Comparative Analysis of Health- and Vision-Related Quality of Life Measures among Trinidadians with Low Vision and Normal Vision—A Cross-Sectional Matched Sample Study
Abstract
1. Introduction
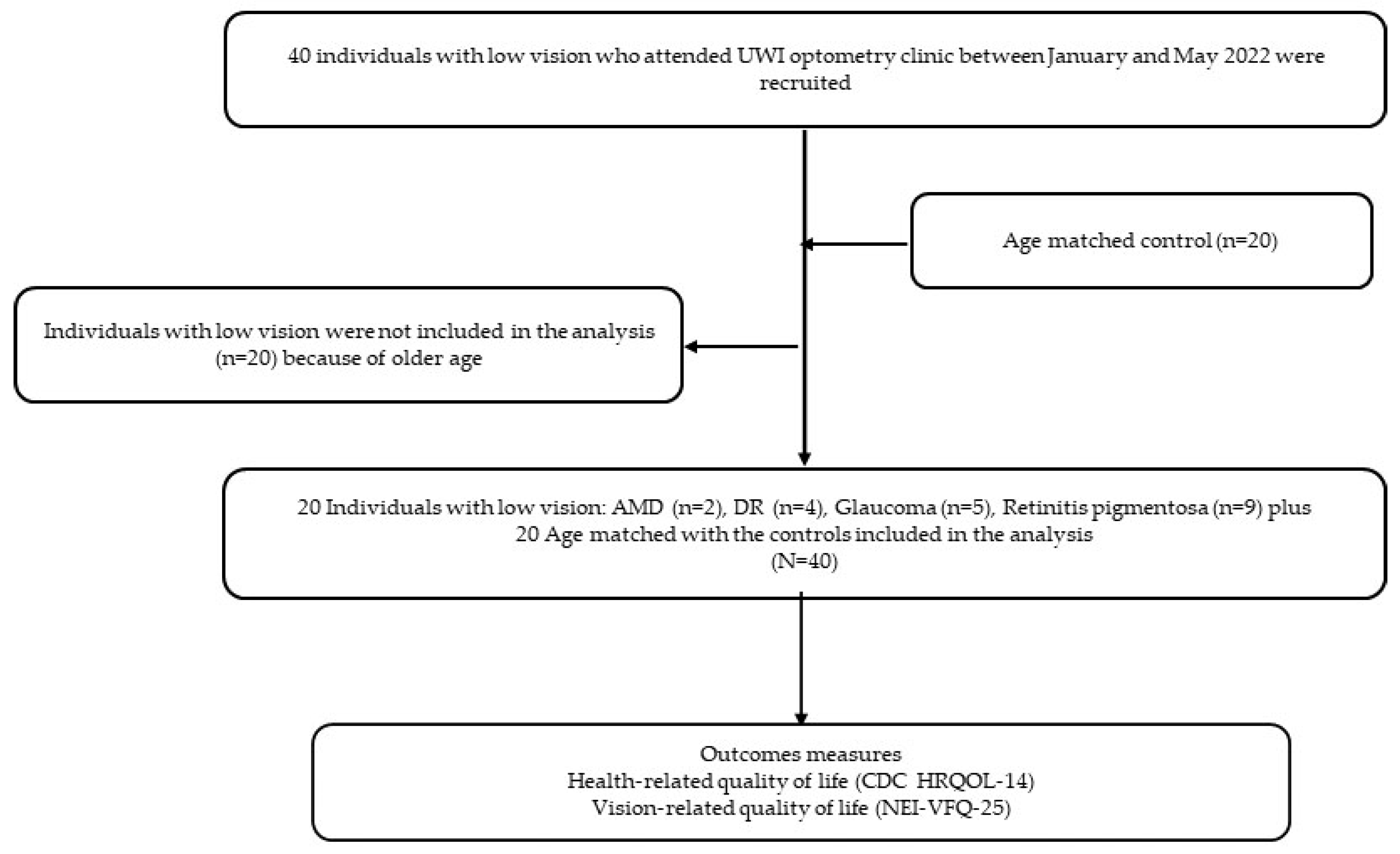
2. Materials and Methods
2.1. Setting and Design
2.2. Participants and Eligibility Criteria
2.3. Procedures
2.4. Measures
2.5. Statistical Analysis
3. Results
3.1. Demographic Characteristics of the Sample
3.2. Differences in HRQoL between the Low-Vision and Control Groups
3.3. Differences in VR-QoL between the Low-Vision and Control Groups
3.4. HRQoL Differences among the Ocular Condition Groups
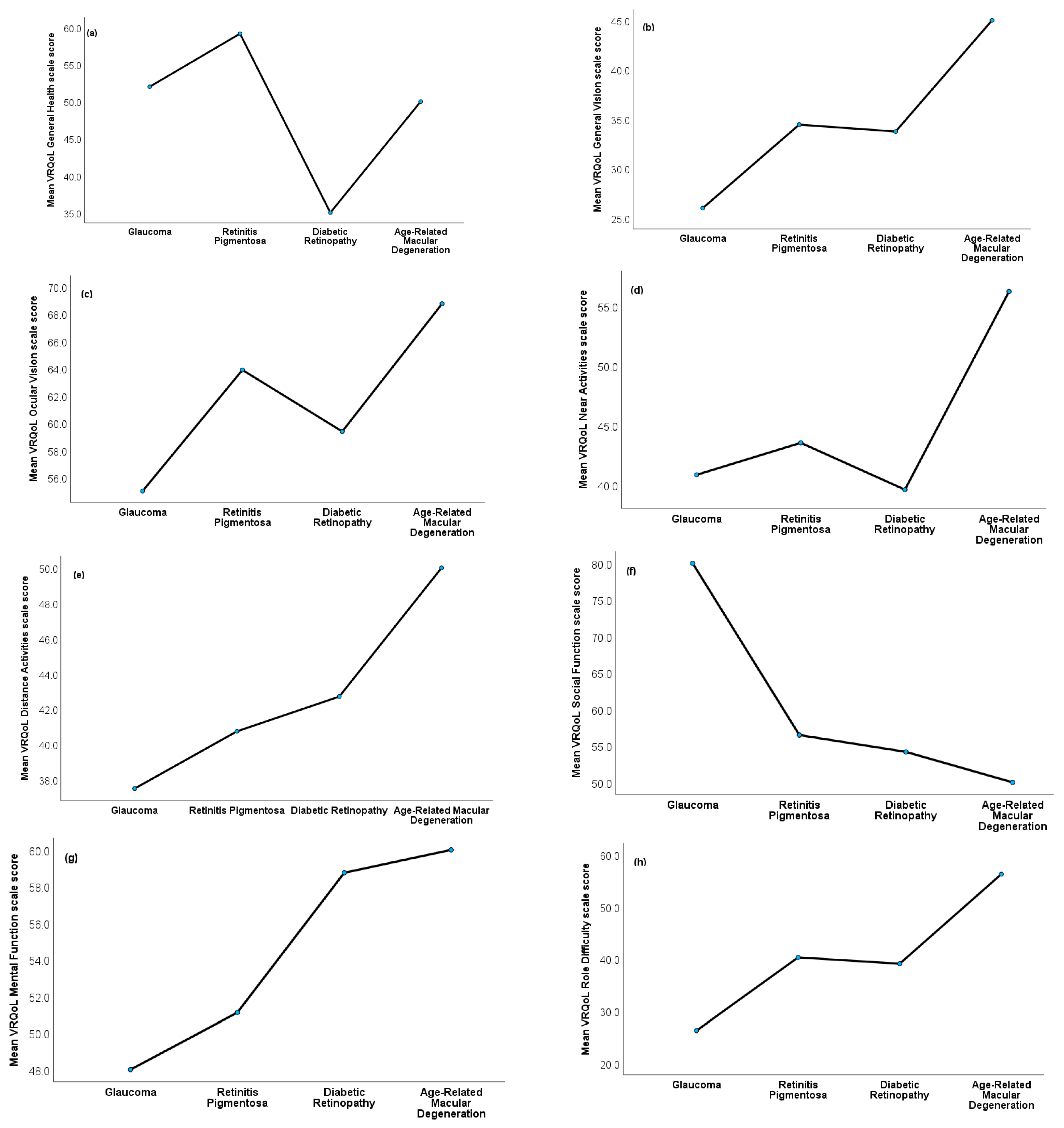
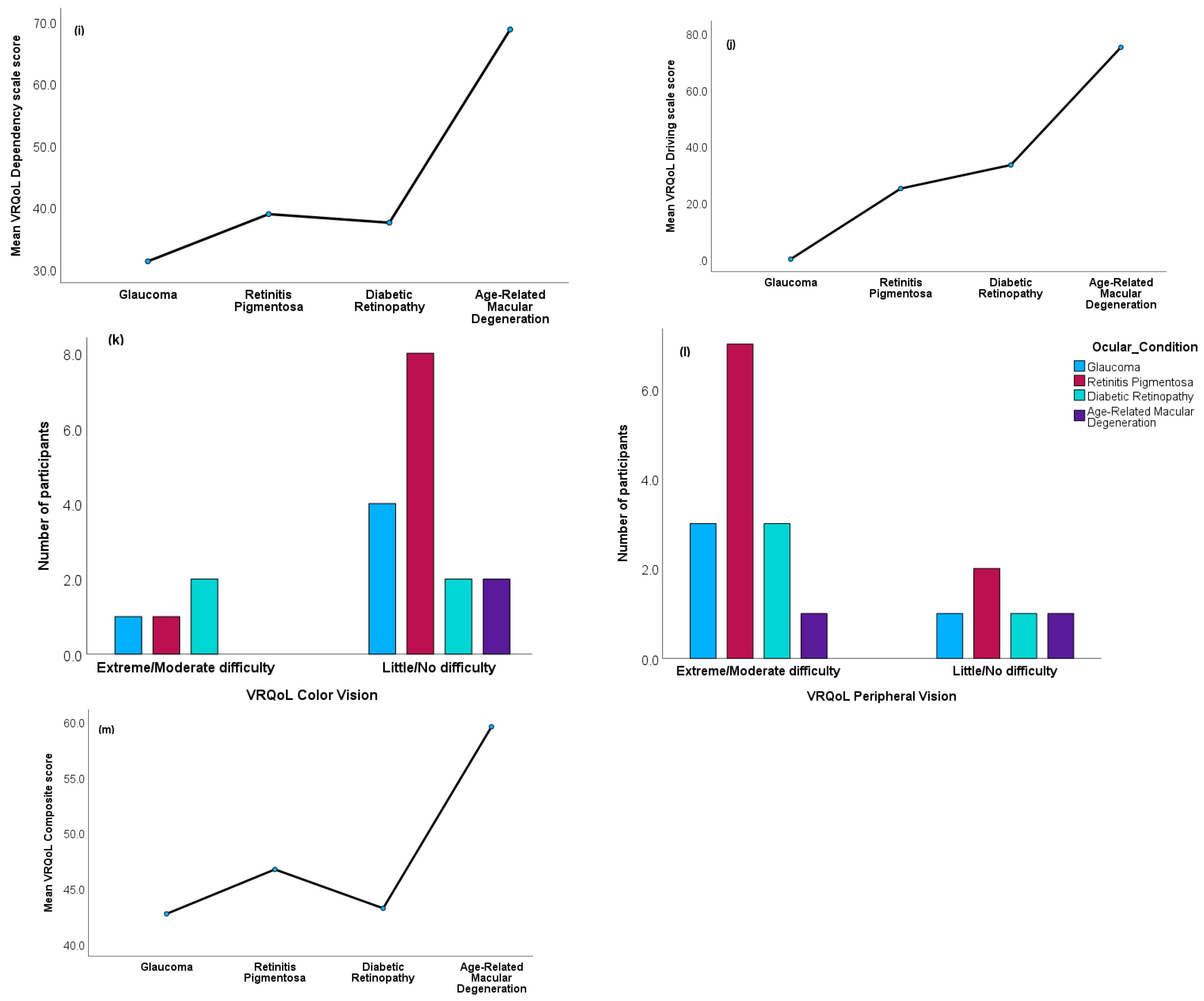
3.5. VRQoL Differences among the Ocular Condition Groups
4. Discussion
4.1. Strengths and Limitations of Study
4.2. Policy and Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burton, M.J.; Ramke, J.; Marques, A.P.; A Bourne, R.R.; Congdon, N.; Jones, I.; Tong, B.A.M.A.; Arunga, S.; Bachani, D.; Bascaran, C.; et al. The Lancet global health Commission on global eye health: Vision beyond 2020. Lancet Glob. Health 2021, 9, e489–e551. [Google Scholar]
- WHO. International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10)-WHO Version for 2016. Diseases of the Eye and Adnexa (H00–H59). Visual Disturbances and Blindness (H53–H54). Geneva: World Health Organization. 2016. Available online: https://icd.who.int/browse10/2016/en#/H53-H54 (accessed on 4 April 2023).
- Yibekal, B.T.; Alemu, D.S.; Anbesse, D.H.; Alemayehu, A.M.; Alimaw, Y.A. Vision-related quality of life among adult patients with visual impairment at University of Gondar, Northwest Ethiopia. J. Ophthalmol. 2020, 2020, 9056097. [Google Scholar] [CrossRef]
- Choi, S.U.; Chun, Y.S.; Lee, J.K.; Kim, J.T.; Jeong, J.H.; Moon, N.J. Comparison of vision-related quality of life and mental health between congenital and acquired low-vision patients. Eye 2019, 33, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Amedo, A.O.; Adade, S.; Koomson, N.Y.; Osae, E.A. Influence of visual impairment on the quality of life: A survey of patients reporting at the low vision centre of the eastern regional hospital of Ghana. J. Ophthalmic Sci. 2016, 1, 1–13. [Google Scholar] [CrossRef]
- Nayeni, M.; Dang, A.; Mao, A.J.; Malvankar-Mehta, M.S. Quality of life of low vision patients: A systematic review and meta-analysis. Can. J. Ophthalmol. 2021, 56, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.R.; Persad, V.; Farnon, N. A retrospective study of causes of visual impairment and use of low vision devices in the low vision clinic in Trinidad and Tobago. J. Optom. 2021, 14, 335–341. [Google Scholar] [CrossRef]
- Bourne, R.R.; Flaxman, S.; Braithwaite, T.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; Leasher, J.L.; Limburg, H.; Naidoo, K.S.; Pesudovs, K.; et al. Global prevalence of blindness and distance and near vision impairment: Magnitude, temporal trends, and projections. Investig. Ophthalmol. Vis. Sci. 2017, 58, 840. [Google Scholar]
- Ejiakor, I.; Achigbu, E.; Onyia, O.; Edema, O.; Florence, U.N. Impact of visual impairment and blindness on quality of life of patients in Owerri, Imo State, Nigeria. Middle East Afr. J. Ophthalmol. 2019, 26, 127. [Google Scholar]
- Fenwick, E.K.; Pesudovs, K.; Khadka, J.; Dirani, M.; Rees, G.; Wong, T.Y.; Lamoureux, E.L. The impact of diabetic retinopathy on quality of life: Qualitative findings from an item bank development project. Qual. Life Res. 2012, 21, 1771–1782. [Google Scholar] [CrossRef]
- Braithwaite, T.; Verlander, N.Q.; Peto, T.; Bartholomew, D.; Deomansingh, F.; Bridgemohan, P.; Saei, A.; Sharma, S.; Singh, D.; Ramsewak, S.S.; et al. National Eye Survey of Trinidad and Tobago (NESTT): Prevalence, causes and risk factors for presenting vision impairment in adults over 40 years. Br. J. Ophthalmol. 2020, 104, 74–80. [Google Scholar] [CrossRef]
- Prem Senthil, M.; Khadka, J.; Pesudovs, K. Seeing through their eyes: Lived experiences of people with retinitis pigmentosa. Eye 2017, 31, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Central Statistical Office. Trinidad and Tobago 2011 Population and Housing Census Demographic Report; Central Statistical Office Port of Spain: Port of Spain, Trinidad and Tobago, 2012.
- Centers of Disease Control and Prevention. Health Related Quality of Life. CDC HRQOL-14 “Healthy Days Measure”; Centers of Disease Control and Prevention: Hyattsville, MD, USA, 2011.
- Mangione, C.M.; Lee, P.P.; Gutierrez, P.R.; Spritzer, K.; Berry, S.; Hays, R.D. Development of the 25-list-item national eye institute visual function questionnaire. Arch. Ophthalmol. 2001, 119, 1050–1058. [Google Scholar] [CrossRef]
- Mangione, C.M.; Lee, P.P.; Pitts, J.; Gutierrez, P.; Berry, S.; Hays, R.D. Psychometric properties of the National Eye Institute visual function questionnaire (NEI-VFQ). Arch. Ophthalmol. 1998, 116, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Nutheti, R.; Shamanna, B.R.; Nirmalan, P.K.; Keeffe, J.E.; Krishnaiah, S.; Rao, G.N.; Thomas, R. Impact of impaired vision and eye disease on quality of life in Andhra Pradesh. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4742–4748. [Google Scholar] [CrossRef]
- Hodge, S.; Barr, W.; Bowen, L.; Leeven, M.; Knox, P. Exploring the role of an emotional support and counselling service for people with visual impairments. Br. J. Vis. Impair. 2013, 31, 5–19. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, J.S.; Heo, H.; Suh, Y.-W.; Kim, S.-H.; Lim, K.H.; Moon, N.J.; Lee, S.J.; Park, S.H.; Baek, S.-H.; et al. A nationwide population-based study of low vision and blindness in South Korea. Investig. Ophthalmol. Vis. Sci. 2015, 56, 484–493. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luu, W.; Kalloniatis, M.; Bartley, E.; Tu, M.; Dillon, L.; Zangerl, B.; Ly, A. A holistic model of low vision care for improving vision-related quality of life. Clin. Exp. Optom. 2020, 103, 733–741. [Google Scholar] [CrossRef]
- Sturrock, B.A.; Xie, J.; Holloway, E.E.; Lamoureux, E.L.; Keeffe, J.E.; Fenwick, E.K.; Rees, G. The influence of coping on vision-related quality of life in patients with low vision: A prospective longitudinal study. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2416–2422. [Google Scholar] [CrossRef]
- Habsyiyah, H.; Lestari, Y.D.; Ariawan, I.; Gondhowiardjo, T.D. Relationship of socioeconomic factors with vision-related quality of life on severe low vision and blind population in Indonesia. Med. J. Indones. 2015, 24, 245–251. [Google Scholar] [CrossRef]
- Nickels, S.; Schuster, A.K.; Singer, S.; Wild, P.S.; Laubert-Reh, D.; Schulz, A.; Finger, R.P.; Michal, M.; Beutel, M.E.; Münzel, T.; et al. The National eye Institute 25-item visual function questionnaire (NEI VFQ-25)–reference data from the German population-based Gutenberg health study (GHS). Health Qual. Life Outcomes 2017, 15, 156. [Google Scholar] [CrossRef]
- Wang, M.Y.; Rousseau, J.; Boisjoly, H.; Schmaltz, H.; Kergoat, M.-J.; Moghadaszadeh, S.; Djafari, F.; Freeman, E.E. Activity limitation due to a fear of falling in older adults with eye disease. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7967–7972. [Google Scholar] [CrossRef]
- Chaumet-Riffaud, A.E.; Chaumet-Riffaud, P.; Cariou, A.; Devisme, C.; Audo, I.; Sahel, J.-A.; Mohand-Said, S. Impact of retinitis pigmentosa on quality of life, mental health, and employment among young adults. Am. J. Ophthalmol. 2017, 177, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Gothwal, V.K.; Bharani, S. Outcomes of multidisciplinary low vision rehabilitation in adults. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7451–7461. [Google Scholar] [CrossRef] [PubMed]
- van Nispen, R.M.; Virgili, G.; Hoeben, M.; Langelaan, M.; Klevering, J.; Keunen, J.E.; van Rens, G.H. Low vision rehabilitation for better quality of life in visually impaired adults. Cochrane Database Syst. Rev. 2020, 2020, CD006543. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dong, J.; Chen, Y.; Zhang, W.; Tong, S.; Guo, J. Low vision rehabilitation in improving the quality of life for patients with impaired vision: A systematic review and meta-analysis of 52 randomized clinical trials. Medicine 2021, 100, e25736. [Google Scholar] [CrossRef] [PubMed]
- Sengo, D.B.; Marraca, N.A.; Muaprato, A.M.; García-Sanjuan, S.; Caballero, P.; López-Izquierdo, I. Barriers to accessing eye health services in suburban communities in Nampula, Mozambique. Int. J. Environ. Res. Public Health 2022, 19, 3916. [Google Scholar] [CrossRef]
- McCloud, C.; Khadka, J.; Gilhotra, J.S.; Pesudovs, K. Divergence in the lived experience of people with macular degeneration. Optom. Vis. Sci. 2014, 91, 966–974. [Google Scholar] [CrossRef]
- Bartlett, R.; Acton, J.H.; Ryan, B.; Man, R.; Pickles, T.; Nollett, C. Training results in increased practitioner confidence and identification of depression in people with low vision: A mixed methods study. Ophthalmic Physiol. Opt. 2021, 41, 316–330. [Google Scholar] [CrossRef]
- Moore, L.W.; Constantino, R.E.; Allen, M. Severe visual impairment in older women. West. J. Nurs. Res. 2000, 22, 571–595. [Google Scholar] [CrossRef]
- Demmin, D.L.; Silverstein, S.M. Visual Impairment and Mental Health: Unmet Needs and Treatment Options. Clin. Ophthalmol. 2020, 14, 4229–4251. [Google Scholar] [CrossRef]
| Unadjusted Models | Adjusted Models | |||||||
|---|---|---|---|---|---|---|---|---|
| CDC HRQOL-14 Item | B | SE | 95% CI | p | β | SE | 95% CI | p |
| Healthy days core module | ||||||||
| Poor general health days | −1.3 | 0.9 | −3.1, 0.4 | 0.133 | −0.8 | 1.1 | −3.0, 1.4 | 0.476 |
| Poor physical health days | −2.8 | 1.2 | −5.2, −0.3 | 0.027 | −2.1 | 1.7 | −5.4, 1.2 | 0.221 |
| Poor mental health days | 4.6 | 1.8 | 1.0, 8.2 | 0.012 | 1.7 | 2.4 | −3.0, 6.4 | 0.481 |
| Impact on activities days | −0.1 | 1.8 | −2.4, 2.2 | 0.933 | −1.6 | 1.6 | −4.7, 1.4 | 0.298 |
| Activities limitation module | ||||||||
| Experience activity limitation | −2.8 | 0.8 | −4.3, −1.2 | <0.001 | −3.7 | 1.3 | −6.3, −1.1 | 0.006 |
| Main problems/impairment | −0.5 | 0.1 | −0.8, −0.3 | <0.001 | −0.4 | 0.1 | −0.7, −0.1 | 0.005 |
| Problem duration | −4.1 | 2.7 | −9.4, 1.3 | 0.140 | −3.2 | 4.3 | −11.7, 5.3 | 0.463 |
| Need personal care support | −3.7 | 4.3 | −12.1, 4.7 | 0.386 | −0.1 | 0.1 | −0.3, 0.0 | 0.125 |
| Need support for routines | −4.1 | 4.3 | −12.5, 4.3 | 0.340 | −0.1 | 0.1 | −0.3, 0.1 | 0.159 |
| Healthy days symptoms module | ||||||||
| Days of feeling pain | 9.0 | 5.9 | −2.6, 20.6 | 0.129 | 13.3 | 6.9 | −0.3, 26.9 | 0.055 |
| Days of feeling moody | 0.5 | 2.7 | −4.9, 5.9 | 0.855 | −2.1 | 3.1 | −8.2, 3.9 | 0.491 |
| Days of feeling anxious | 13.9 | 4.9 | 4.2, 23.5 | 0.005 | 14.4 | 6.0 | 2.6, 26.1 | 0.017 |
| Days of sleep problems | 11.3 | 5.6 | 0.4, 22.2 | 0.042 | 11.0 | 6.8 | −2.4, 24.3 | 0.109 |
| Days of feeling energetic | −8.0 | 5.8 | −19.4, 3.4 | 0.167 | −8.0 | 7.1 | −21.8, 5.9 | 0.260 |
| Unadjusted Models | Adjusted Models | |||||||
|---|---|---|---|---|---|---|---|---|
| NEI-VFQ-25 Subscale | B | SE | 95% CI | p | β | SE | 95% CI | p |
| General health | 27.3 | 5.2 | 44.4, 58.9 | <0.001 | 53.2 | 5.4 | 42.6, 63.8 | <0.001 |
| General vision | 55.2 | 4.0 | 47.4, 63.3 | <0.001 | 53.2 | 5.4 | 42.6, 63.8 | <0.001 |
| Ocular pain | 29.4 | 4.0 | 21.5, 37.3 | <0.001 | 24.1 | 5.4 | 13.6, 34.6 | <0.001 |
| Near activities | 55.0 | 4.8 | 45.7, 64.3 | <0.001 | 51.3 | 6.4 | 38.6, 63.9 | <0.001 |
| Distance activities | 56.9 | 4.3 | 48.5, 65.2 | <0.001 | 50.6 | 5.6 | 39.6, 61.7 | <0.001 |
| Social function | 37.9 | 6.6 | 24.9, 50.9 | <0.001 | 28.2 | 8.9 | 11.0, 45.5 | 0.001 |
| Mental function | 42.8 | 3.3 | 36.2, 49.3 | <0.001 | 41.7 | 4.6 | 32.8, 50.6 | <0.001 |
| Role difficulty | 61.9 | 3.9 | 54.2, 69.6 | <0.001 | 63.0 | 5.4 | 52.5, 73.5 | <0.001 |
| Dependency | 60.3 | 5.3 | 49.9, 70.7 | <0.001 | 56.8 | 7.2 | 42.7, 70.9 | <0.001 |
| Driving | 62.3 | 8.9 | 44.8, 79.8 | <0.001 | 62.3 | 11.4 | 40.1, 84.6 | <0.001 |
| Color vision | 2.1 | 1.5 | −0.7, 5.0 | 0.141 | 1.8 | 1.7 | -1.5, 5.2 | 0.280 |
| Extreme/Moderate difficulty | ||||||||
| Little/No difficulty | ||||||||
| Peripheral vision | 4.5 | 1.4 | 1.7, 7.4 | 0.002 | 3.8 | 1.5 | 0.8, 6.7 | 0.012 |
| Extreme/Moderate difficulty | ||||||||
| Little/No difficulty | ||||||||
| Composite score | 48.8 | 3.4 | 42.1, 55.5 | <0.001 | 45.7 | 4.6 | 36.7, 54.7 | <0.001 |
| CDC HRQOL-14 Item | Glaucoma n (%) | Retinitis Pigmentosa n (%) | Diabetes Retinopathy n (%) | Macular Degeneration n (%) | p | ES |
|---|---|---|---|---|---|---|
| Healthy days core module | ||||||
| General health days | 0.592 | 0.309 | ||||
| Good to excellent | 4 (80.0) | 6 (66.7) | 2 (50) | 2 (100) | ||
| Fair to poor | 1 (20.0) | 3 (30.0) | 6 (60.0) | 5 (50.0) | ||
| Poor physical health days (M, SD) | 5.0 (8.7) | 3.6 (4.2) | 3.0 (3.6) | 5.0 (0.0) | 0.993 | 0.026 |
| Poor mental health days (M, SD) | 4.0 (4.2) | 1.7 (3.5) | 1.3 (2.5) | 0 | 0.471 | 0.142 |
| Impact on activity days (M, SD) | 1.0 (2.2) | 3.0 (5.6) | 3.0 (3.6) | 3.5 (5.0) | 0.849 | 0.047 |
| Activities limitation module | ||||||
| Experienced activity limitation | 4 (80.0) | 8 (88.9) | 3 (75.0) | 1 (50.0) | 0.652 | 0.286 |
| Main problems/Impairment | 0.051 | 0.624 | ||||
| Eye/vision problem | 4 (80.0) | 5 (55.6) | 0 | 0 | ||
| Two or more problems | 1 (20.0) | 4 (44.4) | 4 (100.0) | 2 (100.0) | ||
| Problem duration (years) | 5 (100.0) | 9 (100.0) | 4 (100.0) | 2 (100.0) | - | - |
| Need personal care support | 1 (20.0) | 4 (44.4) | 2 (50.0) | 0 | 0.590 | 0.341 |
| Need support for routines | 2 (40.0) | 7 (77.8) | 2 (50.0) | 0 | 0.185 | 0.491 |
| Healthy days symptoms module | ||||||
| Days of feeling pain (M, SD) | 3.0 (6.7) | 2.1 (3.6) | 7.3 (6.1) | 3.5 (5.0) | 0.436 | 0.152 |
| Days of feeling moody (M, SD) | 5.0 (2.2) | 1.7 (3.5) | 1.3 (2.5) | 1.5 (2.1) | 0.382 | 0.169 |
| Days of feeling anxious (M, SD) | 2.2 (2.2) | 1.7 (3.5) | 4.8 (6.6) | 1.5 (2.1) | 0.622 | 0.102 |
| Days of sleep problems (M, SD) | 5.0 (7.1) | 5.1 (5.2) | 4.8 (6.6) | 2.5 (3.5) | 0.952 | 0.020 |
| Days of feeling energetic (M, SD) | 20.0 (10.0) | 22.8 (6.7) | 18.3 (9.0) | 22.5 (3.5) | 0.785 | 0.063 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekemiri, K.K.; Botchway, E.N.; Ezinne, N.E.; Sirju, N.; Persad, T.; Masemola, H.C.; Chidarikire, S.; Ekemiri, C.C.; Osuagwu, U.L. Comparative Analysis of Health- and Vision-Related Quality of Life Measures among Trinidadians with Low Vision and Normal Vision—A Cross-Sectional Matched Sample Study. Int. J. Environ. Res. Public Health 2023, 20, 6436. https://doi.org/10.3390/ijerph20146436
Ekemiri KK, Botchway EN, Ezinne NE, Sirju N, Persad T, Masemola HC, Chidarikire S, Ekemiri CC, Osuagwu UL. Comparative Analysis of Health- and Vision-Related Quality of Life Measures among Trinidadians with Low Vision and Normal Vision—A Cross-Sectional Matched Sample Study. International Journal of Environmental Research and Public Health. 2023; 20(14):6436. https://doi.org/10.3390/ijerph20146436
Chicago/Turabian StyleEkemiri, Kingsley K., Edith N. Botchway, Ngozika E. Ezinne, Nikolai Sirju, Tea Persad, Hlabje Carel Masemola, Sherphard Chidarikire, Chioma C. Ekemiri, and Uchechukwu Levi Osuagwu. 2023. "Comparative Analysis of Health- and Vision-Related Quality of Life Measures among Trinidadians with Low Vision and Normal Vision—A Cross-Sectional Matched Sample Study" International Journal of Environmental Research and Public Health 20, no. 14: 6436. https://doi.org/10.3390/ijerph20146436
APA StyleEkemiri, K. K., Botchway, E. N., Ezinne, N. E., Sirju, N., Persad, T., Masemola, H. C., Chidarikire, S., Ekemiri, C. C., & Osuagwu, U. L. (2023). Comparative Analysis of Health- and Vision-Related Quality of Life Measures among Trinidadians with Low Vision and Normal Vision—A Cross-Sectional Matched Sample Study. International Journal of Environmental Research and Public Health, 20(14), 6436. https://doi.org/10.3390/ijerph20146436







