Riverine Microplastic Pollution: Insights from Cagayan de Oro River, Philippines
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Microplastic Extraction and Identification
2.3. Quality Control
2.4. Data Analysis
3. Results
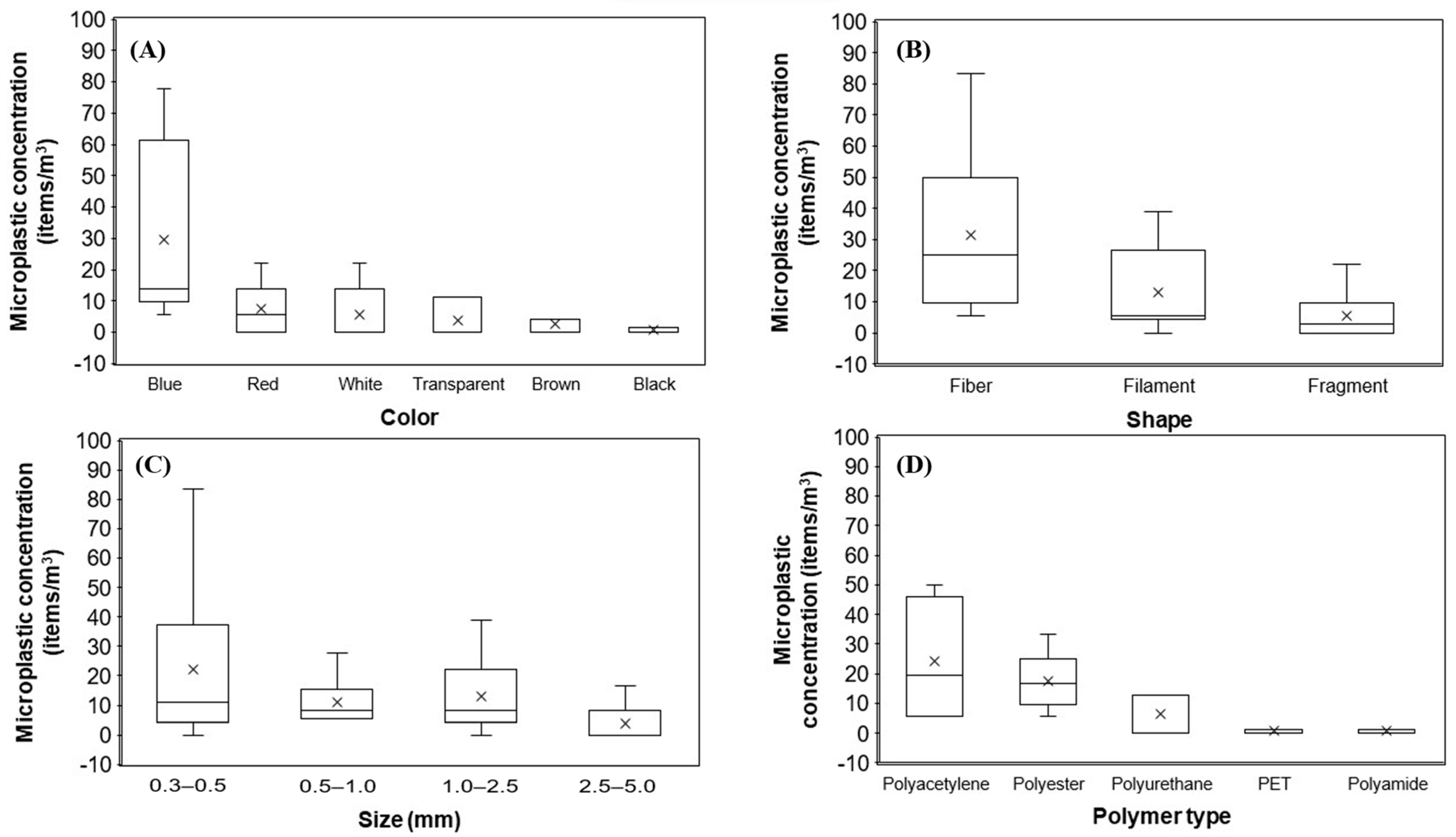
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horton, A.A. Plastic Pollution: When Do We Know Enough? J. Hazard. Mater. 2022, 422, 126885. [Google Scholar] [CrossRef] [PubMed]
- Moad, G.; Solomon, D.H. The Critical Importance of Adopting Whole-of-Life Strategies for Polymers and Plastics. Sustainability 2021, 13, 8218. [Google Scholar] [CrossRef]
- Bajt, O. From Plastics to Microplastics and Organisms. FEBS Open Bio 2021, 11, 954–966. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, P.; Sreekala, M.S. Recycling of Plastics. In Recycling of Polymers: Methods, Characterization, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Mitrano, D.M.; Wagner, M. A Sustainable Future for Plastics Considering Material Safety and Preserved Value. Nat. Rev. Mater. 2021, 7, 71–73. [Google Scholar] [CrossRef]
- de Sousa, F.D.B. The Role of Plastic Concerning the Sustainable Development Goals: The Literature Point of View. Clean. Responsible Consum. 2021, 3, 100020. [Google Scholar] [CrossRef]
- Durance, I.; Bruford, M.W.; Chalmers, R.; Chappell, N.A.; Christie, M.; Cosby, B.J.; Noble, D.; Ormerod, S.J.; Prosser, H.; Weightman, A.; et al. The Challenges of Linking Ecosystem Services to Biodiversity. In Advances in Ecological Research; Elsevier: Amsterdam, The Netherlands, 2016; Volume 54, pp. 87–134. [Google Scholar] [CrossRef]
- Vári, Á.; Podschun, S.A.; Erős, T.; Hein, T.; Pataki, B.; Iojă, I.-C.; Adamescu, C.M.; Gerhardt, A.; Gruber, T.; Dedić, A.; et al. Freshwater Systems and Ecosystem Services: Challenges and Chances for Cross-Fertilization of Disciplines. Ambio 2022, 51, 135–151. [Google Scholar] [CrossRef]
- Otieno, M.O. How much of the Earth is covered by freshwater? World Atlas. Available online: https://www.worldatlas.com/articles/how-much-of-the-earth-is-covered-by-freshwater.html (accessed on 20 February 2023).
- Román-Palacios, C.; Moraga-López, D.; Wiens, J.J. The Origins of Global Biodiversity on Land, Sea and Freshwater. Ecol. Lett. 2022, 25, 1376–1386. [Google Scholar] [CrossRef]
- Superio, M.D.A.; Abreo, N.A.S. Plastic in Freshwater Ecosytems: A Looming Crisis in the Philippines. Philipp. Sci. Lett. 2020, 13, 1–5. [Google Scholar]
- van Emmerik, T.; Strady, E.; Kieu-Le, T.-C.; Nguyen, L.; Gratiot, N. Seasonality of Riverine Macroplastic Transport. Sci. Rep. 2019, 9, 13549. [Google Scholar] [CrossRef]
- van Emmerik, T.; Mellink, Y.; Hauk, R.; Waldschläger, K.; Schreyers, L. Rivers as Plastic Reservoirs. Front. Water 2022, 3, 786936. [Google Scholar] [CrossRef]
- Vriend, P.; van Calcar, C.; Kooi, M.; Landman, H.; Pikaar, R.; van Emmerik, T. Rapid Assessment of Floating Macroplastic Transport in the Rhine. Front. Mar. Sci. 2020, 7, 10. [Google Scholar] [CrossRef]
- Ahmad, J.; Majdi, A.; Babeker Elhag, A.; Deifalla, A.F.; Soomro, M.; Isleem, H.F.; Qaidi, S. A Step towards Sustainable Concrete with Substitution of Plastic Waste in Concrete: Overview on Mechanical, Durability and Microstructure Analysis. Crystals 2022, 12, 944. [Google Scholar] [CrossRef]
- Iroegbu, A.O.C.; Ray, S.S.; Mbarane, V.; Bordado, J.C.; Sardinha, J.P. Plastic Pollution: A Perspective on Matters Arising: Challenges and Opportunities. ACS Omega 2021, 6, 19343–19355. [Google Scholar] [CrossRef]
- Van Sebille, E.; Spathi, C.; Gilbert, A. The Ocean Plastic Pollution Challenge: Towards Solutions in the UK; Technical Report; Imperial College London: London, UK, 2016. [Google Scholar]
- Widyaningrum, S.; Alkyana, M.S.; Kartini, E. Indonesia Strategy to Reduce Land-Based Sources Pollution to Achieve the SDG Target on Life below Water; AIP Publishing: South Tangerang, Indonesia, 2022; p. 070008. [Google Scholar] [CrossRef]
- Sun, L.; Chiu, M.-H.; Xu, C.; Lin, P.; Schwehr, K.A.; Bacosa, H.; Kamalanathan, M.; Quigg, A.; Chin, W.-C.; Santschi, P.H. The Effects of Sunlight on the Composition of Exopolymeric Substances and Subsequent Aggregate Formation during Oil Spills. Mar. Chem. 2018, 203, 49–54. [Google Scholar] [CrossRef]
- Duan, J.; Bolan, N.; Li, Y.; Ding, S.; Atugoda, T.; Vithanage, M.; Sarkar, B.; Tsang, D.C.W.; Kirkham, M.B. Weathering of Microplastics and Interaction with Other Coexisting Constituents in Terrestrial and Aquatic Environments. Water Res. 2021, 196, 117011. [Google Scholar] [CrossRef]
- Bacosa, H.P.; Ancla, S.M.B.; Arcadio, C.G.L.A.; Dalogdog, J.R.A.; Ellos, D.M.C.; Hayag, H.D.A.; Jarabe, J.G.P.; Karim, A.J.T.; Navarro, C.K.P.; Palma, M.P.I.; et al. From Surface Water to the Deep Sea: A Review on Factors Affecting the Biodegradation of Spilled Oil in Marine Environment. J. Mar. Sci. Eng. 2022, 10, 426. [Google Scholar] [CrossRef]
- Andrady, A.L. Weathering and Fragmentation of Plastic Debris in the Ocean Environment. Mar. Pollut. Bull. 2022, 180, 113761. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.; Jaafar, N.F.; Aileen Tan, S.H.; Mohd Zanuri, N.B. A Review of Plastic and Microplastic Pollution towards the Malaysian Marine Environment. IOP Conf. Ser. Earth Environ. Sci. 2022, 1013, 012012. [Google Scholar] [CrossRef]
- Zhang, K.; Hamidian, A.H.; Tubić, A.; Zhang, Y.; Fang, J.K.H.; Wu, C.; Lam, P.K.S. Understanding Plastic Degradation and Microplastic Formation in the Environment: A Review. Environ. Pollut. 2021, 274, 116554. [Google Scholar] [CrossRef]
- Winton, D.J.; Anderson, L.G.; Rocliffe, S.; Loiselle, S. Macroplastic Pollution in Freshwater Environments: Focusing Public and Policy Action. Sci. Total Environ. 2020, 704, 135242. [Google Scholar] [CrossRef]
- Lebreton, L.C.M.; van der Zwet, J.; Damsteeg, J.-W.; Slat, B.; Andrady, A.; Reisser, J. River Plastic Emissions to the World’s Oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef] [PubMed]
- Meijer, L.J.J.; van Emmerik, T.; van der Ent, R.; Schmidt, C.; Lebreton, L. More than 1000 Rivers Account for 80% of Global Riverine Plastic Emissions into the Ocean. Sci. Adv. 2021, 7, eaaz5803. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic Waste Inputs from Land into the Ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Gaboy, S.M.M.; Guihawan, J.Q.; Leopardas, V.E.; Bacosa, H.P. Unravelling Macroplastic Pollution in Seagrass Beds of Iligan City, Mindanao, Philippines. Mar. Pollut. Bull. 2022, 185, 114233. [Google Scholar] [CrossRef]
- Sajorne, R.E.; Cayabo, G.D.B.; Gajardo, L.J.A.; Mabuhay-Omar, J.A.; Creencia, L.A.; Bacosa, H.P. Disentangling Microplastic Pollution on Beach Sand of Puerto Princesa, Palawan Island, Philippines: Abundance and Characteristics. Sustainability 2022, 14, 15303. [Google Scholar] [CrossRef]
- Acot, F.T.; Sajorne, R.E.; Omar, N.-A.K.; Suson, P.D.; Rallos, L.E.E.; Bacosa, H.P. Unraveling Macroplastic Pollution in Rural and Urban Beaches in Sarangani Bay Protected Seascape, Mindanao, Philippines. J. Mar. Sci. Eng. 2022, 10, 1532. [Google Scholar] [CrossRef]
- Inocente, S.A.; Bacosa, H. Assessment of Macroplastic Pollution on Selected Tourism Beaches of Barobo, Surigao Del Sur, Philippines. J. Mar. Isl. Cult. 2022, 11, 203–214. [Google Scholar] [CrossRef]
- Morales, I.D.G.; Macusi, E.D.; Jondonero, M.A.P.; Guihawan, J.Q.; Bacosa, H.P.; Amparado, R.F. Facemask: Protection or Threat? Mar. Pollut. Bull. 2023, 188, 114681. [Google Scholar] [CrossRef] [PubMed]
- Pacilan, C.J.; Bacosa, H. Assessment of Macroplastic Litter on the Coastal Seabeds of Sultan Naga Dimaporo, Lanao Del Norte, Philippines. J. Mar. Isl. Cult. 2022, 11, 13–25. [Google Scholar] [CrossRef]
- Requiron, J.C.; Bacosa, H. Macroplastic Transport and Deposition in the Environs of Pulauan River, Dapitan City, Philippines. Philipp. J. Sci. 2022, 151, 1211–1220. [Google Scholar] [CrossRef]
- Escañan, A.; Bacosa, H. Assessment of Riverine Plastic Flux in Pulot River and Its Tributary in Sofronio Española, Palawan, Philippines. J. Mar. Isl. Cult. 2022, 11, 1–12. [Google Scholar] [CrossRef]
- Gallo, F.; Fossi, C.; Weber, R.; Santillo, D.; Sousa, J.; Ingram, I.; Nadal, A.; Romano, D. Marine Litter Plastics and Microplastics and Their Toxic Chemicals Components: The Need for Urgent Preventive Measures. Environ. Sci. Eur. 2018, 30, 13. [Google Scholar] [CrossRef] [PubMed]
- Kristanti, R.A.; Wong, W.L.; Darmayati, Y.; Hatmanti, A.; Wulandari, N.F.; Sibero, M.T.; Afianti, N.F.; Hernandes, E.; Lopez-Martinez, F. Characteristics of Microplastic in Commercial Aquatic Organisms. Trop. Aquat. Soil Pollut. 2022, 2, 134–158. [Google Scholar] [CrossRef]
- McCormick, A.R.; Hoellein, T.J.; London, M.G.; Hittie, J.; Scott, J.W.; Kelly, J.J. Microplastic in Surface Waters of Urban Rivers: Concentration, Sources, and Associated Bacterial Assemblages. Ecosphere 2016, 7, e01556. [Google Scholar] [CrossRef]
- da Costa, J.P.; Avellan, A.; Mouneyrac, C.; Duarte, A.; Rocha-Santos, T. Plastic Additives and Microplastics as Emerging Contaminants: Mechanisms and Analytical Assessment. TrAC Trends Anal. Chem. 2023, 158, 116898. [Google Scholar] [CrossRef]
- Hermabessiere, L.; Dehaut, A.; Paul-Pont, I.; Lacroix, C.; Jezequel, R.; Soudant, P.; Duflos, G. Occurrence and Effects of Plastic Additives on Marine Environments and Organisms: A Review. Chemosphere 2017, 182, 781–793. [Google Scholar] [CrossRef]
- Luo, H.; Liu, C.; He, D.; Sun, J.; Li, J.; Pan, X. Effects of Aging on Environmental Behavior of Plastic Additives: Migration, Leaching, and Ecotoxicity. Sci. Total Environ. 2022, 849, 157951. [Google Scholar] [CrossRef]
- Sewwandi, M.; Wijesekara, H.; Rajapaksha, A.U.; Soysa, S.; Vithanage, M. Microplastics and Plastics-Associated Contaminants in Food and Beverages; Global Trends, Concentrations, and Human Exposure. Environ. Pollut. 2023, 317, 120747. [Google Scholar] [CrossRef]
- Sridhar, A.; Kannan, D.; Kapoor, A.; Prabhakar, S. Extraction and Detection Methods of Microplastics in Food and Marine Systems: A Critical Review. Chemosphere 2022, 286, 131653. [Google Scholar] [CrossRef]
- Vitali, C.; Peters, R.J.B.; Janssen, H.-G.; Nielen, M.W.F. Microplastics and Nanoplastics in Food, Water, and Beverages; Part I. Occurrence. TrAC Trends Anal. Chem. 2023, 159, 116670. [Google Scholar] [CrossRef]
- Caruso, G. Microplastics as Vectors of Contaminants. Mar. Pollut. Bull. 2019, 146, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Geng, S.; Wu, C.; Song, K.; Sun, F.; Visvanathan, C.; Xie, F.; Wang, Q. Microplastics Contamination in Different Trophic State Lakes along the Middle and Lower Reaches of Yangtze River Basin. Environ. Pollut. 2019, 254, 112951. [Google Scholar] [CrossRef]
- Perveen, S.; Pablos, C.; Reynolds, K.; Stanley, S.; Marugán, J. Microplastics in Fresh- and Wastewater Are Potential Contributors to Antibiotic Resistance—A Minireview. J. Hazard. Mater. Adv. 2022, 6, 100071. [Google Scholar] [CrossRef]
- Galarpe, V.R.K.R.; Jaraula, C.M.B.; Paler, M.K.O. The Nexus of Macroplastic and Microplastic Research and Plastic Regulation Policies in the Philippines Marine Coastal Environments. Mar. Pollut. Bull. 2021, 167, 112343. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as Contaminants in the Marine Environment: A Review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Mani, T.; Hauk, A.; Walter, U.; Burkhardt-Holm, P. Microplastics Profile along the Rhine River. Sci. Rep. 2015, 5, 17988. [Google Scholar] [CrossRef]
- Tong, H.; Zhong, X.; Duan, Z.; Yi, X.; Cheng, F.; Xu, W.; Yang, X. Micro- and Nanoplastics Released from Biodegradable and Conventional Plastics during Degradation: Formation, Aging Factors, and Toxicity. Sci. Total Environ. 2022, 833, 155275. [Google Scholar] [CrossRef]
- Lin, L.; Pan, X.; Zhang, S.; Li, D.; Zhai, W.; Wang, Z.; Tao, J.; Mi, C.; Li, Q.; Crittenden, J.C. Distribution and Source of Microplastics in China’s Second Largest Reservoir—Danjiangkou Reservoir. J. Environ. Sci. 2021, 102, 74–84. [Google Scholar] [CrossRef]
- Argamino, C.R.; Janairo, J.I.B. Qualitative Assessment and Management of Microplastics in Asian Green Mussels (Perna Viridis) Cultured in Bacoor Bay, Cavite, Phillipines. EnvironmentAsia 2016, 9, 48–54. [Google Scholar]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef] [PubMed]
- Bucol, L.A.; Romano, E.F.; Cabcaban, S.M.; Siplon, L.M.D.; Madrid, G.C.; Bucol, A.A.; Polidoro, B. Microplastics in Marine Sediments and Rabbitfish (Siganus Fuscescens) from Selected Coastal Areas of Negros Oriental, Philippines. Mar. Pollut. Bull. 2020, 150, 110685. [Google Scholar] [CrossRef]
- Esquinas, G.G.M.S.; Mantala, A.P.; Atilano, M.G.; Apugan, R.P.; Galarpe, V.R.K.R. Physical Characterization of Litter and Microplastic along the Urban Coast of Cagayan de Oro in Macajalar Bay, Philippines. Mar. Pollut. Bull. 2020, 154, 111083. [Google Scholar] [CrossRef] [PubMed]
- Kalnasa, M.L.; Lantaca, S.M.O.; Boter, L.C.; Flores, G.J.T.; Galarpe, V.R.K.R. Occurrence of Surface Sand Microplastic and Litter in Macajalar Bay, Philippines. Mar. Pollut. Bull. 2019, 149, 110521. [Google Scholar] [CrossRef]
- Paler, M.K.O.; Malenab, M.C.T.; Maralit, J.R.; Nacorda, H.M. Plastic Waste Occurrence on a Beach off Southwestern Luzon, Philippines. Mar. Pollut. Bull. 2019, 141, 416–419. [Google Scholar] [CrossRef]
- Arcadio, C.G.L.A.; Navarro, C.K.P.; Similatan, K.M.; Inocente, S.A.T.; Ancla, S.M.B.; Banda, M.H.T.; Capangpangan, R.Y.; Torres, A.G.; Bacosa, H.P. Microplastics in Surface Water of Laguna de Bay: First Documented Evidence on the Largest Lake in the Philippines. Environ. Sci. Pollut. Res. 2022, 30, 29824–29833. [Google Scholar] [CrossRef] [PubMed]
- Deocaris, C.C.; Allosada, J.O.; Ardiente, L.T.; Bitang, L.G.G.; Dulohan, C.L.; Lapuz, J.K.I.; Padilla, L.M.; Ramos, V.P.; Padolina, J.B.P. Occurrence of Microplastic Fragments in the Pasig River. H2Open J. 2019, 2, 92–100. [Google Scholar] [CrossRef]
- Osorio, E.D.; Tanchuling, M.A.N.; Diola, M.B.L.D. Microplastics Occurrence in Surface Waters and Sediments in Five River Mouths of Manila Bay. Front. Environ. Sci. 2021, 9, 719274. [Google Scholar] [CrossRef]
- Navarro, C.K.P.; Arcadio, C.G.L.A.; Similatan, K.M.; Inocente, S.A.T.; Banda, M.H.T.; Capangpangan, R.Y.; Torres, A.G.; Bacosa, H.P. Unraveling Microplastic Pollution in Mangrove Sediments of Butuan Bay, Philippines. Sustainability 2022, 14, 14469. [Google Scholar] [CrossRef]
- Romarate, R.A.; Ancla, S.M.B.; Patilan, D.M.M.; Inocente, S.A.T.; Pacilan, C.J.M.; Sinco, A.L.; Guihawan, J.Q.; Capangpangan, R.Y.; Lubguban, A.A.; Bacosa, H.P. Breathing Plastics in Metro Manila, Philippines: Presence of Suspended Atmospheric Microplastics in Ambient Air. Environ. Sci. Pollut. Res. 2023, 30, 53662–53673. [Google Scholar] [CrossRef]
- Cabansag, J.B.P.; Olimberio, R.B.; Villanobos, Z.M.T. Microplastics in Some Fish Species and Their Environs in Eastern Visayas, Philippines. Mar. Pollut. Bull. 2021, 167, 112312. [Google Scholar] [CrossRef]
- Requiron, J.C.; Gutierrez, C.S.; Inocente, S.A.T.; Pacilan, C.J.M.; Gaboy, S.M.M.; Sison, C.P.; Amparado, R.F.; Bacosa, H.P. Aquaculture Farmers’ Perception and Level of Awareness of Plastic Litter in San Pedro, Dapitan City, Mindanao, The Philippines. J. Sustain. Sci. Manag. 2023, 18, 77–91. [Google Scholar]
- Lo, D.S.; Oreta, W.C. Seismic Risk Mapping at Micro-Scale: The Case of Barangay Carmen, Cagayan de Oro City, Philippines. In Proceedings of the Harnessing Lessons Towards an Earthquake-Resilient Nation, Sydney, Australia, August 8–12 2010. [Google Scholar]
- Sinco, A.L.; Sendaydiego, J.P.; Saab, L.L.; Mojica, G.R.; Tampus, G.G.; Rondez, A.S. Riverine Biota as Indicators of Water Quality in Tropical Cagayan de Oro River, Philippines. Adv. Environ. Sci. 2014, 6, 157–167. [Google Scholar]
- Mirasol, J.M. Exploring the Confluence of the Cagayan de Oro River’s Ecosystems. Liceo J. High. Educ. Res. 2014, 10, 73–82. [Google Scholar] [CrossRef]
- Meijer, L.; van Emmerik, T.; Lebreton, L.; Schmidt, C.; van der Ent, R. Over 1000 Rivers Accountable for 80% of Global Riverine Plastic Emissions into the Ocean. Engineering 2019. preprint. [Google Scholar] [CrossRef]
- Japos, G.V.; Lubos, L.C. Practices, Behaviors, and Action on Climate Change and Environmental Protection and Conservation of Settlers along the Banks of Oro River, Northern Mindanao, Philippines. Asian J. Biodivers 2014, 5, 109–135. [Google Scholar] [CrossRef]
- Lubos, L.C.; Amoroso, V.B.; Coritico, F.; Demetillo, M. Species Richness and Riparian Vegetation of Plants in Cagayan de Oro River, Mindanao, Philippines. Asian J. Biodivers. 2016, 6, 41–68. [Google Scholar] [CrossRef]
- Chen, H.L.; Gibbins, C.N.; Selvam, S.B.; Ting, K.N. Spatio-Temporal Variation of Microplastic along a Rural to Urban Transition in a Tropical River. Environ. Pollut. 2021, 289, 117895. [Google Scholar] [CrossRef]
- Han, M.; Niu, X.; Tang, M.; Zhang, B.-T.; Wang, G.; Yue, W.; Kong, X.; Zhu, J. Distribution of Microplastics in Surface Water of the Lower Yellow River near Estuary. Sci. Total Environ. 2020, 707, 135601. [Google Scholar] [CrossRef]
- Egessa, R.; Nankabirwa, A.; Ocaya, H.; Pabire, W.G. Microplastic Pollution in Surface Water of Lake Victoria. Sci. Total Environ. 2020, 741, 140201. [Google Scholar] [CrossRef]
- Masura, J.; Baker, J.; Foster, G.; Arthur, C. Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments. NOAA Technical Memorandum NOS-OR&R-48. OceanBestPractices 2015, 1–31. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Su, L.; Xue, Y.; Li, L.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in Taihu Lake, China. Environ. Pollut. 2016, 216, 711–719. [Google Scholar] [CrossRef]
- Abidli, S.; Toumi, H.; Lahbib, Y.; Trigui El Menif, N. The First Evaluation of Microplastics in Sediments from the Complex Lagoon-Channel of Bizerte (Northern Tunisia). Water. Air. Soil Pollut. 2017, 228, 262. [Google Scholar] [CrossRef]
- Toumi, H.; Abidli, S.; Bejaoui, M. Microplastics in Freshwater Environment: The First Evaluation in Sediments from Seven Water Streams Surrounding the Lagoon of Bizerte (Northern Tunisia). Environ. Sci. Pollut. Res. 2019, 26, 14673–14682. [Google Scholar] [CrossRef]
- Primpke, S.; Christiansen, S.H.; Cowger, W.; De Frond, H.; Deshpande, A.; Fischer, M.; Holland, E.B.; Meyns, M.; O’Donnell, B.A.; Ossmann, B.E.; et al. Critical Assessment of Analytical Methods for the Harmonized and Cost-Efficient Analysis of Microplastics. Appl. Spectrosc. 2020, 74, 1012–1047. [Google Scholar] [CrossRef]
- Tirkey, A.; Upadhyay, L.S.B. Microplastics: An Overview on Separation, Identification and Characterization of Microplastics. Mar. Pollut. Bull. 2021, 170, 112604. [Google Scholar] [CrossRef]
- Cunsolo, S.; Williams, J.; Hale, M.; Read, D.S.; Couceiro, F. Optimising Sample Preparation for FTIR-Based Microplastic Analysis in Wastewater and Sludge Samples: Multiple Digestions. Anal. Bioanal. Chem. 2021, 413, 3789–3799. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Gao, L.; Gao, H.; Zou, D. Characteristics and Distribution of Microplastics in the Surface Water of the Songhua River in China. Environ. Sci. Pollut. Res. 2021, 28, 64268–64277. [Google Scholar] [CrossRef]
- Buwono, N.R.; Risjani, Y.; Soegianto, A. Distribution of Microplastic in Relation to Water Quality Parameters in the Brantas River, East Java, Indonesia. Environ. Technol. Innov. 2021, 24, 101915. [Google Scholar] [CrossRef]
- Ta, A.T.; Babel, S. Occurrence and Spatial Distribution of Microplastic Contaminated with Heavy Metals in a Tropical River: Effect of Land Use and Population Density. Mar. Pollut. Bull. 2023, 191, 114919. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; You, J.; Li, Y.; Zhang, J.; He, Y.; Breider, F.; Tao, S.; Liu, W. Insights into the Horizontal and Vertical Profiles of Microplastics in a River Emptying into the Sea Affected by Intensive Anthropogenic Activities in Northern China. Sci. Total Environ. 2021, 779, 146589. [Google Scholar] [CrossRef]
- Mai, L.; You, S.-N.; He, H.; Bao, L.-J.; Liu, L.-Y.; Zeng, E.Y. Riverine Microplastic Pollution in the Pearl River Delta, China: Are Modeled Estimates Accurate? Environ. Sci. Technol. 2019, 53, 11810–11817. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zuo, L.-Z.; Peng, J.-P.; Cai, L.-Q.; Fok, L.; Yan, Y.; Li, H.-X.; Xu, X.-R. Occurrence and Distribution of Microplastics in an Urban River: A Case Study in the Pearl River along Guangzhou City, China. Sci. Total Environ. 2018, 644, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Bruge, A.; Dhamelincourt, M.; Lanceleur, L.; Monperrus, M.; Gasperi, J.; Tassin, B. A First Estimation of Uncertainties Related to Microplastic Sampling in Rivers. Sci. Total Environ. 2020, 718, 137319. [Google Scholar] [CrossRef] [PubMed]
- Lestari, P.; Trihadiningrum, Y.; Wijaya, B.A.; Yunus, K.A.; Firdaus, M. Distribution of Microplastics in Surabaya River, Indonesia. Sci. Total Environ. 2020, 726, 138560. [Google Scholar] [CrossRef]
- Campanale, C.; Stock, F.; Massarelli, C.; Kochleus, C.; Bagnuolo, G.; Reifferscheid, G.; Uricchio, V.F. Microplastics and Their Possible Sources: The Example of Ofanto River in Southeast Italy. Environ. Pollut. 2020, 258, 113284. [Google Scholar] [CrossRef]
- Liu, R.; Li, Z.; Liu, F.; Dong, Y.; Jiao, J.; Sun, P.; Rm, E.-W. Microplastic Pollution in Yellow River: Current Status and Research Progress of Biotoxicological Effects. China Geol. 2021, 4, 585–592. [Google Scholar] [CrossRef]
- Lambert, S.; Scherer, C.; Wagner, M. Ecotoxicity Testing of Microplastics: Considering the Heterogeneity of Physicochemical Properties: Ecotoxicity Testing of Microplastics. Integr. Environ. Assess. Manag. 2017, 13, 470–475. [Google Scholar] [CrossRef]
- Akkajit, P.; Tipmanee, D.; Cherdsukjai, P.; Suteerasak, T.; Thongnonghin, S. Occurrence and Distribution of Microplastics in Beach Sediments along Phuket Coastline. Mar. Pollut. Bull. 2021, 169, 112496. [Google Scholar] [CrossRef]
- Kasamesiri, P. Microplastics Ingestion by Freshwater Fish in the Chi River, Thailand. Int. J. GEOMATE 2020, 18, 114–119. [Google Scholar] [CrossRef]
- Xu, P.; Peng, G.; Su, L.; Gao, Y.; Gao, L.; Li, D. Microplastic Risk Assessment in Surface Waters: A Case Study in the Changjiang Estuary, China. Mar. Pollut. Bull. 2018, 133, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Clere, I.K.; Ahmmed, F.; Remoto, P.I.J.G.; Fraser-Miller, S.J.; Gordon, K.C.; Komyakova, V.; Allan, B.J.M. Quantification and Characterization of Microplastics in Commercial Fish from Southern New Zealand. Mar. Pollut. Bull. 2022, 184, 114121. [Google Scholar] [CrossRef] [PubMed]
- Ory, N.C.; Sobral, P.; Ferreira, J.L.; Thiel, M. Amberstripe Scad Decapterus Muroadsi (Carangidae) Fish Ingest Blue Microplastics Resembling Their Copepod Prey along the Coast of Rapa Nui (Easter Island) in the South Pacific Subtropical Gyre. Sci. Total Environ. 2017, 586, 430–437. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, G.; Wang, W.; Wang, J. Microplastic Pollution Research Methodologies, Abundance, Characteristics and Risk Assessments for Aquatic Biota in China. Environ. Pollut. 2020, 266, 115098. [Google Scholar] [CrossRef]
- Li, W.; Chen, X.; Li, M.; Cai, Z.; Gong, H.; Yan, M. Microplastics as an Aquatic Pollutant Affect Gut Microbiota within Aquatic Animals. J. Hazard. Mater. 2022, 423, 127094. [Google Scholar] [CrossRef]
- Egbeocha, C.; Malek, S.; Emenike, C.; Milow, P. Feasting on Microplastics: Ingestion by and Effects on Marine Organisms. Aquat. Biol. 2018, 27, 93–106. [Google Scholar] [CrossRef]
- Rebelein, A.; Int-Veen, I.; Kammann, U.; Scharsack, J.P. Microplastic Fibers—Underestimated Threat to Aquatic Organisms? Sci. Total Environ. 2021, 777, 146045. [Google Scholar] [CrossRef]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of Different Shapes of Microplastics Initiates Intestinal Injury and Gut Microbiota Dysbiosis in the Gut of Zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef]
- Faulstich, L.; Prume, J.A.; Arendt, R.; Reinhardt-Imjela, C.; Chifflard, P.; Schulte, A. Microplastics in Namibian River Sediments—A First Evaluation. Microplast. Nanoplast. 2022, 2, 24. [Google Scholar] [CrossRef]
- Wong, G.; Löwemark, L.; Kunz, A. Microplastic Pollution of the Tamsui River and Its Tributaries in Northern Taiwan: Spatial Heterogeneity and Correlation with Precipitation. Environ. Pollut. 2020, 260, 113935. [Google Scholar] [CrossRef]
- Lehtiniemi, M.; Hartikainen, S.; Näkki, P.; Engström-Öst, J.; Koistinen, A.; Setälä, O. Size Matters More than Shape: Ingestion of Primary and Secondary Microplastics by Small Predators. Food Webs 2018, 17, e00097. [Google Scholar] [CrossRef]
- Desai, A.; Srimuruganandam, B.; Nanajkar, M.; Kumar, M.; Saha, M.; Rathore, C.; Gupta, P.; Naik, A. Abundance and Quantification of Microplastics in Water and Sediments of Sal River and an Approach to Study the Degradation of Microplastics. In Proceedings of the Ocean Society of India Conference (OSICON–19), Kochi, India, 12–14 December 2019. [Google Scholar]
- Gupta, P.; Saha, M.; Rathore, C.; Suneel, V.; Ray, D.; Naik, A.; Unnikrishnan, K.; Dhivya, M.; Daga, K. Spatial and Seasonal Variation of Microplastics and Possible Sources in the Estuarine System from Central West Coast of India. Environ. Pollut. 2021, 288, 117665. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, T.; Nihei, Y.; Kudou, K.; Hinata, H. Assessment of the Sources and Inflow Processes of Microplastics in the River Environments of Japan. Environ. Pollut. 2019, 244, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Naik, A.; Desai, A.; Nanajkar, M.; Rathore, C.; Kumar, M.; Gupta, P. Microplastics in Seafood as an Emerging Threat to Marine Environment: A Case Study in Goa, West Coast of India. Chemosphere 2021, 270, 129359. [Google Scholar] [CrossRef]
- Dagani, T. Organic Battery Uses Polyacetylene Electrodes. Chem. Inf. 1981, 12, 39–40. [Google Scholar] [CrossRef]
- Rathinamoorthy, R.; Raja Balasaraswathi, S. Investigations on the Impact of Handwash and Laundry Softener on Microfiber Shedding from Polyester Textiles. J. Text. Inst. 2022, 113, 1428–1437. [Google Scholar] [CrossRef]
- Xia, W.; Rao, Q.; Deng, X.; Chen, J.; Xie, P. Rainfall Is a Significant Environmental Factor of Microplastic Pollution in Inland Waters. Sci. Total Environ. 2020, 732, 139065. [Google Scholar] [CrossRef]
- Yin, L.; Wen, X.; Huang, D.; Zhou, Z.; Xiao, R.; Du, L.; Su, H.; Wang, K.; Tian, Q.; Tang, Z.; et al. Abundance, Characteristics, and Distribution of Microplastics in the Xiangjiang River, China. Gondwana Res. 2022, 107, 123–133. [Google Scholar] [CrossRef]
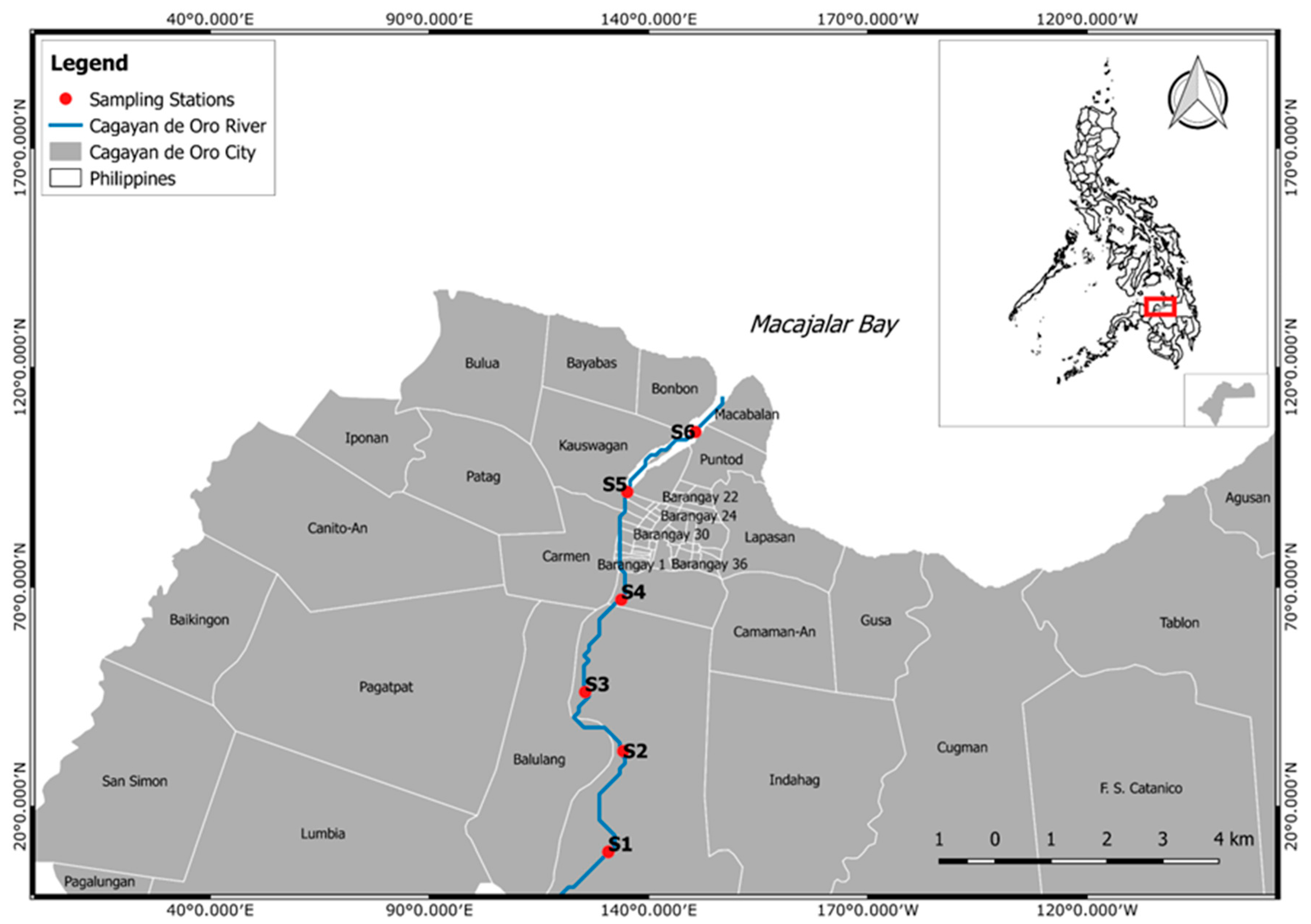



| Sampling Station | Sampling Point | Coordinates | Characteristics | No. of MPs | Mean Concentration (Items/m3 ± SD) |
|---|---|---|---|---|---|
| S1 | P1 P2 P3 | 8.429735 N, 124.638408 E 8.429853 N, 124.638133 E 8.430017 N, 124.637802 E | Fishing, Recreational | 2 4 0 | 200 ± 66.67 abc |
| S2 | P1 P2 P3 | 8.446211 N, 124.640646 E 8.446159 N, 124.640544 E 8.446132 N, 124.640422 E | Fishing, Recreational | 1 3 4 | 266.7 ± 50.92 abc |
| S3 | P1 P2 P3 | 8.455706 N, 124.634622 E 8.455736 N, 124.634365 E 8.455706 N, 124.634140 E | Non-residential | 0 1 1 | 66.7 ± 19.25 c |
| S4 | P1 P2 P3 | 8.470570 N, 124.64066 E 8.470710 N, 124.64024 E 8.470918 N, 124.63982 E | Non-residential | 0 0 3 | 100 ± 57.74 bc |
| S5 | P1 P2 P3 | 8.488010 N, 124.64183 E 8.488176 N, 124.64121 E 8.488471 N, 124.64058 E | Fishing, Residential | 8 3 6 | 566.7 ± 83.89 ab |
| S6 | P1 P2 P3 | 8.497271 N, 124.653097 E 8.427902 N, 124.652271 E 8.498613 N, 124.651289 E | Fishing, Residential | 7 7 4 | 600 ± 57.74 a |
| Study Area | Mesh Size/Pore Size (mm) | MP Concentration (Items/m3) | Reference |
|---|---|---|---|
| Cagayan de Oro River (Philippines) | 0.3 | 300 | This study |
| Cañas River (Philippines) | 0.075 | 1580 | Osorio et al. [62] |
| Pasig River (Philippines) | 0.075 | 3405 | Osorio et al. [62] |
| Parañaque River (Philippines) | 0.075 | 5015 | Osorio et al. [62] |
| Tullahan River (Philippines) | 0.075 | 11,475 | Osorio et al. [62] |
| Meycauayan River (Philippines) | 0.075 | 57,665 | Osorio et al. [62] |
| Gave de Pau River (France) | 0.33 | 3.26 | Bruge et al. [90] |
| Surabaya River (Indonesia) | 0.333 | 1.47–43.11 | Lestari et al. [91] |
| Ofanto River (Italy) | 0.333 | 0.9–13 | Campanale et al. [92] |
| Pearl River Delta (China) | 0.33 | 0.005–0.7 | Mai et al. [88] |
| Pearl River (China) | 0.02 | 379–7924 | Lin et al. [83] |
| Yellow River (China) | 0.05 | 5358–595,270 | Liu et al. [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabriel, A.D.; Amparado, R.F., Jr.; Lubguban, A.A.; Bacosa, H.P. Riverine Microplastic Pollution: Insights from Cagayan de Oro River, Philippines. Int. J. Environ. Res. Public Health 2023, 20, 6132. https://doi.org/10.3390/ijerph20126132
Gabriel AD, Amparado RF Jr., Lubguban AA, Bacosa HP. Riverine Microplastic Pollution: Insights from Cagayan de Oro River, Philippines. International Journal of Environmental Research and Public Health. 2023; 20(12):6132. https://doi.org/10.3390/ijerph20126132
Chicago/Turabian StyleGabriel, Aiza D., Ruben F. Amparado, Jr., Arnold A. Lubguban, and Hernando P. Bacosa. 2023. "Riverine Microplastic Pollution: Insights from Cagayan de Oro River, Philippines" International Journal of Environmental Research and Public Health 20, no. 12: 6132. https://doi.org/10.3390/ijerph20126132
APA StyleGabriel, A. D., Amparado, R. F., Jr., Lubguban, A. A., & Bacosa, H. P. (2023). Riverine Microplastic Pollution: Insights from Cagayan de Oro River, Philippines. International Journal of Environmental Research and Public Health, 20(12), 6132. https://doi.org/10.3390/ijerph20126132









