in Portugal. BMC Health Serv. Res. 2020, 20, 754. [Google Scholar] [CrossRef] [PubMed]
- Kickbusch, I.; Gleicher, D. Governance for Health in the 21st Century; WHO/Europe: Copenhagen, Denmark, 2012; Available online: https://www.euro.who.int/en/publications/abstracts/governance-for-health-in-the-21st-century (accessed on 15 December 2022).
- European Observatory on Health Systems and Policies. Eurohealth 2012. 18 (3). Available online: https://www.euro.who.int/__data/assets/pdf_file/0017/174410/EuroHealth-v18-n3.pdf (accessed on 15 December 2022).
- World Health Organization Regional Office for Europe. Population-Based Model Kaiser Permanente Pyramid. Integrated Care Models: An Overview. Available online: http://www.euro.who.int/__data/assets/pdf_file/0005/322475/Integrated-care-models-overview.pdf (accessed on 15 December 2022).
- Blalock, S.J.; Roberts, A.W.; Lauffenburger, J.C.; Thompson, T.; O’Connor, S.K. The effect of community pharmacy-based interventions on patient health outcomes: A systematic review. Med. Care Res. Rev. 2013, 70, 235–266. [Google Scholar] [CrossRef] [PubMed]
- Chisholm-Burns, M.A.; Kim Lee, J.; Spivey, C.A.; Slack, M.; Herrier, R.N.; Hall-Lipsy, E.; Graff Zivin, J.; Abraham, I.; Palmer, J.; Martin, J.R.; et al. US pharmacists’ effect as team members on patient care: Systematic review and meta-analyses. Med. Care 2010, 48, 923–933. [Google Scholar] [CrossRef]
- Rotta, I.; Salgado, T.M.; Silva, M.L.; Correr, C.J.; Fernandez-Llimos, F. Effectiveness of clinical pharmacy services: An overview of systematic reviews (2000–2010). Int. J. Clin. Pharm. 2015, 37, 687–697. [Google Scholar] [CrossRef]
- Carter, B.L.; Ardery, G.; Dawson, J.D.; James, P.A.; Bergus, G.R.; Doucette, W.R.; Chrischilles, E.A.; Franciscus, C.L.; Xu, Y. Physician and pharmacist collaboration to improve blood pressure control. Arch. Intern. Med. 2009, 169, 1996–2002. [Google Scholar] [CrossRef]
- Carter, B.L.; Rogers, M.; Daly, J.; Zheng, S.; James, P.A. The potency of team-based care interventions for hypertension: A meta-analysis. Arch. Intern. Med. 2009, 169, 1748–1755. [Google Scholar] [CrossRef]
- Charrois, T.L.; Zolezzi, M.; Koshman, S.L.; Pearson, G.; Makowsky, M.; Durec, T.; Tsuyuki, R.T. A systematic review of the evidence for pharmacist care of patients with dyslipidemia. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2012, 32, 222–233. [Google Scholar] [CrossRef]
- Ellis, S.L.; Carter, B.L.; Malone, D.C.; Billups, S.J.; Okano, G.J.; Valuck, R.J.; Barnette, D.J.; Sintek, C.D.; Covey, D.; Mason, B.; et al. Clinical and economic impact of ambulatory care clinical pharmacists in management of dyslipidemia in older adults: The IMPROVE study. Pharmacotherapy 2000, 20, 1508–1516. [Google Scholar] [CrossRef]
- Machado, M.; Bajcar, J.; Guzzo, G.C.; Einarson, T.R. Sensitivity of patient outcomes to pharmacist interventions. Part II: Systematic review and meta-analysis in hypertension management. Ann. Pharmacother. 2007, 41, 1770–1781. [Google Scholar] [CrossRef]
- Machado, M.; Nassor, N.; Bajcar, J.M.; Guzzo, G.C.; Einarson, T.R. Sensitivity of Patient Outcomes to Pharmacist Interventions. Part III: Systematic Review and Meta-Analysis in Hyperlipidemia Management. Ann. Pharmacother. 2008, 42, 1195–1207. [Google Scholar] [CrossRef]
- Robinson, J.D.; Segal, R.; Lopez, L.M.; Doty, R.E. Impact of a pharmaceutical care intervention on blood pressure control in a chain pharmacy practice. Ann. Pharmacother. 2010, 44, 88–96. [Google Scholar] [CrossRef]
- Santschi, V.; Chiolero, A.; Colosimo, A.L.; Platt, R.W.; Taffé, P.; Burnier, M.; Burnand, B.; Paradis, G. Improving blood pressure control through pharmacist interventions: A meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2014, 3, e000718. [Google Scholar] [CrossRef]
- Straka, R.J.; Taheri, R.; Cooper, S.L.; Smith, J.C. Achieving cholesterol target in a managed care organization (ACTION) trial. Pharmacotherapy 2005, 25, 360–371. [Google Scholar] [CrossRef]
- Costa, S.O. Impacto dos Cuidados Farmacêuticos em Doentes Hipertensos The Impact of Pharmaceutical Care in Hypertensive Patients. Master’s Thesis, NOVA Medical School, Universidade NOVA de Lisboa, Lisbon, Portugal, 2001. (In Portuguese). [Google Scholar]
- Chabot, I.; Moisan, J.; Grégoire, J.-P.; Milot, A. Pharmacist intervention program for control of hypertension. Ann. Pharmacother. 2003, 37, 1186–1193. [Google Scholar] [CrossRef]
- Rogers, E. Diffusion of Innovations, 3rd ed.; New York Free Press: New York, NY, USA, 1983. [Google Scholar]
- Bully, P.; Sánchez, Á.; Zabaleta-del-Olmo, E.; Pombo, H.; Grandes, G. Evidence from interventions based on theoretical models for lifestyle modification (physical activity, diet, alcohol and tobacco use) in primary care settings: A systematic review. Prev. Med. 2015, 76, S76–S93. [Google Scholar] [CrossRef]
- Holmes, E.A.F.; Hughes, D.A.; Morrison, V.L. Predicting adherence to medications using health psychology theories: A systematic review of 20 years of empirical research. Value Health 2014, 17, 863–876. [Google Scholar] [CrossRef]
- Bodenheimer, T.; Wagner, E.H.; Grumbach, K. Improving Primary Care for Patients With Chronic Illness. JAMA 2002, 288, 1775. [Google Scholar] [CrossRef]
- Costa, S. Economic Evaluation of Pharmacy Services in Portugal. In Economic Evaluation of Pharmacy Services; Babar, Z.U., Ed.; Elsevier: London, UK, 2017; pp. 183–192. [Google Scholar] [CrossRef]
- Costa, S.; Cary, M.; Helling, D.K.; Pereira, J.; Mateus, C. An overview of systematic reviews of economic evaluations of pharmacy-based public health interventions: Addressing methodological challenges. Syst. Rev. 2019, 8, 272. [Google Scholar] [CrossRef]
- Costa, S.; Guerreiro, J.; Teixeira, I.; Helling, D.K.; Pereira, J.; Mateus, C. Cost-Effectiveness and Cost-Utility of Hypertension and Hyperlipidemia Collaborative Management between Pharmacies and Primary Care in Portugal Alongside a Trial Compared With Usual Care (USFarmácia®). Front. Pharmacol. 2022, 13, 903270. [Google Scholar] [CrossRef]
- Costa, S.; Guerreiro, J.; Teixeira, I.; Helling, D.K.; Mateus, C.; Pereira, J. Patient Preferences and Cost-Benefit of Hypertension and Hyperlipidemia Collaborative Management Model Between Pharmacies and Primary Care in Portugal: A Discrete Choice Experiment Alongside a Trial (USFarmácia®). In Value in Health, Proceedings of the ISPOR Europe 2022, Vienna, Austria, 6–9 November 2022; Elsevier: Amsterdam, The Netherlands, 2022; Volume 25, p. S447. [Google Scholar]
- Des Jarlais, D.C.; Lyles, C.; Crepaz, N.; TREND Group. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: The TREND statement. Am. J. Public Health 2004, 94, 361–366. [Google Scholar] [CrossRef]
- Hoffmann, T.C.; Glasziou, P.P.; Boutron, I.; Milne, R.; Perera, R.; Moher, D.; Altman, D.G.; Barbour, V.; Macdonald, H.; Johnston, M.; et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014, 348, g1687. [Google Scholar] [CrossRef]
- Zwarenstein, M.; Treweek, S.; Gagnier, J.J.; Altman, D.G.; Tunis, S.; Haynes, B.; Oxman, A.D.; Moher, D.; CONSORT Group; Pragmatic Trials in Healthcare (Practihc) Group. Improving the reporting of pragmatic trials: An extension of the CONSORT statement. BMJ 2008, 337, a2390. [Google Scholar] [CrossRef]
- Schwartz, D.; Lellouch, J. Explanatory and pragmatic attitudes in therapeutical trials. J. Clin. Epidemiol. 2009, 62, 499–505. [Google Scholar] [CrossRef]
- Loudon, K.; Treweek, S.; Sullivan, F.; Donnan, P.; Thorpe, K.E.; Zwarenstein, M. The PRECIS-2 tool: Designing trials that are fit for purpose. BMJ 2015, 350, h2147. [Google Scholar] [CrossRef]
- Zuidgeest, M.G.P.; Goetz, I.; Groenwold, R.H.H.; Irving, E.; van Thiel, G.J.M.W.; Grobbee, D.E. GetReal Work Package 3. Series: Pragmatic trials and real world evidence: Paper 1. Introduction. J. Clin. Epidemiol. 2017, 88, 7–13. [Google Scholar] [CrossRef]
- Mcdonough, R.P.; Doucette, W.R. Developing Collaborative Working Relationships Between Pharmacists and Physicians. J. Am. Pharm. Assoc. 2001, 41, 682–692. [Google Scholar]
- Helling, D.K.; Nelson, K.M.; Ramirez, J.E.; Humphries, T.L. Kaiser Permanente Colorado Region Pharmacy Department: Innovative leader in pharmacy practice. J. Am. Pharm. Assoc. 2006, 46, 67–76. [Google Scholar] [CrossRef]
- Niquille, A.; Ruggli, M.; Buchmann, M.; Jordan, D.; Bugnon, O. The nine-year sustained cost-containment impact of swiss pilot physicians-pharmacists quality circles. Ann. Pharmacother. 2010, 44, 650–657. [Google Scholar] [CrossRef]
- Chen, T.F.; de Almeida Neto, A.C. Exploring elements of interprofessional collaboration between pharmacists and physicians in medication review. Pharm. World Sci. 2007, 29, 574–576. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.r-project.org/ (accessed on 20 May 2020).
- Sievert, C. Plotly for R. Available online: https://plotly-r.com (accessed on 20 May 2020).
- Zelen, M. A new design for randomized clinical trials. N. Engl. J. Med. 1979, 300, 1242–1245. [Google Scholar] [CrossRef]
- Hutton, J.L. Are distinctive ethical principles required for cluster randomized controlled trials? Stat. Med. 2001, 20, 473–488. [Google Scholar] [CrossRef]
- Giraudeau, B.; Caille, A.; Le Gouge, A.; Ravaud, P. Participant Informed Consent in Cluster Randomized Trials: Review. PLoS ONE 2012, 7, e40436. [Google Scholar] [CrossRef]
- Pharmacist Services Technical Advisory Coalition. Medication Therapy Management Service Codes. Available online: http://www.pstac.org/services/mtms-codes.html (accessed on 15 December 2022).
- Rathod, T.; Belcher, J.; Montgomery, A.A.; Salisbury, C.; Foster, N.E. Health services changes: Is a run-in period necessary before evaluation in randomised clinical trials? Trials 2014, 15, 41. [Google Scholar] [CrossRef]
- Eurostat. Equivalised Disposable Income. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Glossary:Equivalised_disposable_income (accessed on 20 May 2020).
- International Consortium for Health Outcomes Measurement. ICHOM Standard Set for Hypertension in Low and Middle Income Countries. 2017. Available online: https://connect.ichom.org/standard-sets/hypertension-in-low-and-middle-income-countries/ (accessed on 20 May 2020).
- Pratt, N.L.; Kerr, M.; Barratt, J.D.; Kemp-Casey, A.; Kalisch Ellett, L.M.; Ramsay, E.; Roughead, E.E. The validity of the Rx-Risk Comorbidity Index using medicines mapped to the Anatomical Therapeutic Chemical (ATC) Classification System. BMJ Open 2018, 8, e021122. [Google Scholar] [CrossRef]
- Carter, B.L.; Coffey, C.S.; Ardery, G.; Uribe, L.; Ecklund, D.; James, P.; Egan, B.; Vander Weg, M.; Chrischilles, E.; Vaughn, T. Cluster-randomized trial of a physician/pharmacist collaborative model to improve blood pressure control. Circ. Cardiovasc. Qual. Outcomes 2015, 8, 235–243. [Google Scholar] [CrossRef]
- Hirsch, J.D.; Steers, N.; Adler, D.S.; Kuo, G.M.; Morello, C.M.; Lang, M.; Singh, R.F.; Wood, Y.; Kaplan, R.M.; Mangione, C.M. Primary care-based, pharmacist-physician collaborative medication-therapy management of hypertension: A randomized, pragmatic trial. Clin. Ther. 2014, 36, 1244–1254. [Google Scholar] [CrossRef]
- Garção, J.A.; Cabrita, J. Evaluation of a pharmaceutical care program for hypertensive patients in rural Portugal. J. Am. Pharm. Assoc. 2002, 42, 858–864. [Google Scholar] [CrossRef]
- Bunting, B.A.; Smith, B.H.; Sutherland, S.E. The Asheville Project: Clinical and economic outcomes of a community-based long-term medication therapy management program for hypertension and dyslipidemia. J. Am. Pharm. Assoc. 2008, 48, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Nola, K.M.; Gourley, D.R.; Portner, T.S.; Gourley, G.K.; Solomon, D.K.; Elam, M.; Regel, B. Clinical and humanistic outcomes of a lipid management program in the community pharmacy setting. J. Am. Pharm. Assoc. 2000, 40, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Bundy, J.D.; Li, C.; Stuchlik, P.; Bu, X.; Kelly, T.N.; Mills, K.T.; He, H.; Chen, J.; Whelton, P.K.; He, J. Systolic Blood Pressure Reduction and Risk of Cardiovascular Disease and Mortality: A Systematic Review and Network Meta-analysis. JAMA Cardiol. 2017, 2, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Gould, A.L.; Davies, G.M.; Alemao, E.; Yin, D.D.; Cook, J.R. Cholesterol reduction yields clinical benefits: Meta-analysis including recent trials. Clin. Ther. 2007, 29, 778–794. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on Data Monitoring Committees. Doc Ref EMEA/CHMP/EWP/5872/03 Corr. London: EMEA. 2005. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-data-monitoring-committees_en.pdf (accessed on 15 December 2022).
- Kairalla, J.A.; Coffey, C.S.; Thomann, M.A.; Muller, K.E. Adaptive trial designs: A review of barriers and opportunities. Trials 2012, 13, 145. [Google Scholar] [CrossRef]
- Bernal, J.L.; Cummins, S.; Gasparrini, A. Interrupted time series regression for the evaluation of public health interventions: A tutorial. Int. J. Epidemiol. 2017, 46, 348–355, Corrigendum: Int. J. Epidemiol. 2020, 49, 1414. [Google Scholar] [CrossRef]
- Bernal, J.L.; Cummins, S.; Gasparrini, A. The use of controls in interrupted time series studies of public health interventions. Int. J. Epidemiol. 2018, 47, 2082–2093. [Google Scholar] [CrossRef]
- Portaria No 281/2016. [Ordinance No. 281/2016]. Diário da República Ia Série. 206 (2016-10-26). Available online: https://diariodarepublica.pt/dr/detalhe/portaria/281-2016-75606253 (accessed on 15 December 2022). (In Portuguese).
- Decreto-Lei No 73/2017. [Decree-Law No. 73/2017]. Diário da República Ia Série. 118 (2017-06-21). Available online: https://diariodarepublica.pt/dr/detalhe/decreto-lei/73-2017-107541409 (accessed on 15 December 2022). (In Portuguese).
- Portaria No 212/2017. [Ordinance No. 212/2017]. Diário da República Ia Série. 138 (2017-07-19). Available online: https://diariodarepublica.pt/dr/detalhe/portaria/212-2017-107709510 (accessed on 15 December 2022). (In Portuguese).
- Boutell, J.; Craig, P.; Lewsey, J.; Robinson, M.; Popham, F. Synthetic control methodology as a tool for evaluating population-level health interventions. J. Epidemiol. Community Health 2018, 72, 673–678. [Google Scholar] [CrossRef]
- Kodner, D.L.; Spreeuwenberg, C. Integrated Care: Meaning, Logic, Applications, and Implications—A discussion Paper. Int. J. Integr. Care 2002, 2. Available online: https://www.ijic.org/article/10.5334/ijic.67/ (accessed on 15 December 2022). [CrossRef]
- Isetts, B.J.; Buffington, D.E.; Carter, B.L.; Smith, M.; Polgreen, L.A.; James, P.A. Evaluation of Pharmacists’ Work in a Physician-Pharmacist Collaborative Model for the Management of Hypertension. Pharmacotherapy 2016, 36, 374–384. [Google Scholar] [CrossRef]
- Decreto-Lei No 62/2016. [Decree-Law No. 62/2016]. Diário da República Ia Série. 175(2016-09-12). Available online: https://diariodarepublica.pt/dr/detalhe/decreto-lei/62-2016-75307907 (accessed on 29 January 2023). (In Portuguese).
- Bilhete de Identidade dos Cuidados de Saúde Primários (BI-CSP). Available online: https://bicsp.min-saude.pt/pt/Paginas/default.aspx (accessed on 20 March 2018).
- Perfis Locais de Saúde Região Centro 2016. Available online: https://www.arscentro.min-saude.pt/saude-publica/perfis-locais-de-saude/ (accessed on 20 March 2018).
| Measures | Timepoint Recorded | Data Sources |
|---|---|---|
| Effectiveness Outcomes | ||
| Primary Care * PCU point-of-care measurements | All available data points: 6 ± 2 M before patient enrollment and 6 ± 2 M after patient enrollment | Primary care Electronic Medical Record (EMR) software (SClínico®) |
| Process | ||
| Pharmacy * Pharmacy visits Pharmacy point-of-care measurements and tests | All available data points: 6 ± 2 M after patient enrollment (no intervention before enrollment) | Pharmacy dispensing claims software (Sifarma®) |
| Primary Care * GP visits Nurse visits PCU point-of-care measurements and tests | All available data points: 6 ± 2 M before patient enrollment and 6 ± 2 M after patient enrollment | Primary care Electronic Medical Record (EMR) software (SClínico®) |
| Medication * Prescribed antihypertensive/lipid-lowering medication | All available data points: 6 ± 2 M before patient enrollment and 6 ± 2 M after patient enrollment | Primary care prescription claims software (PEM®) |
| Economic, Quality, and Preference Outcomes | ||
| Quality of Life ** Quality of life | 0 and 6 months | Patient telephone survey (EQ-5D-3L™) |
| Use of Healthcare Resources ** Primary care + hospital ER visits Hospital outpatient visits Days in hospital Working days lost Travel + waiting time to PCU/Pharmacy Means of transport + km or cost | 0 and 6 months | Patient telephone survey |
| Behaviors ** Medication adherence | All available data points: 6 ± 2 M before patient enrollment and 6 ± 2 M after patient enrollment 0 and 6 months | Primary care prescription claims software (PEM®) Patient telephone survey (MAT-7®) |
| Patient Experience ** Patient focus group | After the end of the trial | Focus group |
| Patient Experience ** Patient preferences | After the end of the trial | Patient telephone preference survey |
| Patient Experience ** Satisfaction with pharmacy care | 0 and 6 months | Patient telephone survey |
| Demographics and Case Mix at Baseline | Intervention (n = 131) | Control (n = 72) | p-Value for Difference (a) |
|---|---|---|---|
| Gender (Telephone baseline survey) | |||
| Female, n (%) | 82 (62.6%) | 43 (59.7%) | 0.6872 |
| Male, n (%) | 49 (37.4%) | 29 (40.3%) | |
| Age, years (mean ± SD) (Telephone baseline survey) | 66.4 ± 10.8 | 64.0 ± 9.8 | 0.0497 |
| Education (Telephone baseline survey) | |||
| No. years compulsory education, mean (SD) | 9.1 (4.6) | 7.5 (4.6) | 0.0214 |
| Education ≤ elementary school 3rd cycle (current 9th grade/former 5th grade/technical schools), n (%) | 64 (58.7%) | 45 (75.0%) | 0.0343 |
| NR | 22 | 12 | |
| Employment status (Telephone baseline survey) | |||
| Retired/pensioner + permanently disabled + unemployed + household tasks, n (%) | 81 (74.3%) | 41 (68.4%) | 0.4065 |
| NR | 22 | 12 | |
| Income status (Telephone baseline survey) | |||
| Approx. monthly equivalent income per person (=household income average threshold/ no. of equalized individuals in household) in EUR (SD) NR = 74 | 846.30 (569.14) | 614.52 (411.54) | 0.0058 |
| Approx. monthly household income (= household income average threshold) in EUR (SD) NR = 72 | 1282.76 (854.84) | 943.48 (602.83) | 0.0204 |
| ≤EUR 501.20 (n, %) | 23 (26.7%) | 22 (48.9%) | 0.0113 |
| NR | 45 | 27 | |
| Municipality Purchasing Power Index | 95 | 92,5 | |
| Smoking status (n, %) (Telephone baseline survey) | |||
| Smoker (Yes) | 11 (9.9%) | 5 (8.3%) | 0.7355 |
| NR | 20 | 12 | |
| Body Mass Index (mean kg/m2 ± SD) (Telephone baseline survey) | 27.0 ± 4.2 | 28.4 ± 4.5 | 0.028l5 |
| Comorbidities (NR = 7) * | |||
| No. comorbidities per patient (mean ± SD) | 1.9 (1.7) | 2.3 (1.8) | 0.0988 |
| ≥1 (n,%) | 97 (75.8) | 62 (91.2) | |
| Ischemic heart disease: hypertension | 27 (21.1) | 27 (39.7) | 0.0055 |
| Gastroesophageal reflux disease | 29 (22.7) | 16 (23.5) | 0.8900 |
| Anxiety | 29 (22.7) | 13 (19.1) | 0.5655 |
| Depression | 27 (21.1) | 13 (19.1) | 0.7439 |
| Congestive heart failure | 18 (14.1) | 12 (17.6) | 0.5070 |
| No. regular medicines per patient (Primary care software) | |||
| Mean, (SD) | 4.5 (2.7) | 4.8 (2.8) | 0.3916 |
| Minimum–maximum | 0–13 | 0–13 | |
| Medication profile (Pharmacy and primary care software, telephone baseline survey) | |||
| Antihypertensive medication (n, %) | 44 (33.6%) | 19 (26.4%) | 0.0117 |
| Lipid-lowering medication (n, %) | 36 (27.5%) | 10 (13.9%) | |
| Antihypertensive and lipid-lowering medication (n, %) | 51 (38.9%) | 43 (59.7%) | |
| Number of years since onset (mean ± SD) (Telephone baseline survey) | |||
| Antihypertensive medication | 5.4 (5.6) | 6.3 (6.7) | 0.8698 |
| Lipid-lowering medication | 4.4 (4.4) | 5.9 (6.8) | 0.4041 |
| Antihypertensive medication (Primary care software) | |||
| No. antihypertensive medicines per patient (mean ± SD) | 1.5 (0.7) | 1.7 (0.9) | 0.3950 |
| ACEi/ARB (C09) | 83 (64.8%) | 51 (75.0%) | 0.1456 |
| Alpha-blocker (C02CA) | 1 (0.8%) | 1 (1.5%) | 1.0000 |
| Beta-blocker (C07) | 23 (18.0%) | 16 (23.5%) | 0.3533 |
| Loop diuretics (C03CA + C03CA) | 12 (9.4%) | 9 (13.2%) | 0.4056 |
| Thiazides (C03A) | 0 (0.0%) | 0 (0.0%) | NA |
| Calcium channel blocker (C08) | 11 (8.6%) | 14 (20.6%) | 0.0166 |
| Other (C02 + C03)-(C02CA + C03A + C03CA + C03CB) | 11 (8.6%) | 6 (8.8%) | 0.9566 |
| Lipid-lowering medication (Primary care software) | |||
| Statin (C10AA + C10BA + C10BX) | 67 (52.3%) | 38 (55.9%) | 0.6363 |
| Ezetimibe (C10AX09) | 1 (0.8%) | 1 (1.5%) | 1.0000 |
| Fibrates (C10AB) | 2 (1.6%) | 2 (2.9%) | 0.6107 |
| Other (C10-(C10AA + C10BA + C10BX + C10AB + C10AX09) | 0 (0.0%) | 0 (0.0%) | NA |
| Outcome | Model 1 Unadjusted Effect (95% CI) | p-Value | Model 2 Adjusted Effect (95% CI) * | p-Value |
|---|---|---|---|---|
| All patients | ||||
| Systolic BP, mm Hg | −2.08 (−12.09; 7.94) | 0.6844 | 7.48 (−4.17; 19.33) | 0.2062 |
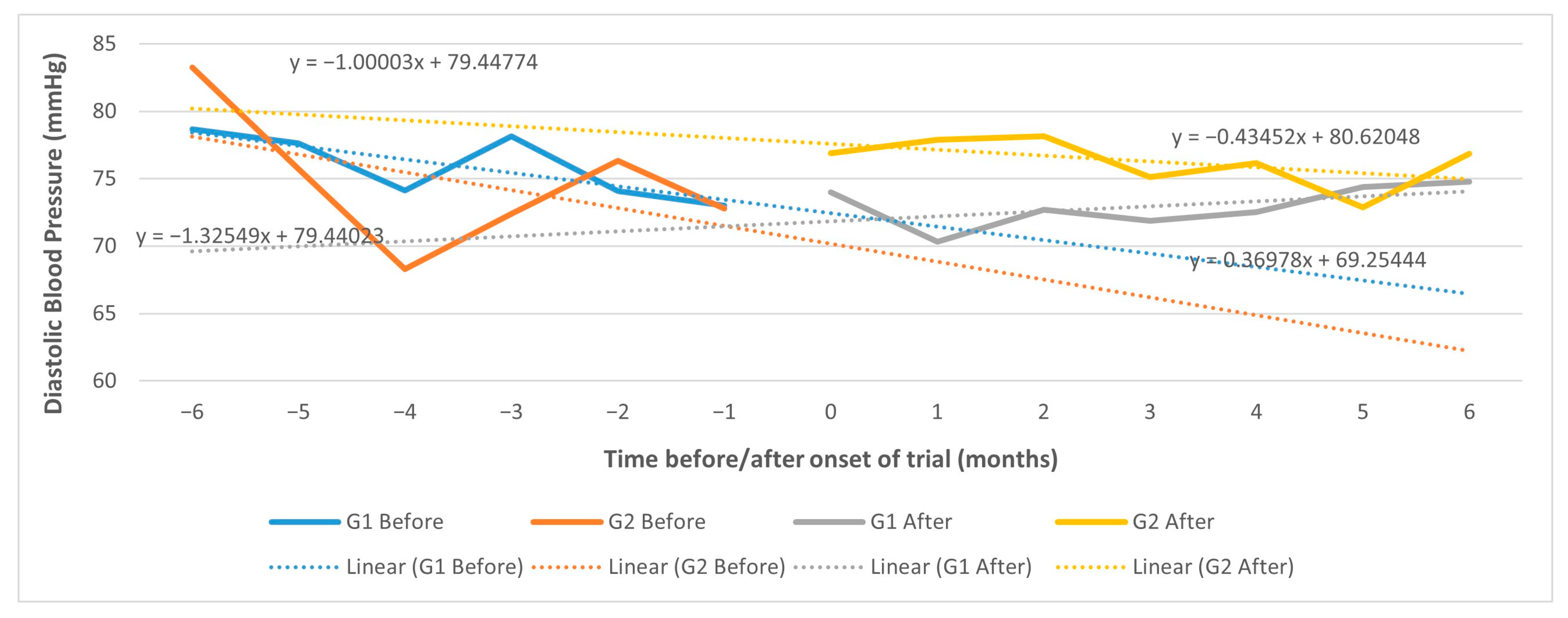
| Diastolic BP, mm Hg | −0.42 (−7.74; 6.90) | 0.9100 | 1.66 (−6.46; 9.78) | 0.6887 |
| BP control ** | 1.14 (0.31; 4.24) | 0.8477 | 0.85 (0.17; 4.32) | 0.8444 |
| TC, mg/dL | 12.84 (−19.70; 45.38) | 0.4392 | 22.12 (−15.37; 59.61) | 0.2475 |
| TC control ** | 0.22 (0.01; 3.17) | 0.2629 | - | - |
| Subgroup uncontrolled at baseline | ||||
| Systolic BP, mm Hg | −2.47 (−16.60; 11.65) | 0.7315 | 5.41 (−8.10; 18.92) | 0.4325 |
| Diastolic BP, mm Hg | −1.23 (−13.63; 11.17) | 0.8458 | 1.34 (−13.34; 16.03) | 0.8579 |
| TC, mg/dL | 44.88 (−4.58; 94.34) | 0.0753 | 36.93 (0.57; 73.28) | 0.0465 |
| Subgroup most income deprived (≤501.20 €/month) | ||||
| Systolic BP, mm Hg | 10.88 (−5.17; 26.94) | 0.1840 | 10.85 (−5.77; 27.47) | 0.2006 |
| Diastolic BP, mm Hg | 6.79 (−4.50; 18.07) | 0.2384 | 6.11 (−5.47; 17.69) | 0.3009 |
| BP control ** | 0.79 (0.07; 8.45) | 0.8484 | 1.05 (0.09; 12.64) | 0.9705 |
| TC, mg/dL | 19.61 (−26.87; 66.10) | 0.4082 | −1.41 (−52.64; 49.83) | 0.9571 |
| TC control ** | - | - | - | - |
| Pharmacy Profiling * | Intervention Pharmacies (n = 7) | Control Pharmacies (n = 13) | p-Value |
|---|---|---|---|
| Ratio of pharmacists/pharmacy, mean (SD) | 4.4 (2.6) | 3.7 (1.5) | 0.6011 |
| Number of daily dispensed POM and NPM per pharmacist, mean (SD) | 62.3 (17.6) | 122.5 (43.9) | 0.0015 |
| Number of operating hours per week, mean (SD) | 63.6 (16.2) | 76.7 (29.6) | 0.1770 |
| Engagement of pharmacy director in local/regional leadership, n (%) | 1 (14.3) | 1 (7.7) | 1.0000 |
| Active status in Kaizen™, n (%) | 5 (71.4) | 11 (84.6) | 0.5868 |
| Engagement in at least one national/regional professional initiative, n (%) | 6 (85.7) | 12 (92.3) | 1.0000 |
| Active status in Pharmacy Customer Loyalty Program Saúda®, n (%) | 5 (71.4) | 11 (84.6) | 0.5868 |
| Active status in MED180® (refill text reminder), n (%) | 6 (87.5) | 0 (0.0) | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, S.; Biscaia, J.L.; Horta, M.R.; Romano, S.; Guerreiro, J.; Heudtlass, P.; Cary, M.; Romão, M.; Teixeira Rodrigues, A.; Miranda, A.; et al. Real-World Effectiveness in Hypertension and Hyperlipidemia Collaborative Management between Pharmacies and Primary Care in Portugal: A Multicenter Pragmatic Controlled Trial (USFarmácia®). Int. J. Environ. Res. Public Health 2023, 20, 6496. https://doi.org/10.3390/ijerph20156496
Costa S, Biscaia JL, Horta MR, Romano S, Guerreiro J, Heudtlass P, Cary M, Romão M, Teixeira Rodrigues A, Miranda A, et al. Real-World Effectiveness in Hypertension and Hyperlipidemia Collaborative Management between Pharmacies and Primary Care in Portugal: A Multicenter Pragmatic Controlled Trial (USFarmácia®). International Journal of Environmental Research and Public Health. 2023; 20(15):6496. https://doi.org/10.3390/ijerph20156496
Chicago/Turabian StyleCosta, Suzete, José Luís Biscaia, Maria Rute Horta, Sónia Romano, José Guerreiro, Peter Heudtlass, Maria Cary, Mariana Romão, António Teixeira Rodrigues, Ana Miranda, and et al. 2023. "Real-World Effectiveness in Hypertension and Hyperlipidemia Collaborative Management between Pharmacies and Primary Care in Portugal: A Multicenter Pragmatic Controlled Trial (USFarmácia®)" International Journal of Environmental Research and Public Health 20, no. 15: 6496. https://doi.org/10.3390/ijerph20156496
APA StyleCosta, S., Biscaia, J. L., Horta, M. R., Romano, S., Guerreiro, J., Heudtlass, P., Cary, M., Romão, M., Teixeira Rodrigues, A., Miranda, A., Martins, A. P., Bento, A. S., Pereira, J., Mateus, C., & Helling, D. K. (2023). Real-World Effectiveness in Hypertension and Hyperlipidemia Collaborative Management between Pharmacies and Primary Care in Portugal: A Multicenter Pragmatic Controlled Trial (USFarmácia®). International Journal of Environmental Research and Public Health, 20(15), 6496. https://doi.org/10.3390/ijerph20156496









