Effect of Individualized Coaching at Home on Quality of Life in Subacute Stroke Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Participants
2.2. Interventions
2.3. Measurements
2.4. Statistical Analysis
3. Results
3.1. Descriptive Analysis of the Sample
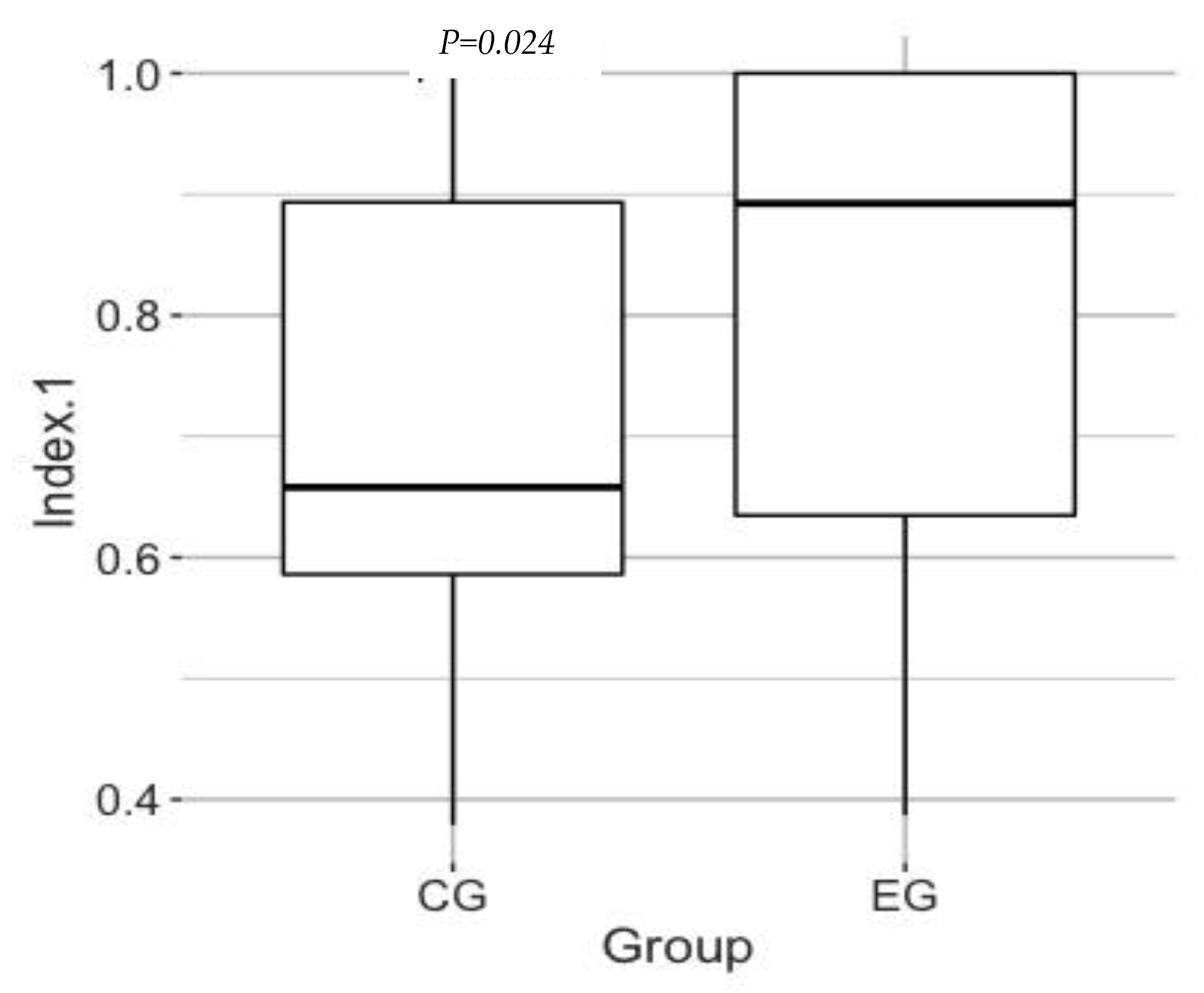
3.2. Effect of the Program on the QOL
3.3. Effects on Other Outcomes
4. Discussion
4.1. Effect of PA Incentive Program on QOL
4.2. Effect of PA Incentive Program on QOL Domains
4.3. Relation between QOL Assessment and Level of Physical Activity during Daily Living
4.4. Limits of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2016 Causes of Death Collaborators. Global, Regional, and National Age-Sex Specific Mortality for 264 Causes of Death, 1980–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef] [PubMed]
- De Morand, A. Pratique de la Rééducation Neurologique. Available online: https://www.elsevier-masson.fr/pratique-de-la-reeducation-neurologique-9782294744020.html (accessed on 10 March 2022).
- Marulanda-Londoño, E.; Chaturvedi, S. Stroke Due to Large Vessel Atherosclerosis. Neurol Clin. Pract. 2016, 6, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Colle, F.; Bonan, I.; Gellez Leman, M.-C.; Bradai, N.; Yelnik, A. Fatigue after Stroke. Ann. Readapt. Med. Phys. 2006, 49, 272–276. [Google Scholar] [CrossRef] [PubMed]
- MacKay-Lyons, M.J.; Howlett, J. Exercise Capacity and Cardiovascular Adaptations to Aerobic Training Early after Stroke. Top. Stroke Rehabil. 2005, 12, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Masson, E. Activité Physique de Loisir et Qualité de Vie. Available online: https://www.em-consulte.com/es/article/201032/figures/activite-physique-de-loisir-et-qualite-de-vie (accessed on 10 April 2022).
- Haute Autorité de Santé. Consultation et Prescription Médicale d’activité Physique à des fins de Santé chez L’adulte. Available online: https://www.has-sante.fr/upload/docs/application/pdf/2018-10/guide_aps_vf.pdf (accessed on 13 July 2022).
- Studenski, S.; Duncan, P.W.; Perera, S.; Reker, D.; Lai, S.M.; Richards, L. Daily Functioning and Quality of Life in a Randomized Controlled Trial of Therapeutic Exercise for Subacute Stroke Survivors. Stroke 2005, 36, 1764–1770. [Google Scholar] [CrossRef] [PubMed]
- Chaiyawat, P.; Kulkantrakorn, K.; Sritipsukho, P. Effectiveness of Home Rehabilitation for Ischemic Stroke. Neurol. Int. 2009, 1, e10. [Google Scholar] [CrossRef] [PubMed]
- Chaiyawat, P.; Kulkantrakorn, K. Effectiveness of Home Rehabilitation Program for Ischemic Stroke upon Disability and Quality of Life: A Randomized Controlled Trial. Clin. Neurol. Neurosurg. 2012, 114, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, E.; Gosman-Hedström, G.; Lindström, B.; Wester, P. What Is the Benefit of a High-Intensive Exercise Program on Health-Related Quality of Life and Depression after Stroke? A Randomized Controlled Trial. Adv. Physiother. 2010, 12, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Flansbjer, U.-B.; Lexell, J.; Brogårdh, C. Long-Term Benefits of Progressive Resistance Training in Chronic Stroke: A 4-Year Follow-Up. J. Rehabil. Med. 2012, 44, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Macko, R.F.; Ivey, F.M.; Forrester, L.W.; Hanley, D.; Sorkin, J.D.; Katzel, L.I.; Silver, K.H.; Goldberg, A.P. Treadmill Exercise Rehabilitation Improves Ambulatory Function and Cardiovascular Fitness in Patients with Chronic Stroke: A Randomized, Controlled Trial. Stroke 2005, 36, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, D.; Daviet, J.-C.; Borel, B.; Kammoun, B.; Salle, J.-Y.; Tchalla, A.; Mandigout, S. Home-Based Physical Activity Incentive and Education Program in Subacute Phase of Stroke Recovery (Ticaa’dom): Study Protocol for a Randomized Controlled Trial. Trials 2018, 19, 68. [Google Scholar] [CrossRef] [PubMed]
- Daviet, J.C.; Bonan, I.; Caire, J.M.; Colle, F.; Damamme, L.; Froger, J.; Leblond, C.; Leger, A.; Muller, F.; Simon, O.; et al. Therapeutic patient education for stroke survivors: Non-pharmacological management. A literature review. Ann. Phys. Rehabil. Med. 2012, 55, 641–656. [Google Scholar] [CrossRef] [PubMed]
- EuroQol Research Foundation. EQ-5D User Guides—EQ-5D. Available online: https://euroqol.org/publications/user-guides/ (accessed on 7 September 2022).
- Barthel Index for Stroke Trials: Development, Properties, and Application—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/21372310/ (accessed on 10 April 2022).
- Collin, C.; Wade, D. Assessing Motor Impairment after Stroke: A Pilot Reliability Study. J. Neurol. Neurosurg. Psychiatry 1990, 53, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Brun, V.; Mousbeh, Z.; Jouet-Pastre, B.; Benaim, C.; Kunnert, J.E.; Dhoms, G.; d’Angeli-Chevassut, M.; Torres, B.; Pélissier, J. Évaluation clinique de la marche de l’hémiplégique vasculaire: Proposition d’une modification de la functional ambulation classification. Ann. Readapt. Med. Phys. 2000, 43, 14–20. [Google Scholar] [CrossRef]
- American Thoracic Society. ATS Statement: Guidelines for the Six-Minute Walk Test—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/12091180/ (accessed on 10 April 2022).
- Mandigout, S.; Chaparro, D.; Borel, B.; Kamoun, B.; Salle, J.Y.; Compagnat, M.; Daviet, J.C. Effet of individualized coaching at home on walking capacity in subacute stroke patients: A randomized controlled trial (Ticaa’dom). Ann. Phys. Rehabil. Med. 2021, 64, 101453. [Google Scholar] [CrossRef] [PubMed]
- Duncan, P.; Studenski, S.; Richards, L.; Gollub, S.; Lai, S.M.; Reker, D.; Perera, S.; Yates, J.; Koch, V.; Rigler, S.; et al. Randomized Clinical Trial of Therapeutic Exercise in Subacute Stroke. Stroke 2003, 34, 2173–2180. [Google Scholar] [CrossRef] [PubMed]
- Mead, G.E.; Greig, C.A.; Cunningham, I.; Lewis, S.J.; Dinan, S.; Saunders, D.H.; Fitzsimons, C.; Young, A. Stroke: A Randomized Trial of Exercise or Relaxation. J. Am. Geriatr. Soc. 2007, 55, 892–899. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | n = 83 | |
|---|---|---|
| Mean age (years) ± SD | 62.2 ± 13.6 | |
| Gender (n = ; M/F) | 56/27 | |
| Mean time post-stroke (days) ± SD | 77.9 ± 45.1 | |
| BMI (kg.m−2) | 26.1 (5.4) | |
| Nature of the stroke (%) | Ischemic | 62 (75) |
| Hemorrhagic | 21 (25) | |
| Location of the stroke (%) | Cerebellar | 6 (7) |
| Parietal | 1 (1) | |
| Middle cerebral | 54 (65) | |
| Anterior brain | 4 (5) | |
| Posterior cerebral | 3 (4) | |
| Brain stem | 14 (17) | |
| Ventricle | 1 (1) | |
| Side reached (%) | Right | 38 (46) |
| Left | 45 (54) | |
| Background (n = /83) | Smoker | 12 |
| HBP | 40 | |
| Diabetes | 7 | |
| Depression | 4 | |
| Heart disease | 15 | |
| Mean Blood pressure (mm/Hg) ± SD | Systolic | 137.6 ± 13.5 |
| Diastolic | 80.1 ± 7.5 | |
| Parameters | Median | Lower Quartile | Superior Quartile | Rank (Min/Max) |
|---|---|---|---|---|
| Barthel Index (/100) | 100 | 95 | 100 | 55/100 |
| mFAC (/8) | 6 | 6 | 8 | 2/8 |
| MDI (/100) | 94 | 77 | 100 | 32/100 |
| 6MWT (m) | 358 | 270 | 485 | 30/658 |
| EQ-5D-5L index | 0.760 | 0.575 | 0.875 | 338/1000 |
| Number Step | 4081 | 1453 | 6153 | 33/16,084 |
| TEE (Kcal) | 1680 | 1482 | 1953 | 850/2415 |
| TAEE (Kcal) | 444 | 263 | 653 | 2.8/1049.3 |
| Total Group (n = 83) | Experimental Group (n = 42) | Control Group (n = 41) | ||||
|---|---|---|---|---|---|---|
| Dimensions | T0 | T1 | T0 | T1 | T0 | T1 |
| Mobility; n (%) | p = 0.01 | p = 0.23 | p = 0.60 | |||
| No problems | 50 (60.2) | 54 (65.1) | 27 (64.3) | 32 (76.2) | 23 (56.1) | 22 (53.7) |
| Slight problems | 33 (39.8) | 28 (33.7) | 15 (35.7) | 10 (23.8) | 18(43.9) | 18 (43.9) |
| Moderate problems | 0 (0) | 1 (1.2) | 0 (0) | 0 (0) | 0 (0) | 1 (2.4) |
| Severe problems | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Unable to walk about | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Self-care; n (%) | NS | p = 0.14 | p = 0.71 | |||
| No problems | 61 (73.4) | 68 (81.9) | 31 (73.8) | 35 (83.3) | 30 (73.2) | 353 (80.5) |
| Slight problems | 14 (16.9) | 13 (15.7) | 5 (11.9) | 6 (14.3) | 10 (24.4) | 7 (17.1) |
| Moderate problems | 8 (9.6) | 2 (2.4) | 6 (14.3) | 1 (2.4) | 1 (2.4) | 1 (2.4) |
| Severe problems | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Unable to wash or dress | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Usual activities; n (%) | p = 0.03 | p = 0.10 | p = 0.97 | |||
| No problems | 33 (39.8) | 46 (55.4) | 19 (45.2) | 26 (61.9) | 19 (46.3) | 20 (48.8) |
| Slight problems | 39 (46.9) | 32 (38.6) | 20 (47.6) | 26 (38.1) | 17 (41.5) | 16 (39.0) |
| Moderate problems | 11 (13.3) | 5 (6) | 3 (7.2) | 0 (0) | 5 (12.2) | 5 (12.2) |
| Severe problems | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Unable to do usual activities | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Pain/discomfort; n (%) | p = 0.01 | p = 0.42 | p = 0.68 | |||
| No pain/discomfort | 31 (37.3) | 45 (54.2) | 20 (47.6) | 26 (61.9) | 19 (46.3) | 18 (46.3) |
| Slight pain/discomfort | 42 (50.6) | 32 (38.6) | 19 (45.2) | 14 (33.3) | 20 (48.8) | 18 (43.9) |
| Moderate pain/discomfort | 10 (12.1) | 6 (7.2) | 3 (7.2) | 2 (4.8) | 2 (4.9) | 4 (9.8) |
| Severe pain/discomfort | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Extreme pain/discomfort | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Anxiety/depression; n (%) | p = 0.01 | p = 0.21 | p = 0.51 | |||
| Not anxious/depressed | 26 (31.3) | 46 (55.4) | 17 (40.5) | 25 (59.5) | 16 (39.0) | 20 (48.8) |
| Slightly anxious/depressed | 49 (59.1) | 35 (42.2) | 24 (57.1) | 17 (40.5) | 22 (53.7) | 20 (48.8) |
| Moderately anxious/depressed | 8 (9.6) | 2 (2.4) | 1 (2.4) | 0 (0) | 3 (7.3) | 1 (2.4) |
| Severely anxious/depressed | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Extremely anxious/depressed | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| EG; n = 42 | CG; n = 41 | p | |||||
|---|---|---|---|---|---|---|---|
| T0 | T1 | T0 | T1 | G | T | G × T | |
| 6MWT | 361.9 ± 148.4 | 430.8 ± 145.1 | 379.9 ± 147.8 | 391.6 ± 152.5 | 0.4 | 0.01 | 0.01 |
| BI | 94.8 ± 9.6 | 97.2 ± 6.5 | 94.7 ± 8.5 | 96.1 ± 9.9 | 0.7 | 0.2 | 0.5 |
| MDI | 90.4 ± 11.9 | 93.6 ± 10.1 | 85.0 ± 17.7 | 89.6 ± 15.2 | 0.05 | 0.1 | 0.7 |
| mFAC | 6.5 ± 1.6 | 7.2 ± 0.8 | 6.6 ± 1.2 | 6.5 ± 1.5 | 0.25 | 0.17 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Telfils, R.; Gelineau, A.; Daviet, J.-C.; Lacroix, J.; Borel, B.; Toulgui, E.; Compagnat, M.; Mandigout, S. Effect of Individualized Coaching at Home on Quality of Life in Subacute Stroke Patients. Int. J. Environ. Res. Public Health 2023, 20, 5908. https://doi.org/10.3390/ijerph20105908
Telfils R, Gelineau A, Daviet J-C, Lacroix J, Borel B, Toulgui E, Compagnat M, Mandigout S. Effect of Individualized Coaching at Home on Quality of Life in Subacute Stroke Patients. International Journal of Environmental Research and Public Health. 2023; 20(10):5908. https://doi.org/10.3390/ijerph20105908
Chicago/Turabian StyleTelfils, Rodeline, Axelle Gelineau, Jean-Christophe Daviet, Justine Lacroix, Benoit Borel, Emna Toulgui, Maxence Compagnat, and Stéphane Mandigout. 2023. "Effect of Individualized Coaching at Home on Quality of Life in Subacute Stroke Patients" International Journal of Environmental Research and Public Health 20, no. 10: 5908. https://doi.org/10.3390/ijerph20105908
APA StyleTelfils, R., Gelineau, A., Daviet, J.-C., Lacroix, J., Borel, B., Toulgui, E., Compagnat, M., & Mandigout, S. (2023). Effect of Individualized Coaching at Home on Quality of Life in Subacute Stroke Patients. International Journal of Environmental Research and Public Health, 20(10), 5908. https://doi.org/10.3390/ijerph20105908







